Abstract
Introduction
Currently, the cause of psoriatic arthritis (PsA) is unknown, and the effectiveness of current drug treatments is unsatisfactory. In March 2019, the US Food and Drug Administration (FDA) approved risankizumab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody targeting the p19 subunit of interleukin (IL)-23, for the treatment of PsA in adults. This study aimed to conduct a meta-analysis of double-blind, randomized, placebo-controlled trials to evaluate the effectiveness and safety of risankizumab in moderate-to-severe PsA.
Methods
We conducted a thorough search of relevant databases from the establishment of the databases to October 1, 2023. We conducted a meta-analysis using Stata 12.0 and utilized I2 and Egger tests to assess heterogeneity and publication bias among the studies. Bias assessment was performed using the risk bias map and bias risk summary diagram generated by Revman5.4 software. The review protocols were registered on PROSPERO (CRD42023451894) and adhered to the preferred reporting item of system evaluation (PRISMA) guideline.
Results
Six randomized controlled trials (RCTs) involving 5038 patients with PsA treated with either risankizumab or placebo were included in the analysis. At 24 weeks, the risankizumab group demonstrated a significantly higher American College of Rheumatology-20 (ACR20) response rate compared to the placebo group (RR 1.760, 95% CI 1.568–1.977, P < 0.001). Additionally, the risankizumab group showed a significantly higher Minimal Disease Activity (MDA) response rate compared to the placebo group (RR 1.827, 95% CI 1.048–3.184, P < 0.05). The risankizumab group also exhibited improvement in Short Form 36 Questionnaire (SF-36) score (SMD 0.51, 95% CI 0.33–0.69, P < 0.001), with significantly lower Health Assessment Questionnaire Disability Index (HAQ-DI) score (SMD − 0.27, 95% CI − 0.37 to − 0.17, P < 0.001) and higher Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) score (SMD 0.27, 95% CI 0.20–0.35, P < 0.001) compared to the placebo group. Moreover, the risankizumab group had a significantly lower Psoriasis Area and Severity Index (PASI) score (SMD − 6.12, 95% CI − 10.02 to 2.23, P < 0.001). A study by Mease et al. indicated that patients receiving risankizumab generally demonstrated numerical improvements in the Leeds Enthesitis Index (LEI), although the small sample size limits the evidence. Further research is necessary to provide evidence-based guidelines. There were no significant differences in the incidence of serious adverse events (SAE) and serious treatment-emergent adverse events (STEAE) between the risankizumab and placebo groups (RR 0.76, 95% CI 0.45–1.28, P = 0.31; RR 0.99, 95% CI 0.49–1.99, P = 0.97, respectively), and the overall incidence of adverse events (AE) was not comparable (RR 1.10, 95% CI 0.63–1.94, P = 0.73).
Conclusion
Risankizumab showed superior efficacy across multiple outcome measures compared to placebo, with no significant increase in adverse events. Our findings endorse risankizumab as an excellent treatment option for PsA, offering valuable insights for clinicians and patients when choosing appropriate therapeutic interventions.
Trial Registration
Retrospectively registered (CRD42023451894, 16 August 2023).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Risankizumab, a humanized IgG1 monoclonal antibody that targets the p19 subunit of IL-23, was approved by the US Food and Drug Administration (FDA) in March 2019 for treating PsA in adults |
Although IL-23-targeted therapies have shown promise, there is still an ongoing debate about their effectiveness and safety |
What was learned from the study? |
The risankizumab treatment resulted in significantly higher American College of Rheumatology-20 (ACR20) response rate and Minimal Disease Activity (MDA) response rate compared to the placebo treatment |
The risankizumab treatment led to improved Short Form 36 Questionnaire (SF-36) score, significantly lower PASI score and Health Assessment Questionnaire Disability Index (HAQ-DI) score, as well as higher Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) score compared to the placebo treatment |
Compared to placebo, risankizumab exhibited superior efficacy across various outcome measures, without a significant increase in adverse events |
Introduction
Psoriatic arthritis (PsA) is an immune-mediated inflammatory joint disease affecting up to 30% of patients with psoriasis and up to 0.7% of the general population [1]. The condition primarily affects the skin and joints, leading to painful and stiff joints, irreversible joint damage, and a negative impact on patients’ quality of life [2, 3]. Despite the availability of several treatment options, some patients do not respond adequately to or tolerate current therapies, requiring the development of effective and well-tolerated alternative treatments [3].
