Abstract
Introduction
Psoriatic arthritis (PsA) is associated with increased cardiovascular (CV) risk and mortality. Aortic stiffness measured by carotid-femoral pulse wave velocity (cfPWV) has been shown to predict CV risk in the general population. The present study aimed to examine cfPWV values of patients with PsA compared to healthy controls and to evaluate associations of cfPWV with patient- and disease-associated characteristics, as well as with an established traditional CV prediction score of the European Society of Cardiology (Systemic Coronary Risk Evaluation; SCORE), for the first time.
Methods
cfPWV and SCORE were evaluated in patients with PsA and healthy controls, along with clinical and laboratory disease parameters. Differences in cfPWV measurements between the two groups and associations of cfPWV with patient- and disease-associated characteristics were statistically evaluated.
Results
A total of 150 patients with PsA (PSOCARD cohort) and 88 control subjects were recruited. cfPWV was significantly higher in the PsA group compared to controls, even after adjustment for confounders (padj = 0.034). Moreover, cfPWV was independently associated with disease duration (r = 0.304, p = 0.001), age (rho = 0.688, p < 0.001), systolic arterial pressure (rho = 0.351, p < 0.001), glomerular filtration rate (inverse: rho = − 0.264, p = 0.001), and red cell distribution width, a marker of major adverse CV events (MACE) (rho = 0.190, p = 0.02). SCORE revealed an elevated CV risk in 8.73% of the patients, whereas cfPWV showed increased aortic stiffness and end-organ disease in 16.00% of the same cohort.
Conclusions
In the largest cfPWV/PsA cohort examined to date, patients with PsA exhibited increased aortic stiffness compared to healthy controls. PsA duration was the most important independent disease-associated predictor of increased aortic stiffness, next to traditional CV risk factors. cfPWV measurements may help identify subclinical end-organ disease and abnormal aortic stiffness and thus assist CV risk classification in PsA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study |
Although cardiovascular (CV) disease is one of the most predominant comorbidities in patients with psoriatic arthritis (PsA), no sufficient data exist on precise methodologies for accurately measuring this risk. |
Carotid-femoral pulse wave velocity (cfPWV) has consistently predicted CV risk in the general population; nonetheless, its application as a surrogate marker in PsA remains limited, with scarce publications addressing this topic. |
What was learned from the study |
In the largest cfPWV/PsA cohort to date, increased aortic stiffness was independently predicted by disease duration, indicating an association between chronic complications and CV risk in PsA. |
Essential predictors of increased aortic stiffness among patient-associated characteristics and traditional CV risk factors were identified and a significant association of cfPWV with a marker of major CV events (MACE) was established. |
cfPWV offers a non-invasive method to assess CV risk, aiding in early identification of end-organ disease and prompting timely intervention to improve overall outcomes. |
Introduction
Psoriatic arthritis (PsA) is a complex chronic autoimmune disease with heterogeneous clinical manifestations, characterized by inflammatory arthritis and comorbid skin and/or nail psoriasis [1, 2]. It belongs to the disease group of spondyloarthropathies and affects 0.1–1% of the general population [1, 2]. PsA is associated with painful, swollen joints, functional impairment, and in cases of inadequate treatment, progressive structural damage of the affected joints [3]. Beyond skin and joint involvement, PsA is characterized by a high prevalence of extra-articular manifestations and comorbidities, such as infections, cardiovascular disease (CVD), malignancies, and further autoimmune conditions [4].
Since PsA lacks specific autoantibodies and in some cases laboratory signs of systemic inflammatory activity, diagnosis is often made clinically and with the assistance of the Classification Criteria for Psoriatic Arthritis (CASPAR), which are mainly applied by rheumatologists [5]. These facts combined with the known problem of insufficient resources in several clinical settings can lead to a diagnostic delay of PsA from months to several years [6]. Delay of diagnosis can, however, be associated with major complications, such as physical functional impairment and worse overall long-term disease outcomes [7].
