Abstract
Introduction
Clinical guidelines offer little guidance for treatment selection following inadequate response to conventional synthetic disease-modifying antirheumatic drug (csDMARD) in rheumatoid arthritis (RA). A molecular signature response classifier (MSRC) was validated to predict tumor necrosis factor inhibitor (TNFi) inadequate response. The decision impact of MSRC results on biologic and targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD) selection was evaluated.
Methods
This is an analysis of AIMS, a longitudinal, prospective database of patients with RA tested using the MSRC. This study assessed selection of b/tsDMARDs class after MSRC testing by surveying physicians, the rate of b/tsDMARD prescriptions aligning with MSRC results, and the percentage of physicians utilizing MSRC results for decision-making.
Results
Of 1018 participants, 70.7% (720/1018) had treatment selected after receiving MSRC results. In this MSRC-informed cohort, 75.6% (544/720) of patients received a b/tsDMARD aligned with MSRC results, and 84.6% (609/720) of providers reported using MSRC results to guide treatment selection. The most prevalent reason reported (8.2%, 59/720) for not aligning treatment selection with MSRC results from the total cohort was health insurance coverage issues.
Conclusion
This study showed that rheumatologists reported using the MSRC test to guide b/tsDMARD selection for patients with RA. In most cases, MSRC test results appeared to influence clinical decision-making according to physician self-report. Wider adoption of precision medicine tools like the MSRC could support rheumatologists and patients in working together to achieve optimal outcomes for RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Tumor necrosis factor inhibitors (TNFi) are standard first-line biologics after conventional synthetic disease-modifying antirheumatic drug (DMARD) failure, yet 60% to 82% of patients with rheumatoid arthritis (RA) inadequately respond to TNFi. |
The lack of personalized therapeutic recommendations in current RA guidelines results in trial-and-error biologic prescribing, underscoring the need for predictive biomarkers to enable optimal personalized treatment selection. |
This study assessed the impact of molecular signature response classifier (MSRC) testing on biologic and targeted synthetic DMARD (b/tsDMARD) selection by surveying physicians on prescription alignment with test results and their reported use of the MSRC to guide treatment decisions. |
What was learned from the study? |
For patients whose physicians received MSRC results prior to treatment selection, 75.6% (544/720) of patients received a b/tsDMARD aligned with MSRC results, and 84.6% (609/720) of providers reported using MSRC results to guide treatment selection. |
These results indicate that the MSRC informs treatment selection decisions and enables physicians with actionable information to select a b/tsDMARD for moderate-to-high RA. |
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease that initially causes joint inflammation and pain, potentially leading to disability [1]. Inadequately controlled RA is associated with considerable morbidity and increased mortality [2,3,4]. Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) such as methotrexate are first-line treatments, but only 30% of patients achieve low disease activity or remission with these therapies [5,6,7]. Biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) have expanded treatment options; however, trial-and-error prescribing remains common because of lack of predictive biomarkers [7,8,9]. Tumor necrosis factor inhibitors (TNFi) are the first bDMARDs for 90% of patients who are b/tsDMARD-naïve after csDMARD failure [10]. Inadequate response rates to TNFi are variable, ranging from 30% to 40% in some reports, while others report an inadequate response ranging from 60% to 82% [11,12,13,14]. Current RA treatment guidelines lack recommendations for selecting personalized b/tsDMARD therapies [5, 6]. This gap results in trial-and-error b/tsDMARD prescription until an effective class of drug is found, highlighting the need for predictive biomarkers to promote optimal treatment decisions.
To address this gap in clinical practice, a blood-based molecular signature response classifier (MSRC) has been validated to detect a signature of inadequate response to TNFi therapy [15, 16]. The MSRC is an algorithmic classifier that integrates RNA expression data from 19 genes, three clinical features (body mass index (BMI); sex; patient global assessment (PtGA)), and one laboratory feature (anti-cyclic citrullinated protein (anti-CCP) serostatus). It generates a score from 1 to 25, with higher scores indicating a greater probability of inadequate response to TNFi. Patients with a score ≥ 10.6 are predicted to be inadequate responders to TNFi with a positive predictive value of 87.7% (95% CI 78–97%) [16]. The MSRC has been validated to accurately detect a signature of inadequate response to TNFi in both b/tsDMARD-naïve and TNFi-exposed patients with RA with moderate to high disease activity [15, 16].
