Abstract
Introduction
With scarce comparative data on mortality in Australian patients with rheumatoid arthritis (RA), we investigated temporal changes in standardized mortality rates for patients with RA using longitudinal linked population-wide health data in Western Australia (WA) over the period 1980 to 2015.
Methods
The study included 17,125 patients with a first-time hospital contact for RA (ICD-10-AM M05.00–M06.99 and ICD-9-AM 714.00–714.99) in the study period. Standardized mortality rate ratios (SMRRs) for the RA cohort versus the WA general population was estimated using direct age standardization. We analyzed temporal trends over with dates and causes provided by the WA Death Registry.
Results
During 356,069 patient-years of follow-up, a total of 8955 (52%) deaths occurred in the RA cohort. The SMRR was 2.24 (95% CI 2.15–2.34) in males and 3.09 (95% CI 3.00–3.19) in females over the study period. SMRR decreased since 2000 to 1.59 (95% CI 1.39–1.81) for the period 2011–2015. Median survival was 26.80 years (95% CI 26.30–27.30), where age and comorbidity independently increased the risk of death. The leading causes of deaths were cardiovascular diseases (26.60%), cancer (16.80%), rheumatic diseases (5.80%), chronic pulmonary disease 491 (5.50%), dementia (3.00%), and diabetes 235 (2.6%).
Conclusions
The mortality rate in patients with RA in WA has decreased but remains 1.59-times higher than in community counterparts, suggesting that there is room for further improvement. Comorbidity is the main modifiable risk factor to further reduce mortality in patients with RA.
Similar content being viewed by others
Why carry out this study? |
Rheumatoid arthritis (RA) contributes to excess morbidity and mortality in patients with RA compared with the general population. |
The mortality rate of RA remains higher than the general population, reducing the life expectancy by approximately 10 years. |
There is a paucity of published literature on mortality and morbidity rates in Australian patients with RA. |
What was learned from the study? |
The mortality rate of patients with RA in Western Australia has decreased but remains 1.5-times higher than in community counterparts, suggesting that improved management comorbidity can further reduce mortality in RA. |
Introduction
Mortality rates for patients with rheumatoid arthritis (RA) have reportedly improved in the past decades due to increasing access to specialized rheumatology care [1], early introduction of DMARDs with combination regimens [2], and subsequent biologics therapies [3]. However, the mortality rate remains higher than the general population, reducing the life expectancy by approximately 10 years [4,5,6,7,8,9]. A review found the median standardized mortality rate ratios (SMRRs) for patients with RA between 1953 and 2008 varied from 1.20 and 1.30 in inception cohorts and 1.60 to 1.70 in observational cohorts including patients with longer disease duration [6, 10]. Thus, patients with RA have not experienced the same improvement in survival over time as the general population [11]. To close the mortality gap, it is critical to understand the trend in causes and predictors of RA mortality in order to develop suitable measures [11]. The reported excess mortality in patients with RA may relate to disease severity, the higher prevalence and poorer outcome of cardiovascular disease (CVD) [12,13,14], and/or the total number of comorbidities [15, 16]. There is an evident disparity between patients with RA and the general population in terms of prevention and management of comorbidities [17, 18]. The updated Charlson Comorbidity Index (CCI) has validated International Classification of Diseases (ICD) coding algorithms to identify comorbid conditions as predictors of mortality to predict mortality in various disease sub-groups and the overall CCI and weight risk scores have been validated in hospital discharge data from six countries, including Australia [19]. As linked health data provide the best overall RA case ascertainment [20], and there is a paucity of published literature on mortality and morbidity rates in Australian patients with RA [21], this study estimated temporal standardized mortality rates over a 35-year period and identified survival predictors and leading causes of death in a large RA cohort.
Methods
Study Design
This is a longitudinal population-based study using population-wide linked administrative health data to assess standardized mortality rates and comorbidity accrual for patients with RA in WA from 1980 to 2015.
Data Sources
The Western Australian Rheumatic Disease Epidemiological Registry (WARDER) [22, 23] contains longitudinally linked health data for all hospital-ascertained patients with RA in the WA Hospital Morbidity Data Collection, Emergency Department Data Collection, and the WA death registry. WARDER data were extracted and linked by the WA DLB through probabilistic matching with clerical review based on patient's name, date of birth, sex, and residential address with high linkage accuracy (99.90%) [24]. These linked datasets provide the opportunity to analyze comorbidities and mortality for people with RA.