PsA is associated with a high risk for cardiometabolic disorders, such as hypertension, dyslipidemia, diabetes, obesity, and CVD [8]. Particularly, a meta-analysis of 11 studies found a 43% increased risk of CVD in patients with PsA compared with the general population [9], and CV events have been described as one of the leading causes of death in patients with PsA [8]. In order to estimate the CV risk in the general population, several CV prediction scores such as Systemic Coronary Risk Evaluation (SCORE) [10], Framingham score [11], or PROCAM score [12] have been proposed. However, these scores do not factor in the effects of systemic inflammation and can thus lead to an underestimation of CV risk in patients with autoimmune rheumatic diseases [13]. For this reason, the European Alliance of Associations for Rheumatology (EULAR) guidelines for CV risk management recommend the adaptation of such CV risk prediction models by a 1.5 multiplication factor in the case of rheumatoid arthritis (RA) and other inflammatory arthritides [14]. Nevertheless, no conclusive evidence regarding precise means of CV risk calculation is available in PsA and the task force spoke against the use of 1.5 × SCORE in these patients [2]. For these reasons, new diagnostic markers of CV risk are greatly needed in the field of PsA.
One of the most important causes of CV disease is atherosclerosis [15]. Arterial stiffness is a well-established CV surrogate marker strongly associated with atherosclerosis. Interestingly, stiffness of the aorta can predict CVD in the general population independently of traditional CV risk factors [16]. In clinical practice, carotid-femoral pulse wave velocity (cfPWV) is considered the gold standard for aortic stiffness evaluation and has emerged as a useful method for the diagnosis and risk stratification of CVD [16,17,18,19]. cfPWV can be assessed in a non-invasive manner, without known complications, and is easily replicable. Thus, assessing cfPWV was recommended in the 2013 and 2018 ESC guidelines for the management of arterial hypertension [20, 21]. Arterial stiffness is a reflection of arterial compliance and thus of the elastic properties of the examined arteries. Vlachopoulos et al. highlighted, that in contrast to parameters such as blood pressure, lipids, or glucose, which match the instantaneous intensity of traditional CV risk factors (and can therefore vary highly), cfPWV reflects the long-term effects of established and unknown risk factors together with the individual genetic predisposition of each patient [22].
To date, studies examining established markers of CV risk in PsA, such as arterial stiffness, are scarce. In particular, stiffness of the aortic vasculature has been examined only in a few and small PsA case–control studies [23, 24] despite its known high predictive value in the general population [19, 25] and in patients with other rheumatic diseases, such as rheumatoid arthritis or connective tissue diseases [26,27,28,29,30,31,32].
Thus, the objective of the present study was to compare the aortic stiffness of patients with PsA with healthy controls in a large cohort from Germany and to identify predictors of cfPWV among clinical PsA-associated parameters, various patient characteristics, and traditional CV risk factors.
Methods
Study Participants
The prospective PSOriatic Arthritis CARDiovascular Disease (PSOCARD) cohort consists of patients with PsA being treated at the acute Rheumatology Center Rhineland-Palatinate and the University Medical Center, Mainz, Germany and is part of the multicenter CARD cohort examining CV markers of different rheumatologic diseases. Patients of the PSOCARD cohort have been consecutively enrolled for CV risk classification after being diagnosed with PsA by the CASPAR criteria [33]. Moreover, hospital employees and their circle of acquaintances, without underlying systemic inflammatory diseases, who freely responded to an open call for study participation, served as control subjects. Aortic stiffness was assessed in both groups using cfPWV. Exclusion criteria in both groups were malignancy, pregnancy, age < 18 years, kidney failure, body mass index (BMI) > 45 kg/m2, and active infection. The study has been reviewed and approved by the local standing committee for ethical conduct (Medical Board Rhineland-Palatinate, approval number 13762_2), and adhered to the Declaration of Helsinki. Written informed consent was obtained from all study subjects prior to enrollment.
Data Collection
Epidemiological data (age, gender), current medication, and traditional CV risk factors (diabetes mellitus, hypertension, dyslipidemia, nicotine use) were documented in all subjects. BMI was calculated by dividing the weight by the square of the height of a patient and BMI values ≥ 30 kg/m2 were defined as obesity. Mean arterial pressure (MAP) was calculated in relation to systolic (SAP) and diastolic arterial pressure (DAP) by the formula MAP = DAP + 1/3(SAP − DAP). Arterial hypertension was defined as SAP > 140 mmHg. Moreover, the use of non-biologic disease-modifying antirheumatic drugs (DMARDs), glucocorticoids (GC), non-steroidal anti-inflammatory drugs (NSAIDs), antihypertensive drugs, and statins was documented. Joint swelling and tenderness were evaluated clinically by a trained examiner and disease activity was calculated by the Disease Activity in PSoriatic Arthritis (DAPSA) score and the Disease Activity Score 28 (DAS28).