Prior clinical utility studies have demonstrated improved outcomes when treatment aligned with MSRC results [17, 18]. Compared to standard of care, a significantly higher proportion of patients achieved treatment targets when treatment selection was informed by MSRC results. Although the clinical validity and performance of the TNFi MSRC have been demonstrated, the impact of the classifier on physician decision-making has been directly assessed in only a limited capacity. Clinical decision-making is a complex process that considers a variety of factors, including the interpretability and applicability of diagnostic test results. The impact of actionable precision medicine test results on clinical decision-making for RA treatment remains poorly characterized. Consequently, a decision impact analysis is required to better elucidate the real-world implications of the TNFi MSRC for b/tsDMARD treatment selection in RA.
The objective of this study was to assess self-reported impact of MSRC test results on rheumatologists’ treatment decisions. Physician surveys were used to determine if results from MSRC testing informed choice of b/tsDMARD for patients with RA with moderate-to-high disease activity. This study addresses critical gaps regarding the real-world impact of MSRC testing on clinical decision-making and barriers preventing personalized b/tsDMARD selection for RA management. Investigation of factors affecting alignment between MSRC results and prescribed therapy can provide actionable insights to improve patient outcomes and satisfaction, as well as guide next steps to overcome barriers to precision medicine implementation.
Methods
Data Source: AIMS
AIMS is a prospective, longitudinal, multi-institutional clinical database of patients with RA managed by providers in clinical practice. A total of 72 US private and academic rheumatology practices participate in AIMS under Institutional Review Board (Advarra IRB Services, Columbia, MD, USA; IRB #PRO00044807) approval, with informed consent obtained from all enrolled patients. All patient data is deidentified, and management of clinical data is conducted in accordance with the Health Insurance Portability and Accountability Act (HIPAA) [18].
Description of MSRC Test
The MSRC identifies a molecular signature associated with inadequate achievement of ACR50 to TNFi therapy in patients with a clinical diagnosis of RA [15, 16]. The MSRC test underwent an update during the study period.
For patients tested prior to August 2021, the 23-biomarker MSRC included assessment of single-nucleotide polymorphisms (SNPs), gene expression features, anti-CCP, sex, BMI, PtGA of disease activity, and C-reactive protein (CRP) [19]. After August 2021, the MSRC test was updated and validated to a 19-biomarker version removing the SNP and CRP components and relying on gene expression, anti-CCP status, sex, BMI, and PtGA of disease activity [16].
The MSRC test produces a continuous result from 1 to 25. Pre-defined thresholds along this scale categorize patients as having very high, high, or no signal of inadequate response to TNF inhibitors at 6 months as measured by ACR50 criteria. Patients exceeding a threshold of 10.6 are identified as having a molecular signature of inadequate response. These response categories were optimized during analytical validation of the MSRC [16].
Gene expression feature analysis was performed in PAXgene Blood RNA tubes. Anti-CCP testing was conducted in serum separator tubes. Sample processing and anti-CCP testing was performed in the Scipher Medicine Laboratory (Durham, North Carolina; CAP# 8821838, CLIA# 34D2180776) on the Roche Cobas® system in accordance with standard operating procedures. RNA sequencing was performed at the Ambry Genetics Corporation (Aliso Viejo, CA) under Clinical Laboratory Improvement Amendments laboratory standard operating procedures, as previously described [16]. Algorithmic analysis of the MSRC was performed at the Scipher Medicine Laboratory. The MSRC test was analytically and clinically validated in both biologic-naïve and biologic-exposed patients with RA, demonstrating comparable performance characteristics between these patient populations [15, 16].
Study Design and Statistical Analyses
Study Design and Registry
This study was designed to interrogate the AIMS database for physician use of the MSRC, b/tsDMARD treatment at baseline for each patient, and the subsequent treatment decisions made by physicians. Baseline characteristics, including previous csDMARD treatment, treatment at baseline, patient demographics, and baseline clinical variables were recorded (Table 1). The presence or absence of an inadequate response signature detected by the MSRC and the treatment prescribed after receiving MSRC results were documented for each. To evaluate alignment between treatment selection and MSRC test results, patients were characterized into two groups: (1) patients with a signature of inadequate response to TNFi who were prescribed a TNFi were noted to have received therapy that did not align with MSRC results; (2) patients with a signature of inadequate response to TNFi who were prescribed a non-TNFi as well as patients without a signature of inadequate response to TNFi were considered to have received a b/tsDMARD consistent with MSRC results.