Participants
Patients with RA were identified from the WARDER with the ICD primary or secondary codes for RA (ICD-10-AM M05.00–M06.99 and ICD-9-AM 714.00–714.99) who as having had been admitted at least once in a public or private hospital in WA from 1 January 1980 to 31 December 2014. The WA clinical coding authority confirmed the relevant ICD codes used for extraction. To increase the RA diagnosis accuracy, patients with RA were excluded if they had at least two subsequent health contacts identifying other forms of arthritis, including psoriatic arthritis, ankylosing spondylitis, other spondyloarthropathies and systemic lupus erythematosus, or other connective tissue diseases [25]. This method has been shown to have greater than 90% positive predictive value for identifying patients with a rheumatologist-reported diagnosis of RA [26]. A summary of the characteristics of the RA cohort (n = 17,125, 68% female) is presented in Table 1.
Outcome Measures
The primary outcome was all-cause mortality. Dates and leading causes of deaths were obtained from the WA Death Registry. Crude mortality rates per 1000 person-years by sex and age were calculated for the whole study period and for each period of study entry. The period of study entry was divided into three decades [1980–1990, 1991–2000, and 2001–2010], and the most recent 5 years [2011–2015] in the study. The death rates for the WA general population were extracted from the Australian Bureau of Statistics [27] and SMRR with a 95% confidence interval (CI) were calculated per decade. We used the 2006 WA national census population as a reference, stratified by age and gender. We also examined survival rates for patients with RA from entry into the study and categorized causes of death among patients with RA based on ICD chapter headings of leading causes of deaths.
Statistical Methods
Continuous parametric and nonparametric variables were reported differently as the mean value ± SD and median values with IQR range, respectively. Continuous variables were analyzed using Student's t test (normally distributed data) and the Mann–Whitney U test (non-normally distributed data). Categorical variables were compared using the Pearson χ2 test. Comorbidity was ascertained using the CCI coding algorithms for defining comorbidities in ICD-9 and ICD-10 administrative data based on last observation in each period of study (Supplementary Material Table S1) [19]. Multi-morbidity was defined as the presence of ≥ 2 CCI score. The ARIA is an index of remoteness derived from road distance measures between populated localities and service centers. For this study, the patient's residential postcode was used to calculate the ARIA + score, and patient records were subsequently assigned at baseline to one of three geographical population settings: urban (major cities), rural (inner and outer regional), and remote areas (remote and very remote areas). Survival analysis was conducted for the whole study cohort by the Kaplan–Meier method with follow-up from the first RA health contact until Dec 31, 2014, or death. We measured median years of life lost by subtracting an individual's death age from their remaining life expectancy based on the Australian Government Actuary Life Table [28]. Differences in survival times were assessed using the log-rank test and Cox proportional hazard model was used to estimate hazard ratios of death with corresponding 95% CI. The proportional hazards assumption was assessed by examining plots of the scaled Schoenfeld residuals against survival time for the predictors. Potential predictors of death assessed were sex, age, geographic location, admitted to intensive care units (ICU), number of comorbidities (CCI), and period of study entry [14,15,16, 29, 30]. Multivariate analysis was performed for variables that were significant on univariate analysis. All statistical analyses were performed in R version 4.0.2, with statistical significance set at two-tailed with the alpha level of 0.05.
Ethics Approval
Ethics approval for this study was granted by the WA Department of Health Human Research Ethics Committee (approval number 2016/24) and the Human Research Ethics Committee of UWA (approval number RA/4/20/4070). The study was conducted in accordance with the last revised Helsinki Declaration [31].