Laboratory assessments of the patient group included inflammation markers [erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and red cell distribution width], high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, rheumatoid factor (RF), anti-cyclic citrullinated peptide antibodies (anti-CCP), differential blood counts, and glomerular filtration rate (GFR).
cfPWV
cfPWV measurements were conducted by one trained blinded medical assistant, using a validated non-invasive oscillometric device (Vicorder®, SMT medical, Wuerzburg, Germany). The cfPWV examination protocol was carried out in accordance with the manufacturer’s instructions and the expert consensus document on arterial stiffness [16]. All measurements were performed in a quiet room after 10 min of rest.
cfPWV was measured as the velocity value calculated as 0.8 × the distance between the right common carotid artery and the right femoral artery in meters (m), divided by the time that one pulse wave needed to cover this distance in seconds (Δs/Δt) (m/s) (“foot-to-foot” velocity method) [16]. The average value of three measurements was calculated. A threshold value of cfPWV > 10 m/s was considered as an indicator of increased CV risk [20, 34]. This cutoff value for aortic stiffness was also applied in the current study.
SCORE Calculation
SCORE provides a predictive assessment of the total 10-year risk for a fatal CV event, taking into consideration specific patient characteristics and traditional CV risk factors, such as gender, age, cholesterol, smoking habits, and blood pressure values. In our study, SCORE was assessed in all eligible patients (40–70 years old) and based on the European guidelines for CV disease prevention [35]. As there is no conclusive evidence in PsA regarding the multiplication factor during CV risk assessment, the task force suggested not including the multiplication factor (×1.5) for SCORE results in the PSA disease [2]. For this reason, we did not apply the multiplication factor for the assessed SCORE values. As proposed by the guidelines, patients with SCORE values > 5% denoted high CV risk [36].
Statistical Analysis
The assumption of normality of distribution was evaluated by the Shapiro–Wilk numerical test and quantile–quantile plots. Continuous variables are presented as the mean (SD) if they were normally distributed or the median (25th/75th percentiles) if they were skewed. Categorical variables were summarized as absolute (n) and relative (%) frequencies. A comparison of categorical variables was performed through a chi-squared test.
The differences in cfPWV and SCORE between patients with PsA and controls were evaluated by t test when the variables were normally distributed and by Mann–Whitney U test when they were skewed. To assess the correlation between CV surrogates and continuous characteristics, Spearman’s (rho) or Pearson’s (r) correlation coefficients were used. A p value less than 0.05 was considered significant.
Linear regression was used to assess the difference in cfPWV between PsA and control groups, after adjusting for confounding factors including age, gender, diabetes, nicotine, cholesterol, heart rate, and GFR. Statistical analysis was performed using the SPSS software version using IBM SPSSVR 23.0 software (USA).
Results
Study Populations
Within the framework of this study, cfPWV measurements, clinical, and laboratory assessments were performed in 150 consecutive patients with PsA [61.3% female, age 55 years (47.0–63.0)], as well as in 88 healthy control subjects [85.2% female, age 51 years (36.5–58.0)].
All descriptive characteristics of patients with PsA and healthy controls included in the study are reported in Table 1.
Associations Between Group Status (Psoriatic Arthritis Group vs. Control Group): cfPWV and SCORE
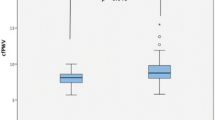
cfPWV median was significantly higher in the patient group compared with the control group [7.80 (6.87–9.32) m/s vs. 6.76 (6.03–7.68) m/s, p < 0.001] (Fig. 1). A linear regression model showed that cfPWV remained significantly higher in the patient group compared to the control group, even after adjusting for the effect of traditional CV risk factors such as age, gender, diabetes, nicotine, cholesterol, heart rate, and GFR (0.457, 95% CI 0.035–0.879, padj = 0.034) (Table 2) and thus pointing to a higher aortic stiffness in patients with PsA independently from cfPWV-influencing factors.