Eligibility Criteria
Patients from the AIMS database who met the following inclusion criteria were included in this study: 18 years of age or older with a clinical diagnosis of RA by a rheumatologist; moderate or high disease activity at baseline [CDAI > 10]; naïve to or with a history of treatment with non-TNFi b/tsDMARD prior to enrollment; currently on a TNFi at the time of MSRC testing; must have baseline and treatment decision visit data available for analyses. Patients treated with non-TNFi b/tsDMARD at the time of MSRC testing were excluded. Patients were also excluded if (1) they were participating in other clinical studies and (2) patients’ physicians reported making a treatment decision prior to receiving MSRC test results.
Interventions and Data Collection
Interventions consisted of the MSRC, described above. Two different versions of a questionnaire probed physician decision-making within the AIMS study, physicians completed: (1) version 1 of the questionnaire for patients enrolled between August 2020 and August 2021; and (2) version 2 for patients enrolled between August 2021 and February 2023. Improvements in questionnaire version 2 reflected an expansion of the MSRC intended use and in addition, versions of the questionnaire differed in how physicians were asked about use of the MSRC to guide treatment: version 1 used a “yes/no” question; version 2 asked if the MSRC was used with “yes/no” response, and category selection of how it was used. If the answer was “yes”, the options were “Start new therapy (new therapies include new products both within class and outside of class)”, “Continue existing therapy as originally prescribed”, and “Continue existing therapy, but modify dose and/or add an additional medication”. Both versions of the questionnaire documented reasons why the MSRC was not used to inform treatment decision-making.
Outcomes
The primary analyses evaluated observed physician behavior, reporting the percentage of patients who were prescribed and received a b/tsDMARD consistent with MSRC results, and the percentage of physicians who prescribed various treatments after receiving test results. Secondary analyses evaluated how physician self-reported perceptions of MSRC impact on decision-making, assessing the percentage of patients for whom their provider reported incorporating the MSRC as a precision medicine tool into their decision-making, and reasons for not incorporating test results into decision-making.
Statistical Analyses
Statistical analyses were performed using R version 3.6.1 (www.rproject.org). Continuous variables were described using mean, standard deviation (SD), or median, interquartile range (IQR), and the number of non-missing observations. Categorical variables were summarized by providing the frequency counts and percentages, including a separate category for missing data. Normality is established using both Shapiro–Wilk test as well as visualization techniques such as Q–Q plot. Student’s t test and chi-squared tests were utilized for continuous and categorical variables, respectively, in the baseline table to determine differences between patient cohort subsets in primary analyses. For non-normally distributed continuous variables, Kruskal–Wallis test was utilized. All tests were conducted with a two-sided setting and, unless specified otherwise, the significance level was set at 0.05. Efforts were made to minimize the amount of missing data; no imputation of missing data was conducted.
Results
Study Population
A total of 1018 patients from the AIMS database met all inclusion and exclusion criteria. MSRC tests were ordered for these patients by 119 participating physicians, of which 82.4% (98/119) prescribed treatment for 720 patients after receiving MSRC results. This MSRC-informed cohort (n = 720) patients had a mean [SD] age of 57.45 [13.31] years and 582 (80.8%) were female (Fig. 1). Median RA disease duration was 4.0 years and 402 (55.8%) were b/tsDMARD-naïve. Among 44.2% of patients (318/720) who had previously received a b/tsDMARD, TNFi was the most common biologic used prior to MSRC testing in 87.4% (278/318) and 72.3% (230/318) were active TNFi users at the time of blood draw. Details of baseline demographic and disease features of patients whose treatment was informed by the MSRC are shown in Table 1.
Flow diagram for patients in this study. The interim analysis cohort consisted of 1018 patients who met inclusion and exclusion criteria. The MSRC-informed cohort included 720 patients for whom the MSRC result was available on or before the date for b/tsDMARD initiation and who were not participating in another clinical trial. b/tsDMARD biologic or targeted synthetic disease-modifying antirheumatic drug; CDAI clinical disease activity index; MSRC molecular signature response classifier; RA rheumatoid arthritis
Physicians for 29.3% of patients (298/1018) elected to prescribe treatment prior to receiving MSRC results and were excluded from analyses. These patients were more likely to be Hispanic or Latino (p = 0.006), b/tsDMARD-naïve (p < 0.001), and have higher baseline disease activity than patients in the MSRC-informed cohort as measured by physician global assessment (52.9 vs 46.5; p < 0.001), tender joint counts (14.9 vs 11.7; p < 0.001), swollen joint counts (9.6 vs 6.3; p < 0.001), CDAI (35.2 vs 28.0; p < 0.001), and a history or active use of prednisone.