Interleukin (IL)-23 is a heterodimer composed of a unique IL-23A (p19) subunit and an IL-12/23B (p40) subunit shared with IL-12, driving the differentiation and activation of T helper 17 (Th17) cells and subsequent production of pro-inflammatory factors such as IL-17A, IL-17F, IL-6, IL-22, and tumor necrosis factor-α (TNFα) to trigger joint and synovial inflammation [4]. IL-23 plays a critical role in regulating immune cell differentiation, activation, and survival, as well as in the induction of arthritis, osteoclastic formation, and the maintenance of bone mass [5].
Risankizumab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody targeting the p19 subunit of IL-23, disrupts IL-23-mediated signaling, activation, and cytokine production, resulting in symptomatic improvement in PsA symptoms [2]. The drug was approved for the treatment of moderate-to-severe PsA in March 2019. Although IL-23-targeted therapies have shown promise, there is still ongoing debate regarding their efficacy and safety. Therefore, the study aimed to conduct a meta-analysis of double-blind, randomized, placebo-controlled trials to assess the efficacy and safety of risankizumab in moderate-to-severe PsA, with the goal of improving clinical decision-making, facilitating rational drug use, and minimizing adverse events associated with IL-23 inhibitors.
Methods
Data Source and Search Strategy Methods
This system evaluation was conducted according to the meta-analysis guidelines of the preferred reporting item of system evaluation (PRISMA) and has been registered in the trial registry of PROSPERO (CRD42023451894). We conducted computer-based retrieval of relevant research published from January 1, 1950 to October 1, 2023 on PubMed, SciDev. Net, Embase, Cochrane Library, Knowledge Net, MEDLINE, Clinical Trials.gov, and FDA.gov, combining subject words and free words. In the retrieval process, we use the terms “Psoriatic arthritis,” “Risankizumab,” and related free words.
Study Selection and Data Extraction
Before conducting the data retrieval process, we established clear inclusion and exclusion criteria. No restrictions were imposed regarding subjects’ gender, age, race/nationality, or publication timeframe. However, studies must have a placebo or historical control group. Animal experiments, letters, comments, and editorials were excluded. Included studies needed to evaluate one or more of the following outcome measures: (1) Short Form 36 Questionnaire(SF-36); (2) Health Assessment Questionnaire Disability Index (HAQ-DI); (3) Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F); (4) American College of Rheumatology-20 (ACR20); (5) Minimal Disease Activity (MDA); (5) Psoriasis Area and Severity Index (PASI); (6) Leeds Enthesitis Index (LEI). Safety outcomes were adverse events (AEs) leading to discontinuation and treatment-emergent AEs (TEAEs).
ACR20 is considered the gold standard for evaluating clinical trial efficacy, but it may not reflect disease activity in a timely manner or allow for comparison between different patients, making it unsuitable for evaluating stable patient conditions. In contrast, MDA assesses the minimum disease activity and was therefore chosen as a primary efficacy measure, along with ACR20. SF-36, FACIT-F, HAQ-DI, PASI, and LEI were selected as secondary efficacy measures to comprehensively evaluate the efficacy and safety of risankizumab in the treatment of PsA. Safety outcomes were also assessed and compared between the two groups, including AE, serious AE (SAE), and serious TEAEs (STEAEs).