Furthermore, an additional multivariate analysis including the variables age, gender, diabetes, nicotine, MAP, obesity, and arterial hypertension was performed showing also a statistically significant difference of cfPWV between the patients and the control group (0.646, 95% CI 0.230–1.062, padj = 0.002) (S1, supplementary material).
cfPWV and SCORE Values of Patients with Estimated High CV Risk
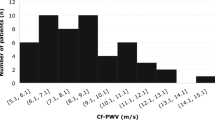
A total of 150 patients with PsA underwent cfPWV measurement, out of whom 24 (16%) had cfPWV values > 10 m/s, indicating end-organ disease and exaggerated CV risk. Of these 150 patients, 103 were eligible for the calculation of SCORE. Interestingly, out of these only 9 (8.73%) showed SCORE values > 5%, indicative of high CV risk. Cohen’s kappa between SCORE and cfPWV was 0.20 showing a poor agreement between those two parameters (p = 0.34).
Associations of cfPWV in Patients with PsA and Controls
Among patients with PsA, cfPWV correlated strongly with age (rho = 0.688, p < 0.001) and moderately with systolic blood pressure (rho = 0.351, p < 0.001) and disease duration (r = 0.304, p = 0.001) (Fig. 2, Table 3). Moreover, we conducted a regression model to ensure that the correlation between cfPWV and disease duration was not due to confounding factors such as older age. Even after the adjustment, the correlation remained significant (0.028, 95% CI 0.011–0.045, padj = 0.020], pointing to an independent relationship between these two variables.
Furthermore, cfPWV is associated with mean arterial pressure (rho = 0.253, p = 0.002) and red cell distribution width (r = 0.190, p = 0.020), as well as inversely with GFR (rho = − 0.264, p = 0.001) (Table 3). No statistically significant associations were found within the patient group between cfPWV and VAS, CRP, or further disease activity parameters (number of swollen joints, DAS28, DAPSA) (all p > 0.05).
However, patients with diabetes had higher cfPWV values compared with those without [9.40 (8.40–10.85) m/s vs. 7.60 (6.80–8.72) m/s, p < 0.001] and subjects with known hypertension also exhibited higher cfPWV values compared with their hypertension-free counterparts [8.40 (6.90–9.70) m/s vs. 7.50(6.70–8.40) m/s, p = 0.003] (Table 3).
Among controls, cfPWV correlated strongly with age (rho = 0.656, p < 0.001), and moderately with BMI (rho = 0.395, p = 0.001), SCORE (rho = 0.412, p < 0.001), and MAP (r = 0.429, p < 0.001), respectively (Table 4).
Effects of Immunosuppressive Medication on cfPWV
We conducted a subgroup analysis to compare the median cfPWV results of patients with PsA who received biologic disease-modifying anti-rheumatic drugs (bDMARDs) or conventional-synthetic disease-modifying anti-rheumatic drugs (csDMARDs) against those who did not, respectively. In both cases, no statistically significant cfPWV differences were found between patients with PsA treated with bDMARDs [7.80 (6.90–9.05) m/s vs. 7.80 (6.04–9.40) m/s; p = 0.236)] or csDMARDs [7.65 (6.90–8.90) m/s vs. 7.90 (6.80–9.40) m/s, (p = 0.635)], and their counterparts, respectively.
Discussion
In the present study, patients with PsA had higher cfPWV values than controls, even after adjustment for confounding factors. Moreover, we were able to show that cfPWV was predicted not only by traditional CV risk factors such as systolic blood pressure but also by disease-related factors, such as disease duration. To the best of our knowledge, this study is the largest to date to examine the gold standard assessment method of aortic stiffness in patients with PsA.
Overall, data concerning markers of CV risk in PsA are scarce. We were able to identify only two previous studies that examined cfPWV in patients with PsA [23, 24]. However, these studies included a low number of patients (n = 9 and n = 20, respectively) making the extraction of concrete statistical results difficult: Soy et al. reported increased cfPWV in nine patients with PsA in comparison with 39 controls [23], and Costa et al. found higher cfPWV values in 20 patients with PsA in a case–control study [24]. Another PsA study of arterial stiffness focused on a marker known as brachial-ankle pulse wave velocity (baPWV), which is less established than cfPWV, and can thus not be directly compared with our exploration, or the results of the two aforementioned works [37]. The high number of included patients in our exploration gave us the possibility to reveal a hitherto undescribed significant association with a disease chronicity parameter like disease duration.