Impact of MSRC on Treatment Selection
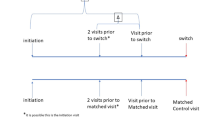
Among the MSRC-informed cohort, TNFi was the most common b/tsDMARD selected (56.9%, 410/720); 41.0% of these patients (168/410) were actively receiving TNFi therapy at baseline and continued the same TNFi after receiving MSRC results. In these 168 patients, 40.5% (68/168) received an MSRC prediction of inadequate response to TNFi and 59.5% (100/168) did not. Among new treatment initiations (76.7%, 552/720), the next most common b/tsDMARDs selected after TNFi therapies (43.8%, 242/552) were JAK inhibitors (24.1%, 133/552), T cell co-stimulation modulator (22.1%, 122/522), IL-6 inhibitors (7.2%, 40/552), and B cell inhibitor (2.7%, 15/552) (Table 2). Physicians made decisions that did not conflict with MSRC results in 75.6% of patients (544/720) (Fig. 2).
Patients with a signature of inadequate response constituted 56.3% of the MSRC-informed cohort (405/720), and 56.5% of such patients (229/405) received a non-TNFi b/tsDMARD. Compared to these patients, those who received a TNFi had lower baseline disease activity assessments such as pain (60.4 vs 65.0, p = 0.048), physician global assessment (47.1 vs 55.31, p < 0.001), swollen joint counts (5.5 vs 7.5, p = 0.002), and CDAI (28.6 vs 32.1, p = 0.02) (Table 1).
Patients with no prediction of inadequate response to TNFi comprised 43.8% of the MSRC-informed cohort (315/720). TNFi was prescribed to 74.3% of these patients (234/315). This subgroup treated with TNFi had a greater proportion of patients who were seronegative (41.6% vs 20.3%, p = 0.002) than those with no prediction of inadequate response who received a non-TNFi.
Physician-Reported Impact of MSRC on Treatment Decision-Making
Two versions of the physician questionnaire were administered: (1) version 1 of the questionnaire for 161 patients enrolled between August 2020 and August 2021; and (2) version 2 for 559 patients enrolled between August 2021 and February 2023. On average, 98 physicians in the MSRC-informed cohort reported data in four patients each (median, IQR [2–10]); and questionnaire responses were not provided in 0.8% of patients (6/720). For each patient, physicians were asked whether MSRC results were used to inform b/tsDMARD selection. Physicians reported incorporating MSRC results into treatment decisions in 84.6% (609/720) of patients (Table 3). A prediction of inadequate response was reported in 56.3% (405/720) of patients and MSRC results were used in treatment decision-making in 82.0% (332/405). TNFi were prescribed to 43.5% (176/405) who received a prediction of inadequate response; within this subgroup, providers reported using MSRC results to inform treatment decision-making in 68.8% (121/176) of patients. No signal of inadequate response was detected in 43.8% of patients (315/720) and MSRC results contributed to treatment decisions in 88.0% (277/315) of this subgroup.
In the MSRC-informed group of patients, the reasons physicians reported not incorporating MSRC results into treatment decision-making (14.6%, 105/720) included health insurance concerns (8.2%, 59/720); patient and provider concerns with treatment options (0.5%, 4/720 and 1.1%, 8/720, respectively); issues pertaining to the interpretation of or action to be taken as recommended by the results (1.1%, 8/720); stable on therapy (1.1%, 8/720); unclassified response (1.1%, 8/720); and unknown (1.4%, 10/720). In the subset of patients whose physicians reported not using MSRC results and who expressed concerns regarding health insurance, 62.7% (37/59) had a prediction of inadequate response and 52.5% (31/59) were prescribed TNFi (Table 3).