Excluded studies comprised animal experiments lacking follow-up and data recording. Extracted data included the study title, first author’s name, publication year, number of patients with PsA in the treatment and placebo groups, administration timing and dosage, patient age, sex ratio, main endpoint, incidence of positive events based on each scoring standard, or their means and standard deviations (SD).
Furthermore, in accordance with the Cochrane Intervention System Evaluation Manual, two researchers independently evaluated the studies for eligibility based on the criteria. We conducted an independent quality assessment of the included studies, considering selection bias (random sequence generation and allocation concealment), performance bias, detection bias, attrition bias, reporting bias, and other potential biases. Any discrepancies were resolved by a third investigator.
Statistical Analysis
For the continuous data (SF-36PCS, HAQ-DI, FACIT-F, PASI) in the data analysis results, we calculated the standardized mean deviation (SMD) and 95% confidence interval (CI) using Stata 12.0. The statistical analysis data of the exposure group and the placebo group are expressed as the mean ± standard deviation. The square value of I (I2) is used to explore the statistical heterogeneity between studies. I2 > 50% indicates medium and high heterogeneity, and a random effects model (REM) is used. For discontinuous data, i.e., the results of binary classification, a fixed effects model is used. In addition, the publication bias (p ≥ 0.05) was evaluated using the Begger and Egger test to check the funnel asymmetry.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study Characteristics
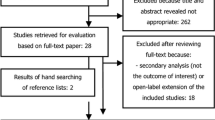
We initially identified 238 studies, and 48 duplicate articles were deleted through EndNoteX9 software. Finally, 10 studies [3, 7,8,9,10,11,12,13,14,15] were included in the final meta-analysis process, and three experiments evaluated the safety of the drug. The flowchart of selecting eligible studies is shown in Fig. 1. Table 1 summarizes the details of the selected study and the characteristics of their corresponding patients (Table 1). All studies were published between 2021 and 2023, five studies evaluated the short-term results of 24 weeks, and one study evaluated the short-term results of 28 weeks. The results are presented according to MDA, ACR20, HAQ-DI, SF36-PCS, FACIT-F, PASI, and LEI. All patients met the PsA classification criteria and were randomly assigned to the risankizumab group and placebo group. Since all the studies are randomized controlled trials (RCT), the quality of the included articles was evaluated using the Cochrane Bias Risk Assessment Tool, and the risk bias map was drawn with Revman5.4 for the evaluation results (Fig. 2).
Efficacy
The meta-analysis results demonstrated significant efficacy of risankizumab compared to placebo in various evaluation indicators of curative effect. At 24 weeks, the ACR20 response rate was significantly higher in the risankizumab group than in the placebo group (RR 1.760, 95% CI 1.568–1.977, P < 0.001) (Fig. 3a). Similarly, the MDA response rate was significantly higher in the risankizumab group (RR 1.827, 95% CI 1.048–3.184, P < 0.05) (Fig. 3b).
Forest plot. a ACR20, b MDA, c SF-36, d HAQ-DI, e FACIT-F, and f PASI compared between patients with PsA and controls. ACR-20 American College of Rheumatology-20, CI confidence interval, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, HAQ-D1 Health Assessment Questionnaire Disability Index, MDA Minimal Disease Activity, PASI Psoriasis Area and Severity Index, PsA Psoriatic arthritis, SF-36 Short Form 36 Questionnaire.
Regarding secondary evaluation indicators, the SF-36 score demonstrated improvement in the risankizumab group (SMD 0.51, 95% CI 0.33–0.69, P < 0.001) (Fig. 3c). Moreover, the risankizumab group exhibited higher scores for each item, indicating better health status compared to the placebo group. The HAQ-DI score was significantly lower in the risankizumab group, indicating lower disability (SMD − 0.27, 95% CI − 0.37 to − 0.17, P < 0.001) (Fig. 3d). Additionally, the FACIT-F score was considerably higher in the risankizumab group, suggesting improved quality of life (SMD 0.27, 95% CI 0.20–0.35, P < 0.001) (Fig. 3e). Moreover the PASI score was significantly lowerin the risankizumab group (SMD − 6.12, 95% CI − 10.02 to 2.23, P < 0.001) (Fig. 3f). Overall, these meta-analysis results provide robust evidence that the risankizumab group exhibited superior efficacy compared to the placebo group across various evaluation indicators.