The age-independent association between disease duration and cfPWV indicates that cumulative inflammatory burden, medication effects, and ultimately chronic damage during the course of the disease might affect developing aortic stiffness and thus high CV risk. Even though this relationship has not been described in PsA until today, Vazquez-Del Mercado et al. came across a similar result examining the arterial status of patients with rheumatoid arthritis [38]. Here, the most pronounced arterial stiffness was found in patients with a disease duration of 10 years or longer [38].
Interestingly, in our study, cfPWV is also associated with red cell distribution width which has been suggested to be a marker for major cardiovascular events (MACE) and a novel psoriasis (PsO)/ PsA disease activity marker [39, 40]. Even though no correlations of cfPWV with other markers of acute inflammation (CRP, ESR) were found in our cohort, an association between aortic stiffness and acute inflammation in the context of autoimmune diseases has been extensively discussed and multiple potential mechanisms concerning the interplay between inflammation and arterial stiffening have been suggested. Increased levels of known inflammatory markers, e.g., interleukin-6 (IL-6), CRP, and interferons, can directly alter the endothelial nitric oxide bioavailability [41] by impairing the vasodilatory effects of NO [42]. Moreover, these mediators trigger the increased production of matrix metalloproteinases with subsequent degeneration of elastin fibers, leading to decreased arterial compliance [43]. Accelerated atherosclerosis due to systemic inflammation-mediated effects may also lead to an increase in arterial stiffness, even though their pathophysiological associations have not been clearly established, mainly due to their complex interplay [44].
Shen et al. evaluated several established CV risk assessment tools, including the Framingham risk score (FRS), SCORE, and the 10-year atherosclerotic cardiovascular disease risk algorithm (ASCVD) in patients with PsA [13]. The study revealed only a moderate discriminative ability of these risk calculators compared to ultrasound-assessed carotid subclinical atherosclerosis (SCA), depicting a possible underestimation of the atheromatosis burden by those CV risk scores. However, since SCA does not directly assess CV risk, more studies on this topic with follow-up examinations are needed. In general, cfPWV has been proven to predict future CV events and it is plausible to assume that it can assess the effects of chronic inflammation in PsA in a more accurate manner than traditional CV tools since the latter do not take inflammatory burden or other disease-specific markers into account [45].
Age was associated with cfPWV not only in our PsA cohort but also in the control group. This statistical association between age and cfPWV is not surprising, since age is a known risk factor for CV disease [46]. The influence of age on aortic stiffness results from structural changes in the media layer of the vessel wall during a person’s lifetime. The mechanical properties of the arterial wall change with increasing age, especially as a result of the loss of elastic fibers and accumulation of collagen [28].
Moreover, cfPWV correlated with diabetes in both groups, signaling the influence of metabolic factors on aortic stiffness and hence the overall CV risk. Poznyak et al. postulated that chronic inflammation may be regarded as one of the possible links between atherosclerosis and diabetes mellitus and supports the hypothesis that systemic inflammation promotes insulin resistance [47]. Furthermore, in our patient group, cfPWV was associated with MAP. Cecelja et al. described MAP as the main factor impacting cfPWV even in the general population [48]. As a result of the elasticity of the aorta, the vessel wall gets stretched if a force is applied to it. The higher the blood pressure is, the higher the stretching of the vessel wall; and by stretching, the vessel wall gets stiffer [28].
Our study has some limitations. First, there were no longitudinal associations of cfPWV with future morbidity or mortality data. Nonetheless, a plethora of studies have assessed the predictive value of cfPWV and this marker has been suggested as a valid CV risk stratification tool by the European Society of Cardiology Working Group on peripheral circulation and the ARTERY Association (level of evidence A, Recommendation IIa) [22]. Second, differences in age, gender, and some traditional CV risk factors were statistically observed between the patient and the control group. To avoid confounding, we have performed linear regression models adjusting the findings for the effects of multiple possible cfPWV-influencing factors, like traditional CV parameters and of course age/gender. These statistical models revealed higher cfPWV values in the PsA group, even after statistical corrections and thus a statistical bias seems unlikely. However, results should be controlled in future studies. A third possible limitation is that cfPWV of patients with PsA was compared with the respective values of controls without rheumatologic diseases and not with a diseased group. However, the aim of our study was to examine comparisons between patients with PsA and controls without rheumatic diseases, given the scarcity of data regarding this research question.