Results
Standardized Mortality Rates for RA Cohort with Odds
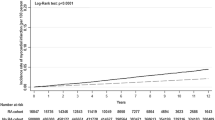
A total of 17,125 patients with RA were included in the RA cohort, and 8955 (52.29%) deaths occurred during 356,069 patient-years of follow-up. Death percentage for female patients with RA was 51.40%, and with a median age at death of 82 years (IQR 75–88), while the death percentage for male patients with RA was 54.20%, and with a median age at death of 78 years. Over the study period, SMRR was 2.50-fold (95% CI 2.52–2.65) higher for patients with RA than for the general population (Table 2), with a sex- and age-specific SMRR of 2.24 (95% CI 2.15–2.34) in males and 3.09 (95% CI 3.00–3.19) in females (Fig. 1). SMRR was 2.50-fold higher than the general population during the first decade of the study, increased between 1991 and 2000 with fivefold higher than the general WA population, and then the SMRR decreased fourfold after in the 2001–2010 period, then dropping to 1.50-fold between 2011 and 2015 (Fig. 2).
Temporal Trends of Sex-Standardized Mortality Rates with Odds
In the earliest decade of study, SMRR for female patients with RA (3.51) was more than double that for males (1.82) in the RA cohort (Supplementary Material Fig. S1–S2). The highest SMRRs stratified by sex were observed in 1991–2000 for both sexes, but with SMRRs were higher in females (5.68) than male patients with RA (4.62) (Supplementary Material Fig. S3–S4). However, SMRR for female patients with RA improved from 4.85-fold in 2001–2010 period to 1.80-fold in the 2011–2015 period (Supplementary Material Fig. S5).
Temporal Trends of Age-Standardized Mortality Rates with Odds
SMRR stratified by age (Fig. 3) was 2.50-fold and 5.00-fold higher in the first and second decades, respectively. In the following decade, SMRR reached fourfold the general population, and by the end of the study period, it was reduced to 1.50-fold (95% CI 1.39–1.81). The SMRRs were increased for patients with RA across all age groups up to the age of 85 (Fig. 3). The highest SMRRs were observed at 27.59 (95% CI 22.42–33.95) in the age group 30–44 years for the 1991–2000 period, then this risk of RA mortality reduced from 27.59-times to 18.60-times for 2001–2010 (Fig. 3). No increased risk of RA mortality was observed by age for 2011–2015.
Survival Analysis
Survival rates for patients with RA following index admission were 91.90% at 5 years, 82.90% at 10 years, 62.50% at 20 years, and 44.8% at 30 years. The median survival of the entire RA cohort was 26.80 years (95% CI 26.30–27.30) and was longer for females 27.7 years (95% CI 27.10–28.30) than males 25.1 years (95% CI 24.50–25.80) with significant difference (p < 0.01, Fig. 4). The estimated rates and median survival time according to patient demographic and clinical characteristics is shown in Supplementary Material Fig. S6–S8.
Years of Life Lost for Patients with RA
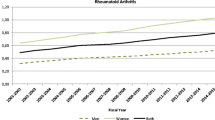
Over the study period, the median life years lost for the RA cohort were 5.7 years for females and 5.1 years for males (Table 3). From 1991 to 2000, life-year loss decreased more in males than in females; the median loss of life decreased from 7.4 to 5.9 years. After 2000, the median lost life decreased further from 5.9 to 5.0 years (15.30%) for males and from 5.4 to 4.6 years (14.80%) for females. During 2011–2015, the median loss of life years in males increased to 5.20 years (4.00%), while in females it declined to 4.3 years (6.50%).
Predicators of RA Mortality
The assumption of proportional hazards was fulfilled for all selected predictors. In Cox multivariable analysis age ≥ 30 years, multimorbidity (CCI ≥ 2), ever ICU admission, living outside urban areas, and study entry before 2000 remained significant predictors of death (Table 4).
Causes of Death
The leading causes of death were diseases of the circulatory system (n = 3646, 40.70%); neoplasms (n = 1586, 17.70%); diseases of the respiratory system (n = 1038, 11.6%); diseases of the musculoskeletal system and connective tissue (n = 599, 6.70%); endocrine, nutritional, and metabolic diseases (n = 414, 4.60%); and diseases of the digestive system (n = 410, 4.60%) (Table 5). Within circulatory diseases, ischemic heart diseases (52.20%) and cerebrovascular diseases (20.10%) were the leading causes of death (Table 5). Based on the age groups, the overall leading causes of death were diseases of the circulatory system, except among 30–44-year-olds in the 1991–2000 period; neoplasm was the leading cause of death (Supplementary Material Table S2–S3).