Safety
Safety data was reported in three studies. One study solely reported AE and SAE, while the other two studies reported TEAE, SAE, and STEAE with similar definitions. The risankizumab group mentioned one death, which occurred as a result of urinary sepsis in an 81-year-old male patient with dementia hospitalized for pneumonia in the eighth week. This death was not directly attributed to the medication (Table 2).
Meta-analysis results of adverse reactions indicated no significant difference in the incidence of SAE (RR 0.76, 95% CI 0.45–1.28, P = 0.31) and STEAE (RR 0.99, 95% CI 0.49–1.99, P = 0.97) between the risankizumab and placebo groups. The incidence of AE also showed no significant difference (RR 1.10, 95% CI 0.63–1.94, P = 0.73). These findings suggest that risankizumab treatment did not lead to an increased risk of adverse consequences compared to the placebo group.
Discussion
This meta-analysis compared the efficacy and safety of risankizumab in the treatment of moderate to severe PsA through a synthesis of randomized clinical trial data. The findings revealed that risankizumab demonstrated superior efficacy while maintaining a favorable safety profile. The main efficacy indicators analyzed in this meta-analysis were the ACR20 response rate and MDA response rate, while the main safety indicators included TEAEs, SAEs, and STEAEs. All included patients met the classification criteria for PsA (CASPAR), and a consistent dosing interval of 6 months was utilized across the studies.
The results of this meta-analysis provide robust evidence for the efficacy and clinical benefits of risankizumab in PsA treatment. The significant improvements observed in ACR20 response rates and other key secondary endpoints, such as enthesitis, dactylitis, cutaneous psoriasis, and nail disease, highlight the therapeutic benefits of risankizumab. IL-23 inhibitors risankizumab binds with high affinity to the p19 subunit of IL-23 functions by triggering the differentiation of Th-17 and Th-22 cells which in turn trigger the inflammatory cascade responsible for the development of psoriatic lesions, blocking the IL-23 signaling pathway and inhibiting the proliferation of Th17 cells; thus the symptoms of PsA were improved [16, 17]. Moreover, the improvement in physical function, fatigue, and health-related quality of life, as assessed by HAQ-DI, FACIT-F, and SF-36 scores, further supports the effectiveness of risankizumab in improving patient outcomes. Furthermore, the PASI score showed that the reduction in biomarkers associated with skin inflammation and IL-23/IL-17 signaling pathways, as well as the decrease in dermal infiltration by immune cells, indicates the mechanism of action of risankizumab in reducing hyperkeratosis, epidermal acanthosis, and inflammation in the dermis and epidermis. The inclusion of well-established experimental data supporting the role of risankizumab in various inflammatory diseases, such as psoriasis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and atopic dermatitis, further reinforces its therapeutic potential [4, 18]. There is only one study on LEI, which is too small and needs to be discussed next.