Discussion
Selecting an effective therapy for achieving low disease activity or remission in RA often takes a long time and requires careful consideration of multiple factors such as the patient’s disease activity, comorbidities, preferences, and response to previous treatments. In fact, many patients do not achieve adequate response to both initial csDMARDs and subsequent b/tsDMARDs, which prolongs the process of finding the optimal therapy. Another contributing factor is the absence of empiric evidence and recommendations from either the American College of Rheumatology or the European Alliance of Associations for Rheumatology regarding selection of TNFi over another b/tsDMARD, although lack of recommendations is largely motivated by a dearth of evidence showing the clear superiority of some RA therapies over others [5, 6]. The trial-and-error approach to prescribing leads to delays in effective treatment initiation, enabling disease progression, escalating costs, deterioration of health-related quality of life, and increased burden on caregivers [20]. To address this unmet and urgent clinical need, the MSRC has been validated to predict inadequate response to TNFi with positive predictive value of 88%, specificity of 77%, and sensitivity of 60% [16].
In this decision impact study, 84.6% of physicians reported using MSRC results to inform treatment decision-making and 75.6% of patients received treatment aligned with their individualized MSRC test. These data are consistent with earlier analyses performed on a smaller set of patients from the AIMS study that reported 70.3% patients received treatment consistent with MSRC results [21]. Mirroring the expanding use of gene expression classifiers in various clinical fields, this study reveals a growing acceptance among physicians of the TNFi MSRC to inform RA treatment [22, 23]. This trend underscores a broader shift toward precision medicine, reflecting increased confidence in genomic assays to enhance patient care. In this context, treatment decisions informed by the MSRC improved outcomes when compared to those informed by the standard of care. Patients who received treatment guided by the MSRC were significantly more likely to achieve CDAI low disease activity/remission, remission (CDAI-REM), or improvements greater than or equal to minimally important differences in CDAI scores (CDAI-MID) [17].
Patients with MSRC results detecting a signature of inadequate response to TNFi constituted 56.3% of patients in this study (405/720), and 56.5% of those (229/405) received a non-TNFi; yet 43.4% (176/405) received TNFi. The results presented here suggest that patients who have a signature of inadequate response to TNFi with higher disease activity are more likely to be treated with non-TNFi b/tsDMARDs. Providers can reassess treatment choices with respect to MSRC results at subsequent patient visits. Such analyses were beyond the scope of the current investigation.
Concern over health insurance coverage was the most frequently cited reason (8.2%, 59/720) for not incorporating MSRC results into clinical decision to inform therapy selection. Of the 59 patients who were not prescribed a therapy informed by MSRC results because of health coverage concerns, 52.5% (31/59) were prescribed a TNFi despite having a signature of inadequate response to TNFi. This suggests that, when faced with concerns over health insurance coverage, physicians may elect to treat patients with RA with a TNFi, even if MSRC results indicate that a different therapy may be more effective. The concern that physicians could not use precision medicine results to inform more effective therapy is warranted, as such health coverage restrictions could lead to suboptimal treatment outcomes. Over 70% of patients with RA are reported to have insurance plans that require step therapy and are less likely to achieve treatment goals and 34% have restrictions to access of medications; other barriers include cost sharing and copayment arrangements, further restricting treatment options [24,25,26]. While many insurers still require “fail first” policies before covering costly therapies, step therapy risks worse outcomes by limiting prescribing options and requiring failed trials of less effective medications before more suitable ones [26,27,28,29]. This study showed high adherence to the biomarker signature, demonstrating its potential to guide more personalized treatment selection rather than trial-and-error.
With recent evidence supporting the clinical utility of the TNFi MSRC, this MSRC-informed approach may provide justification to update fail-first policies and enable precision medicine-directed therapy selection [17, 18, 21]. The type of health insurance a patient had may have played a more subtle role in some treatment decisions. Patients who were prescribed a TNFi despite a signature of inadequate response, when compared with the group of patients who received a signature of inadequate response and were prescribed a b/tsDMARD other than a TNFi, were younger, had a shorter duration of RA, had less severe disease, and were more likely to already be on a TNFi. This is consistent with a previous report that patients prescribed a non-TNFi were more likely to have longer disease duration and more severe disease. In that study, patients who were prescribed a non-TNFi were also more likely to have government insurance rather than private insurance [14].
Determining optimal treatment targets, assessing progress toward those targets, and selecting subsequent therapies present daily challenges in rheumatoid arthritis management. In our study, physicians who did not incorporate MSRC results into treatment decision-making reported that results were unclear or provided a recommendation that might not be actionable for only eight of the 105 patients (7.6%). Of these patients, seven were prescribed a TNFi despite a signature of inadequate response. While this pattern might suggest TNFi cycling at first glance, it could stem from the multifaceted clinical decision-making process rather than conclusively drug cycling. Myriad factors influence treatment selection, including considerations of available options, patient preferences elicited through shared decision-making conversations, and payer restrictions guiding physician prescription choices. Therefore, prescribing TNFi with ambiguous or discordant interpretation of the response signature may reflect the complex interplay of clinical judgment, patient values, and systemic constraints, rather than exclusively TNFi cycling. Our findings underscore the nuances physicians navigate when interpreting precision medicine assays and applying results to personalized treatment decisions within the larger healthcare ecosystem.