Discussion
This study revealed that while the gap in mortality for patients with RA compared to the general population is narrowing over time (particularly since 2000), patients with RA remain at an increased risk of death. A higher comorbidity burden was associated with a greater risk of death in patients with RA where cardiovascular diseases remain the leading cause of death.
The SMRR was 2.58 (95% CI 2.52–2.65) higher for patients with RA in the whole study period. The age- and sex-specific SMRR were two times greater in males and three times greater in females during the study period, which is consistent with the literature [4,5,6,7,8,9]. The excess RA mortality risk in 1980–1990 may be related to less efficacious medications and potentially more toxic medications for patients with RA such as sodium aurothiomalate, penicillamine, and cyclophosphamide [32], before methotrexate became the standard of care and subsidized by Medicare Australia in 1992 [33].
In the current study, a decline in SMRRs was observed, especially after 2000. It is tempting but speculative to relate this narrowing of the mortality gap to changes in RA management toward early [34,35,36], aggressive treatment with DMARDs [37,38,39], the introduction of biological therapy in 2003 [40], and a treat-to-target strategy [41, 42].
Although the death rate of patients with RA has decreased over time, it remains higher than the general population, with 40–50% more fatalities [7]. In a Canadian study [7], the SMRRs were reduced from 1.50 (95% CI 1.43–1.57) in 2001 to 1.41 (95% CI 1.35–1.47) in 2009. Also, a recent Dutch study [8] found that the SMRR for all-cause mortality in the RA population was 1.54 (95% CI 1.41–1.67) in the period 1997–2012. The SMRR was reduced from 1.63 (95% CI 1.15–2.24) in 2003–2007 to 1.29 (95% CI 0.83–1.91) in 2008–2012 [8]. This is in accordance with our findings that the overall mortality of RA after age adjustment was 1.5-fold from 2011 to 2015 and despite a short timeframe. In accordance with our findings, these results suggest improved outcomes may be improved due to the combined changes in disease- and patient-related factors features and advances in specialist care and therapeutics.
The median survival time for the entire RA cohort was 26.80 years over the study period (1980–2015), with a 5-year survival rate of 91.90% for patients with RA. A recent study from Taiwan reported that the mean life expectancy for 29,355 new seropositive RA cases diagnosed between 2003 and 2016 was 26.30 years [43]. In Denmark, a recent study found a 5-year survival rate of 80% for people with RA from national population-based registries in the period 1998–2009, while the 12-year probability of survival was 61% [44]. In the current study, the 12-year probability of survival was 78.90% for people with RA, indicating better survival for people with RA in Australia compared with Denmark in the last decades. However, this finding is dependent upon the extent validity of ICD diagnoses in Denmark-linked registries, which have not been tested yet [44]. The median years lost life was 5 years ranging from 3.50 to 9.00 years over the study period, which is consistent with a single-center observational study conducted in Australia [45].
Survival in patients with RA can be influenced by a range of factors, and we found the hazard of death was independently associated with increasing aged ≥ 30 years, living in rurally areas, multi-morbidity (CCI score ≥ 2), and admissions before 2000 or to ICU or admissions before 2000. These results are in agreement with previous study predictors and suggests risk factors for death in RA are similar across regions where age > 50 years, presence of comorbid conditions, ICU admissions, and living in rural areas increased mortality risk in patients with RA [15, 16, 29, 46].
The accumulative multi-comorbidity was associated with a higher risk of mortality in our study. Using CCI, our study found that 37% of patients with RA had multi-comorbidity and they have a twofold increase in all-cause mortality compared with patients with a low CCI low (< 2 CCI score). These results are in agreement with a recent systematic review that found multimorbidity increases the risk of all-cause mortality in various RA populations [47]. In a UK study, patients with RA with multimorbidity have a 2.20-fold increase in all-cause mortality compared to those without RA and heart disease [48]. Additionally, the study found that cardiovascular disease was associated with a more than twofold increase in all-cause mortality [48].