Our study also provides evidence of the favorable safety profile of risankizumab in PsA treatment. Specifically, no significant difference was observed in the incidence of TEAEs, SAEs, and STEAEs between the risankizumab and placebo groups. The incidence of adverse events was overall similar between the two groups, indicating that patients did not experience more adverse consequences due to medication. This suggests a favorable safety profile for risankizumab in the treatment of PsA. The use of anti-IL-23 agents, such as risankizumab, has advantages over chemical drugs as they block key inflammatory cytokines or cell-surface molecules without being digested in the gastrointestinal tract [19]. However, the risk of serious infections and various adverse events remains a concern when blocking the IL-23 immune axis. It is worth noting that ustekinumab, an anti-IL-12/23-p40 agent, has been widely used with favorable outcomes. Although IL-23p40 antibodies have been approved earlier, they inhibit both IL-12 and IL-23 signaling pathways, which may not be necessary for achieving efficacy in patients with PsA and could pose potential risks [20]. In contrast, risankizumab specifically targets the p19 subunit of IL-23 without disrupting the IL-12 signaling pathway, allowing IL-12 to maintain its beneficial anti-inflammatory effects. So far, no associated risk of malignancy or bacterial/parasite infection has been identified [20, 21]. This suggests that risankizumab has a higher safety profile compared to other IL-23 or IL-12/23 inhibitors. Targeting IL-23p19 selectively could be a promising treatment approach in patients with PsA, achieving a downregulation of Th17 cell responses while minimizing adverse reactions [20].
Regarding the overall quality of the included literature, this meta-analysis operated according to strict literature inclusion and exclusion criteria. Still, there are some limitations to this study. First, PsA is a complex disease involving several clinical domains, so the full efficacy profile of treatments, and the overall value to patients, may not be captured by only assessing ACR, MDA, SF-36PCS, HAQ-DI, FACIT-F, PASI, LEI, AEs, and SAEs. So subsequent studies must evaluate patient-reported and additional clinical outcomes. Secondly, because PsA is a lifelong chronic disease, long-term comparisons should be explored to evaluate the maintenance of treatment response. Our analysis only includes up to 24 weeks of data obtained from clinical trials, so it cannot provide estimates of long-term effectiveness in trials or real-world clinical settings [22]. Finally, only published articles in English and Chinese databases where the original literature was available were included in this study; excluding unpublished articles and articles where the original literature was unavailable might result in publication bias.
Conclusion
Risankizumab, as an IL-23 inhibitor, exhibits significant efficacy and a favorable safety profile in treating PsA. These findings provide substantial support for the inclusion of risankizumab as a highly effective treatment option for PsA, offering valuable guidance to clinicians and patients in their therapeutic decision-making process. Future studies should consider a broader range of outcomes and investigate the long-term effectiveness and safety of risankizumab in real-world settings.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Wirth T, Balandraud N, Boyer L, Lafforgue P, Pham T. Biomarkers in psoriatic arthritis: a meta-analysis and systematic review. Front Immunol. 2022;13:1054539.
McInnes IB, Sawyer LM, Markus K, LeReun C, Sabry-Grant C, Helliwell PS. Targeted systemic therapies for psoriatic arthritis: a systematic review and comparative synthesis of short-term articular, dermatological, enthesitis and dactylitis outcomes. RMD Open. 2022;8(1):e002074.
Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022;81(2):225–31.
Schinocca C, Rizzo C, Fasano S, et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: an overview. Front Immunol. 2021;12:637829.
Adamopoulos IE, Tessmer M, Chao CC, et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol. 2011;187(2):951–9.
Yang K, Oak ASW, Elewski BE. Use of IL-23 inhibitors for the treatment of plaque psoriasis and psoriatic arthritis: a comprehensive review. Am J Clin Dermatol. 2021;22(2):173–92.
Östör A, Van den Bosch F, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann Rheum Dis. 2022;81(3):351–8.
Ostor AJK, Soliman AM, Papp KA, et al. Improved patient-reported outcomes in patients with psoriatic arthritis treated with risankizumab: analysis of the phase 3 trial KEEPsAKE 2. RMD Open. 2022;8(2):e002286.
Merola J, McInnes I, Kavanaugh A, et al. POS1029 effects of treatment with risankizumab on minimal disease activity (MDA) and disease activity in psoriatic arthritis (DAPSA): an analysis of the KEEPsAKE-1 AND -2 Trials. Ann Rheum Dis. 2022;81:827
Mease PJ, Kellner H, Morita A, et al. Long-term efficacy and safety of risankizumab in patients with active psoriatic arthritis: results from a 76-week phase 2 randomized trial. Rheumatol Ther. 2022;9(5):1361–75.