This study has several limitations that warrant consideration. First, the generalizability of the physician survey findings may be affected by respondent bias and regional differences in RA treatment practices. While the survey included a geographically diverse sample representing more than 70 private and academic rheumatology practices, attitudes and clinical experiences influencing interpretability and adoption of the MSRC test results in clinical decision-making may vary. Nonetheless, this study may exhibit a selection bias toward a more intrinsically motivated cohort of patients and healthcare providers. However, surveying adopters of precision medicine yields important self-reported insights into the impact of the MSRC assay on therapeutic decision-making within this actively engaged study population. Although this prospective study collected data on patients’ prior anti-TNF history at baseline, the physician survey did not specifically examine the impact of this treatment experience on physician’s complex decision-making process regarding cycling versus switching biologic therapies, in the context of evolving clinical evidence and other considerations. While insurance coverage issues were cited by rheumatologists as impacting actionability of MSRC results, this study did not account for potential effects of insurance providers’ step therapy requirements. In a study by the Arthritis Foundation, 25% of patients who switched insurance providers were required to repeat step therapy with their new carrier. Finally, this survey provides a cross-sectional view of MSRC test utilization [28]. Long-term studies are needed to elucidate patterns of MSRC adoption among ordering physicians. Despite these limitations, this study offers important data on MSRC test adoption and influence on RA treatment selections.
Conclusions
This decision impact study surveying practicing rheumatologists demonstrates high utilization of the MSRC test for TNFi inadequate response to guide b/tsDMARD selection in patients with RA. Most surveyed rheumatologists endorsed alignment of prescribed b/tsDMARDs with MSRC results and incorporation of the test results into clinical decision-making. Systemic barriers including healthcare coverage and reimbursement issues were obstacles preventing universal adoption of the MSRC to facilitate personalized therapy. Reforming coverage policies, formulary restrictions, and step therapy protocols to enable equitable access to actionable precision medicine tools such as the MSRC could enable clinicians to more effectively utilize precision medicine tools to optimize treatment selection for patients with RA and expedite achievement of treatment targets. Additionally, the study found minimal concerns or questions from patients regarding the test, suggesting physician proficiency in utilizing and communicating results to inform treatment decisions in real-world rheumatology practice. While RA remains incurable, rheumatologists and patients strive to achieve remission or low disease activity. Precision medicine tools such as the TNFi MSRC offer significant opportunities to expedite reaching desirable therapeutic targets.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to proprietary and confidential data.
References
Gravallese EM, Firestein GS. Rheumatoid arthritis—common origins, divergent mechanisms. N Engl J Med. 2023;388:529–42.
Sofat N, Malik O, Higgens CS. Neurological involvement in patients with rheumatic disease. QJM Int J Med. 2006;99:69–79.
De Cock D, Hyrich K. Malignancy and rheumatoid arthritis: epidemiology, risk factors and management. Best Pract Res Clin Rheumatol. 2018;32:869–86.
Popescu D, Rezus E, Badescu MC, et al. Cardiovascular risk assessment in rheumatoid arthritis: accelerated atherosclerosis, new biomarkers, and the effects of biological therapy. Life (Basel). 2023;13:319.
Fraenkel L, Bathon JM, England BR, et al. American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;2021(73):924–39.
Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3–18.
O’Dell JR, Mikuls TR, Taylor TH, et al. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med. 2013;369:307–18.
Ouboussad L, Burska AN, Melville A, Buch MH. Synovial tissue heterogeneity in rheumatoid arthritis and changes with biologic and targeted synthetic therapies to inform stratified therapy. Front Med (Lausanne). 2019;6:45.
Lim SH, Kim K, Choi C-I. Pharmacogenomics of monoclonal antibodies for the treatment of rheumatoid arthritis. J Pers Med. 2022;12:1265.
Johnson KJ, Sanchez HN, Schoenbrunner N. Defining response to TNF-inhibitors in rheumatoid arthritis: the negative impact of anti-TNF cycling and the need for a personalized medicine approach to identify primary non-responders. Clin Rheumatol. 2019;38:2967–76.
Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340(4):253–9.
Plenge RM, Bridges SL. Personalized medicine in rheumatoid arthritis: miles to go before we sleep. Arthritis Rheum. 2011;63:590–3.
Pappas DA, St John G, Etzel CJ, et al. Comparative effectiveness of first-line tumour necrosis factor inhibitor versus non-tumour necrosis factor inhibitor biologics and targeted synthetic agents in patients with rheumatoid arthritis: results from a large US registry study. Ann Rheum Dis. 2021;80:96–102.
Curtis JR, Kremer JM, Reed G, John AK, Pappas DA. TNFi cycling versus changing mechanism of action in TNFi-experienced patients: result of the corona CERTAIN comparative effectiveness study. ACR Open Rheumatol. 2021;4:65–73.
Cohen S, Wells AF, Curtis JR, et al. A molecular signature response classifier to predict inadequate response to tumor necrosis factor-α inhibitors: the NETWORK-004 prospective observational study. Rheumatol Ther. 2021;8:1159–76.
Jones A, Rapisardo S, Zhang L, et al. Analytical and clinical validation of an RNA sequencing-based assay for quantitative, accurate evaluation of a molecular signature response classifier in rheumatoid arthritis. Expert Rev Mol Diagn. 2021;21:1235–43.
Curtis JR, Strand V, Golombek S, et al. Patient outcomes improve when a molecular signature test guides treatment decision-making in rheumatoid arthritis. Expert Rev Mol Diagn. 2022;22:1–10.
Strand V, Zhang L, Arnaud A, Connolly-Strong E, Asgarian S, Withers JB. Improvement in clinical disease activity index when treatment selection is informed by the tumor necrosis factor-α inhibitor molecular signature response classifier: analysis from the study to accelerate information of molecular signatures in rheumatoid arthritis. Expert Opin Biol Ther. 2022;22:801–7.
Mellors T, Withers JB, Ameli A, et al. Clinical validation of a blood-based predictive test for stratification of response to tumor necrosis factor inhibitor therapies in rheumatoid arthritis patients. Netw Syst Med. 2020;3:91–104.
Burgers LE, Raza K, van der Helm-van Mil AH. Window of opportunity in rheumatoid arthritis—definitions and supporting evidence: from old to new perspectives. RMD Open. 2019;5:e000870.
Strand V, Cohen SB, Curtis JR, et al. Clinical utility of therapy selection informed by predicted nonresponse to tumor necrosis factor-α inhibitors: an analysis from the study to accelerate information of molecular signatures (AIMS) in rheumatoid arthritis. Expert Rev Mol Diagn. 2022;22:101–9.
Stover DG, Reinbolt RE, Adams EJ, et al. Prospective decision analysis study of clinical genomic testing in metastatic breast cancer: impact on outcomes and patient perceptions. JCO Precision Oncol. 2019;3:1–11.
Dieci MV, Guarneri V, Zustovich F, et al. Impact of 21-gene breast cancer assay on treatment decision for patients with T1–T3, N0–N1, estrogen receptor-positive/human epidermal growth receptor 2-negative breast cancer: final results of the prospective multicenter ROXANE study. Oncologist. 2019;24:1424–31.
Desai RJ, Rao JK, Hansen RA, Fang G, Maciejewski ML, Farley JF. Predictors of treatment initiation with tumor necrosis factor-α inhibitors in patients with rheumatoid arthritis. JMCP. 2014;20:1110–20.
Polinski JM, Mohr PE, Johnson L. Impact of Medicare part D on access to and cost sharing for specialty biologic medications for beneficiaries with rheumatoid arthritis. Arthritis Rheum. 2009;61:745–54.
Boytsov N, Zhang X, Evans KA, Johnson BH. Impact of plan-level access restrictions on effectiveness of biologics among patients with rheumatoid or psoriatic arthritis. Pharmacoecon Open. 2019;4:105–17.
Park Y, Raza S, George A, Agrawal R, Ko J. The effect of formulary restrictions on patient and payer outcomes: a systematic literature review. J Manage Care Spec Pharm. 2017;23:893–901. https://doi.org/10.18553/jmcp.2017.23.8.893.
Arthritis Foundation. Step therapy. https://www.arthritis.org/advocate/issue-briefs/step-therapy. Accessed 7 Aug 2023.