The cumulative burden of multi-morbidity in patients with RA is not only associated with an increased mortality rate but also a poorer quality of life and increased utilization of healthcare services [47, 49, 50]. A recent study has revealed a significant increase in hospitalization duration for patients with RA plus two other long-term conditions as compared to those with only RA [49]. Therefore, it is important to regularly screen for common comorbidities in patients with RA and manage them adequately through collaboration between multiple care providers in order to improve patients’ long-term outcomes [50]. The leading causes of death were not unexpectedly cardiovascular disease, in accordance with the literature [12,13,14, 51]. This relates to the accelerated rate and/or extent of atherosclerosis observed in patients with RA compared with RA tend to have a higher risk of cardiovascular morbidity than with the general population due to the accelerated atherosclerosis observed in RA [15]. Myocardial infarction (MI) was the most frequent fatal cardiovascular event as in previous studies that found the risk of MI was 1.60 compared with the general population [16, 52]. According to a recent meta-analysis, even after controlling for all traditional cardiovascular risk factors, the risk of incident MI was increased in patients with RA compared with the general population, suggesting that other variables such as systemic inflammation are involved [53].
We found that compared to a CCI score of 1, the hazard ratios for death in our patients with RA were 1.75, 2.25, and 2.90 for CCI scores of 2, 3, and 6, respectively. These findings are in agreement with the Swedish study [16] where the hazard ratios for mortality among patients with RA were increased by 50% per point increase of CCI score. Together, these data demonstrate the importance of screening and management for comorbidities in patients with RA, especially cardiovascular diseases [17].
Our study found an increase in mortality among male patients with RA compared with females in the last period (2011–2015). This was despite adequate treatment options, using treatment-to-target methods, improved cardiovascular comorbidity control in low RA activity [54]. This may be explained by gender differences in cardiovascular risk profile in patients with RA with low disease activity. Based on a recent study, there was significantly more atherosclerosis in male patients with RA despite being of similar age and moderate disease activity (DAS28 3.20) compared to female patients with RA [55]. As a result of conventional CV risk factors, most male patients with RA with low disease activity are at high or extremely high risk of developing fatal cardiovascular disease deaths compared to female patients with RA [55].
The highest SMRRs were observed in patients with RA aged 30–44 years between 1991 and 2000, where malignant neoplasms were the leading causes of death, and lung cancer was the leading cause of cancer mortality. These results align with literature findings that indicate an increased risk of lung and lymphoma malignancies in RA compared with the general population [52]. This may be explained by higher smoking prevalence compared with smoking in other age groups or by more lung complications seen with less efficacious earlier RA therapy [56, 57]. While in later periods, earlier RA diagnosis and more aggressive management, use of biological DMARDs and smoking-cessation policies may have contributed to the reduction in mortality among patients with RA [58].
There are limitations to this study related to a case definition of patients with RA with hospital contacts (admission/ED visits) and did not cover patients with RA treated solely in outpatient settings. However, given the use of a reliable definition for RA in state-wide linked administrative health datasets combined with the finding that patients with RA are hospitalized at least once for any condition over 35-year study period [26], we feel the sample size and power of the study were sufficiently representative of the number of people with RA living in WA and the broader RA population for the whole of Australia [26].
In the literature, the mean number of hospital admissions for patients with RA for disease complications or comorbidity varies from 0.13 to 0.34 per year [59], which lies within the range of our previous published study’s annual RA hospital separations (0.15%) [60]. The prevalence of RA [60] also matched the estimates prevalence derived from Australian general practice data [61]. This suggests that the long-term observation in this study covered the overwhelming majority of RA cases state-wide.
The cohort included patients with juvenile idiopathic arthritis, which can be different from adult RA with regards to symptoms, remission period, and disability [62]. However, we used this age group as an age reference for RA cases [63]. Also, the shorter last observation period (2011–2015) may have skewed our outcome data at the end of follow-up, even though we used SMRR to compensate. The incomplete data for the last period ended in 2015 instead of 2020, which is due to the lengthy and expensive process of accessing linked health data from WA Health (18–24 months). Our access was completed in 2018, but the linked datasets were only available to 2015. In the future, WARDER will be updated to include more recent data, with the latest available data up to 2019. Finally, the nature of this dataset precluded the inclusion of detailed descriptors such as clinical or biological markers of disease activity.