Borroni RG, Malagoli P, Gargiulo L, et al. Real-life effectiveness and safety of risankizumab in moderate-to-severe plaque psoriasis: a 40-week multicentric retrospective study. Acta Derm Venereol. 2021;101(11):adv00605.
Caldarola G, Zangrilli A, Bernardini N, et al. Risankizumab for the treatment of moderate-to-severe psoriasis: a multicenter, retrospective, 1 year real-life study. Dermatol Ther. 2022;35(6):e15489.
Gordon KB, Lebwohl M, Papp KA, et al. Long-term safety of risankizumab from 17 clinical trials in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2022;186(3):466–75.
Megna M, Fabbrocini G, Ruggiero A, Cinelli E. Efficacy and safety of risankizumab in psoriasis patients who failed anti-IL-17, anti-12/23 and/or anti IL-23: preliminary data of a real-life 16-week retrospective study. Dermatol Ther. 2020;33(6):e14144.
Thakre N, D'Cunha R, Goebel A, Liu W, Pang Y, Suleiman AA. Population pharmacokinetics and exposure-response analyses for risankizumab in patients with active psoriatic arthritis. Rheumatol Therapy. 2022;9(6):1587–603.
Haugh IM, Preston AK, Kivelevitch DN, Menter AM. Risankizumab: an anti-IL-23 antibody for the treatment of psoriasis. Drug Des Devel Ther. 2018;12:3879–83.
Girolomoni G, Strohal R, Puig L, et al. The role of IL-23 and the IL-23/T(H) 17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(10):1616–26.
McKeage K, Duggan S. Risankizumab: first global approval. Drugs. 2019;79(8):893–900.
Ru Y, Ding X, Luo Y, et al. Adverse events associated with Anti-IL-23 agents: clinical evidence and possible mechanisms. Front Immunol. 2021;12:670398.
Nguyen CT, Bloch Y, Skladanowska K, Savvides SN, Adamopoulos IE. Pathophysiology and inhibition of IL-23 signaling in psoriatic arthritis: a molecular insight. Clin Immunol. 2019;206:15–22.
Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605–13.
Mease PJ, McInnes IB, Tam LS, et al. Comparative effectiveness of guselkumab in psoriatic arthritis: results from systematic literature review and network meta-analysis. Rheumatology (Oxford). 2021;60(5):2109–21.
Acknowledgements
We thank the participants of the study.
Funding
This project was supported by grants from the National Natural Science Foundation of China (No. 82001740) and the Natural Science Foundation of Shanxi Province (No. 202203021221269). The journal’s Rapid Service Fee was funded by these grants.
Author information
Authors and Affiliations
Contributions
Qin-Yi Su, Hao-Nan Zhou, Guo-Mei Xia and Rui-Yuan Zhang wrote the manuscript, Hong-Yuan Tian, Chang Su, Yu-Xin Liu, He-Yi Zhang, Ting Cheng, Sheng-Xiao Zhang and Yue-Hong Huo designed the figures. Qinyi Su, Shengxiao Zhang, Qian Li and Rui-Yuan Zhang checked and polished the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Qin-Yi Su, Hao-Nan Zhou, Guo-Mei Xia, Rui-Yuan Zhang, Hong-Yuan Tian, Chang Su, Yu-Xin Liu, He-Yi Zhang, Ting Cheng, Yue-Hong Huo, Qian Li and Sheng-Xiao Zhang have no competing interests to declare.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Su, QY., Zhou, HN., Xia, GM. et al. Efficacy and Safety of Risankizumab in Patients with Psoriatic Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Rheumatol Ther 11, 227–237 (2024). https://doi.org/10.1007/s40744-024-00638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-024-00638-5