Fendrick AM, Mease P, Davis M, et al. Continuity of care within a single patient support program for patients with rheumatoid arthritis prescribed second or later line advanced therapy. Adv Ther. 2023;40:990–1004.
Acknowledgements
We thank the health care providers and participants who made this study possible.
Authorship.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Funding
This study was funded by Scipher Medicine Corporation. The Rapid Service Fee is also being paid by Scipher Medicine Corporation.
Author information
Authors and Affiliations
Contributions
Conceptualization—Jeffrey R. Curtis, Vibeke Strand, Steven Golombek, George Karpouzas, Krishna Patel, Jennifer Dines, Viatcheslav R. Akmaev. Methodology—Jeffrey R. Curtis, Vibeke Strand, Steven Golombek, Lixia Zhang, Krishna Patel, Jennifer Dines. Formal Analysis—Lixia Zhang, Angus Wong. Investigation—Jeffrey R. Curtis, Vibeke Strand, Steven Golombek, George Karpouzas, Krishna Patel. Resources—Lixia Zhang, Krishna Patel, Jennifer Dines, Viatcheslav R. Akmaev. Data Curation—Lixia Zhang, Angus Wong, Krishna Patel. Writing—Original Draft—Lixia Zhang, Angus Wong, Krishna Patel. Writing—Review and Editing—Jeffrey R. Curtis, Vibeke Strand, Steven Golombek, George Karpouzas, Lixia Zhang, Angus Wong, Krishna Patel, Jennifer Dines, Viatcheslav R. Akmaev. Visualization—Lixia Zhang, Angus Wong, Krishna Patel. Supervision—Lixia Zhang, Krishna Patel, Jennifer Dines, Viatcheslav R. Akmaev. Project administration—Lixia Zhang, Krishna Patel, Jennifer Dines. Funding acquisition—Jennifer Dines, Viatcheslav R. Akmaev.
Corresponding author
Ethics declarations
Conflict of Interest
Jeffrey Curtis reported financial relationships with AbbVie Pharmaceuticals, Amgen Inc., Bendcare, Bristol Myer Squib Company, CorEvitas, Eli Lilly & Company, Janssen Pharmaceuticals, Myriad Genetics, Novartis, Pfizer Inc., Regeneron Pharmaceuticals Inc., Roche, Scipher Medicine Corp., and UCB. Vibeke Strand reported consulting fees from Abbvie Pharmaceuticals, Alpine Immune Sciences, Alumis, Amgen Inc., Aria Pharmaceuticals, AstraZeneca, Atom Biosciences, Bayer AG, Bioventus Inc., Blackrock Inc., Bristol-Myers Squibb Company, Boehringer Ingelheim, Celltrion Inc., Citryll, Equillium, Ermium, Fortress Biotech, Genentech/Roche Inc., Gilead Sciences Inc., GlaxoSmithKline Pharmaceuticals Ltd, Horizon Therapeutics, Inmedix Inc., Janssen Pharmaceuticals, Kiniksa Pharmaceuticals, Lilly, Merck & Co., MiMedx Group, Novartis Pharmaceuticals Corporation, Omeros, Pfizer Inc., R-Pharm, RAPT, Regeneron Pharmaceuticals Inc., Samsung, Sandoz, Sanofi, Scipher Medicine Corp., Setpoint Medical, SOFUSA, Spherix Global Insights, and Urica. George Karpouzas reported financial support from Pfizer and is a clinical trial adjudicator for Janssen. Steven Golombek reported speaking honoraria from Amgen Inc. and AstraZeneca. Lixia Zhang, Angus Wong, Jennifer Dines, Krishna Patel, and Viatcheslav Akmaev reported financial support provided by Scipher Medicine Corp.
Ethical Approval
AIMS is a prospective, longitudinal, multi-institutional clinical database of patients with RA managed by providers in clinical practice. A total of 72 US private and academic rheumatology practices participate in AIMS under Institutional Review Board (Advarra IRB Services, Columbia, MD, USA; IRB #PRO00044807) approval, with informed consent obtained from all enrolled patients. All patient data is deidentified, and management of clinical data is conducted in accordance with the Health Insurance Portability and Accountability Act (HIPAA) [18].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Curtis, J.R., Strand, V., Golombek, S.J. et al. Decision Impact Analysis to Measure the Influence of Molecular Signature Response Classifier Testing on Treatment Selection in Rheumatoid Arthritis. Rheumatol Ther 11, 61–77 (2024). https://doi.org/10.1007/s40744-023-00618-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00618-1