Conclusions
The mortality gap for patients with RA in WA has diminished since 2000, which is likely due to improvements in the management of RA and comorbid conditions. Nonetheless, the mortality rate in patients with RA in WA still remains 1.59-times higher than their community counterparts, indicating that improved management comorbidity can further reduce mortality in RA.
References
Björnådal L, Baecklund E, Yin L, Granath F, Klareskog L, Ekbom A. Decreasing mortality in patients with rheumatoid arthritis: results from a large population based cohort in Sweden, 1964–95. J Rheumatol. 2002;29(5):906–12.
Kremers HM, Nicola P, Crowson CS, O’Fallon WM, Gabriel SE. Therapeutic strategies in rheumatoid arthritis over a 40-year period. J Rheumatol. 2004;31(12):2366–73.
Carmona L, Descalzo MA, Perez-Pampin E, et al. All-cause and cause-specific mortality in rheumatoid arthritis are not greater than expected when treated with tumour necrosis factor antagonists. Ann Rheum Dis. 2007;66(7):880–5.
Dadoun S, Zeboulon-Ktorza N, Combescure C, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Jt Bone Spine. 2013;80(1):29–33.
Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379–85.
Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26(5 Suppl 51):S35-61.
Widdifield J, Bernatsky S, Paterson JM, et al. Trends in excess mortality among patients with rheumatoid arthritis in Ontario. Canada Arthritis Care Res (Hoboken). 2015;67(8):1047–53.
van den Hoek J, Boshuizen HC, Roorda LD, et al. Mortality in patients with rheumatoid arthritis: a 15-year prospective cohort study. Rheumatol Int. 2017;37(4):487–93.
Lassere M, Rietbergen M, Rappo J, et al. Mortality its predictors in patients with rheumatoid arthritis. Intern Med J. 2004;34(11):A113.
Ward MM. Recent improvements in survival in patients with rheumatoid arthritis: better outcomes or different study designs? Arthritis Rheum. 2001;44(6):1467–9.
Gonzalez A, Maradit Kremers H, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56(11):3583–7.
Radner H, Lesperance T, Accortt NA, Solomon DH. Incidence and prevalence of cardiovascular risk factors among patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis. Arthritis Care Res (Hoboken). 2017;69(10):1510–8.
Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008;121(10 Suppl 1):S9–14.
Radovits B, Fransen J, Al Shamma S, Eijsbouts A, Van Riel P, Laan R. Excess mortality emerges after 10 years in an inception cohort of early rheumatoid arthritis. Arthritis Care Res (Hoboken). 2010;62(3):362–70.
Dadoniene J, Stropuviene S, Stukas R, Venalis A, Sokka-Isler T. Predictors of mortality in patients with rheumatoid arthritis in Lithuania: data from a cohort study over 10 years. Medicina (Kaunas). 2015;51(1):25–31.
Pedersen JK, Holst R, Primdahl J, Svendsen AJ, Horslev-Petersen K. Mortality and its predictors in patients with rheumatoid arthritis: a Danish population-based inception cohort study. Scand J Rheumatol. 2018;47:1–7.
Peters M, Symmons D, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–31.
Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73(1):62–8.
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82.
Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021.
Ackerman IN, Bohensky MA, Pratt C, Gorelik A, Liew D. Counting the cost. Part 1 Healthcare costs: the current and future burden of arthritis. 2016.
Nossent JC, Raymond W, Keen H, Preen DB, Inderjeeth CA. Infection rates before and after diagnosis of IgA vasculitis in childhood: a population-wide study using non-exposed matched controls. J Rheumatol. 2020;47(3):424–30.
Ognjenovic M, Raymond W, Inderjeeth C, Keen H, Preen D, Nossent J. The risk and consequences of vertebral fracture in patients with ankylosing spondylitis: a population-based data linkage study. J Rheumatol. 2020.
Kelman CW, Bass AJ, Holman CD. Research use of linked health data—a best practice protocol. Aust N Z J Public Health. 2002;26(3):251–5.
Lacaille D, Anis AH, Guh DP, Esdaile JM. Gaps in care for rheumatoid arthritis: a population study. Arthritis Rheum. 2005;53(2):241–8.