Conclusion
This is one of the largest surrogate CV marker studies in PsA and the first report of an independent association between aortic stiffness and PsA duration pointing to a possible link with disease-related cumulative damage or comorbidities. Since aortic stiffness can reflect the long-term effects of both traditional CV risk factors and inflammation-associated vascular damage in a non-invasive and radiation-free manner, cfPWV could prove to be a useful tool for the identification of patients with PsA with end-organ disease and thus high CV risk. Further research and follow-up data from longitudinal studies are warranted.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Karmacharya P, Chakradhar R, Ogdie A. The epidemiology of psoriatic arthritis: a literature review. Best Pract Res Clin Rheumatol. 2021;35(2):101692.
Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28.
Garrido-Cumbrera M, Hillmann O, Mahapatra R, et al. Improving the management of psoriatic arthritis and axial spondyloarthritis: roundtable discussions with healthcare professionals and patients. Rheumatol Ther. 2017;4(2):219–31.
Di Minno MN, Ambrosino P, Lupoli R, et al. Cardiovascular risk markers in patients with psoriatic arthritis: a meta-analysis of literature studies. Ann Med. 2015;47(4):346–53.
Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73.
Sorensen J, Hetland ML. Diagnostic delay in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Ann Rheum Dis. 2015;74(3):e12.
Tillett W, Jadon D, Shaddick G, et al. Smoking and delay to diagnosis are associated with poorer functional outcome in psoriatic arthritis. Ann Rheum Dis. 2013;72(8):1358–61.
Karmacharya P, Ogdie A, Eder L. Psoriatic arthritis and the association with cardiometabolic disease: a narrative review. Ther Adv Musculoskelet Dis. 2021. https://doi.org/10.1177/1759720X21998279
Polachek A, Touma Z, Anderson M, Eder L. Risk of cardiovascular morbidity in patients with psoriatic arthritis: a meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2017;69(1):67–74.
Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003.
D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53.
Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105(3):310–5.
Shen J, Lam SH, Shang Q, et al. Underestimation of risk of carotid subclinical atherosclerosis by cardiovascular risk scores in patients with psoriatic arthritis. J Rheumatol. 2018;45(2):218–26.
Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–31.
Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366(1):54–63.
Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–8.
Milan A, Zocaro G, Leone D, et al. Current assessment of pulse wave velocity: comprehensive review of validation studies. J Hypertens. 2019;37(8):1547–57.
De Luca M, Iacono O, Valente V, et al. Can pulse wave velocity (PWV) alone express arterial stiffness? A neglected tool for vascular function assessment. J Basic Clin Physiol Pharmacol. 2021;33(4):373–9.
Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–46.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–219.
Vlachopoulos C, Xaplanteris P, Aboyans V, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241(2):507–32.
Soy M, Yildiz M, Sevki Uyanik M, Karaca N, Gufer G, Piskin S. Susceptibility to atherosclerosis in patients with psoriasis and psoriatic arthritis as determined by carotid-femoral (aortic) pulse-wave velocity measurement. Rev Esp Cardiol. 2009;62(1):96–9.
Costa L, Caso F, D’Elia L, et al. Psoriatic arthritis is associated with increased arterial stiffness in the absence of known cardiovascular risk factors: a case control study. Clin Rheumatol. 2012;31(4):711–5.
Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–27.
Triantafyllias K, Cavagna L, Klonowski A, et al. Possible misclassification of cardiovascular risk by SCORE in antisynthetase syndrome: results of the pilot multicenter study RI.CAR.D.A. Rheumatology (Oxford). 2021;60(3):1300–12.
Triantafyllias K, De Blasi M, Hoffmann I, Thomaidis T, Drees P, Schwarting A. The count of tender rather than swollen joints correlates with aortic stiffness in patients with rheumatoid arthritis. Springerplus. 2016;5:428.
Stortz M, Triantafyllias K, Schwarting A, Weinmann-Menke J. Vascular stiffness: influencing factors on carotid-femoral pulse wave velocity in systemic lupus erythematosus. Clin Exp Rheumatol. 2020;38(1):74–81.
Triantafyllias K, de Blasi M, Lutgendorf F, et al. High cardiovascular risk in mixed connective tissue disease: evaluation of macrovascular involvement and its predictors by aortic pulse wave velocity. Clin Exp Rheumatol. 2019;37(6):994–1002.
Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43(9):1622–30.
Cioffi G, Viapiana O, Ognibeni F, et al. Clinical profile and outcome of patients with rheumatoid arthritis and abnormally high aortic stiffness. Eur J Prev Cardiol. 2016;23(17):1848–59.
Constans J, Germain C, Gosse P, et al. Arterial stiffness predicts severe progression in systemic sclerosis: the ERAMS study. J Hypertens. 2007;25(9):1900–6.
Tillett W, Costa L, Jadon D, et al. The ClASsification for Psoriatic ARthritis (CASPAR) criteria—a retrospective feasibility, sensitivity, and specificity study. J Rheumatol. 2012;39(1):154–6.
Kim HL, Kim SH. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med. 2019;6:41.
De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171(1):145–55.
Ozen G, Sunbul M, Atagunduz P, Direskeneli H, Tigen K, Inanc N. The 2013 ACC/AHA 10-year atherosclerotic cardiovascular disease risk index is better than SCORE and QRisk II in rheumatoid arthritis: is it enough? Rheumatology (Oxford). 2016;55(3):513–22.
Shen J, Shang Q, Li EK, et al. Cumulative inflammatory burden is independently associated with increased arterial stiffness in patients with psoriatic arthritis: a prospective study. Arthritis Res Ther. 2015;17(1):75.
Vazquez-Del Mercado M, Gomez-Banuelos E, Chavarria-Avila E, et al. Disease duration of rheumatoid arthritis is a predictor of vascular stiffness: a cross-sectional study in patients without known cardiovascular comorbidities: a STROBE-compliant article. Medicine (Baltimore). 2017;96(33):e7862.
Conic RR, Damiani G, Schrom KP, et al. Psoriasis and psoriatic arthritis cardiovascular disease endotypes identified by red blood cell distribution width and mean platelet volume. J Clin Med. 2020;9(1):186.
Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. 2011;107(8):1241–5.
Yildiz M. Arterial distensibility in chronic inflammatory rheumatic disorders. Open Cardiovasc Med J. 2010;4:83–8.
Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA. Inflammation and arterial stiffness in humans. Atherosclerosis. 2014;237(2):381–90.
Arnold N, Gori T, Schnabel RB, et al. Relation between arterial stiffness and markers of inflammation and hemostasis—data from the population-based Gutenberg Health Study. Sci Rep. 2017;7(1):6346.
Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1–7.
Triantafyllias K, Thiele LE, Cavagna L, Baraliakos X, Bertsias G, Schwarting A. Arterial stiffness as a surrogate marker of cardiovascular disease and atherosclerosis in patients with arthritides and connective tissue diseases: a literature review. Diagnostics (Basel). 2023;13(11):1870.
Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6(2):19.
Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835.
Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54(6):1328–36.
Acknowledgements
Special thanks to the medical assistants Mrs Nicole Divonskis, Mrs. Susanne Dietz, and Mrs. Melanie Opp of the Rheumatology Center Rhineland-Palatine, Germany. The authors would like to thank the participants of the study for their time and participation.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Conceptualization: Konstantinos Triantafyllias. Methodology: Konstantinos Triantafyllias; Formal analysis and investigation: Stefanie Liverakos, Konstantinos Triantafyllias, Muthuraman Muthuraman; Writing- original draft preparation: Konstantinos Triantafyllias, Stefanie Liverakos; Writing- review and editing: Andreas Schwarting, Ioannis Parodis, Lorenzo Cavagna; Supervision: Konstantinos Triantafyllias. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors, Konstantinos Triantafyllias, Stefanie Liverakos, Muthuraman Muthuraman, Lorenzo Cavagna, Ioannis Parodis and Andreas Schwarting, declare that they have no competing interests.
Andreas Schwarting is an Editorial Board member of Rheumatology and Therapy. Andreas Schwarting was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
The study has been reviewed and approved by the local standing committee for ethical conduct (Medical Board Rhineland-Palatinate, approval number 13762_2), and adhered to the Declaration of Helsinki. Written informed consent was obtained from all study subjects prior to enrollment.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Triantafyllias, K., Liverakos, S., Muthuraman, M. et al. Cardiovascular Risk Evaluation in Psoriatic Arthritis by Aortic Stiffness and the Systemic Coronary Risk Evaluation (SCORE): Results of the Prospective PSOCARD Cohort Study. Rheumatol Ther 11, 897–911 (2024). https://doi.org/10.1007/s40744-024-00676-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-024-00676-z