Almutairi K, Inderjeeth C, Preen DB, Keen H, Rogers K, Nossent J. The accuracy of administrative health data for identifying patients with rheumatoid arthritis: a retrospective validation study using medical records in Western Australia. Rheumatol Int. 2021;41(4):741–50.
Australian Bureau of statistics. Population by age and sex. http://stat.data.abs.gov.au/Index.aspx?DataSetCode=ABS_ERP_ASGS2016. Accessed 15 Mar 2022.
The Australian Government Actuary. Australian Life Tables 2015–17. https://aga.gov.au/publications/life-tables/australian-life-tables-2015-17. Accessed 15 Mar 2022.
Fujiwara T, Tokuda K, Momii K, et al. Prognostic factors for the short-term mortality of patients with rheumatoid arthritis admitted to intensive care units. BMC Rheumatol. 2020;4(1):64.
Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The prevalence of rheumatoid arthritis: a systematic review of population-based studies. J Rheumatol. 2020;48: jrheum.200367.
World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310(20):2191–4.
Papadopoulos NG, Alamanos Y, Papadopoulos IA, Tsifetaki N, Voulgari PV, Drosos AA. Disease-modifying antirheumatic drugs in early rheumatoid arthritis: a long-term observational study. J Rheumatol. 2002;29(2):261–6.
Chan V, Tett SE. Changes in use of disease-modifying anti-rheumatic drugs in Australia over the period 1992–2004. Pharmacoepidemiol Drug Saf. 2006;15(7):462–8.
Public Health Division CDoH, Services F. The National Public Health partnership-commonwealth and state and territory co-operation. Health Promot J Aust. 1996;6(3).
National Public Health Partnership. Preventing chronic disease: a strategic framework. https://commed.vcu.edu/Chronic_Disease/2015/NPHPProject.pdf. Accessed 15 Mar 2022.
Van der Horst-Bruinsma I, Speyer I, Visser H, Breedveld F, Hazes J. Diagnosis and course of early-onset arthritis: results of a special early arthritis clinic compared to routine patient care. Br J Rheumatol. 1998;37(10):1084–8.
Irvine S, Munro R, Porter D. Early referral, diagnosis, and treatment of rheumatoid arthritis: evidence for changing medical practice. Ann Rheum Dis. 1999;58(8):510–3.
Boers M, Verhoeven AC, Markusse HM, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350(9074):309–18.
Keyszer G, Keysser C, Keysser M. Efficacy and safety of a combination therapy of methotrexate, chloroquine and cyclophosphamide in patients with refractory rheumatoid arthritis: results of an observational study with matched-pair analysis. Clin Rheumatol. 1999;18(2):145–51.
Almutairi K, Nossent J, Preen DB, Keen H, Inderjeeth C. The temporal association between hospital admissions, biological therapy usage and direct health care costs in rheumatoid arthritis patients. Rheumatol Int. 2021.
Wechalekar MD, Quinn S, Lester S, et al. A treat-to-target strategy preserves work capacity in a rheumatoid arthritis inception cohort treated with combination conventional DMARD therapy. J Clin Rheumatol. 2017;23(3):131–7.
Hock ES, James M-S, Wailoo A, et al. Treat-to-target strategies in rheumatoid arthritis: a systematic review and cost-effectiveness analysis. SN Compr Clin Med. 2021;3(3):838–54.
Chiu YM, Lu YP, Lan JL, Chen DY, Wang JD. Lifetime risks, life expectancy, and health care expenditures for rheumatoid arthritis: a nationwide cohort followed up from 2003 to 2016. Arthritis Rheumatol. 2021;73(5):750–8.
Løppenthin K, Esbensen BA, Østergaard M, Ibsen R, Kjellberg J, Jennum P. Morbidity and mortality in patients with rheumatoid arthritis compared with an age- and sex-matched control population: a nationwide register study. J Comorb 2019;9:2235042X19853484–2235042X.
Lassere MN, Rappo J, Portek IJ, Sturgess A, Edmonds JP. How many life years are lost in patients with rheumatoid arthritis? Secular cause-specific and all-cause mortality in rheumatoid arthritis, and their predictors in a long-term Australian cohort study. Intern Med J. 2013;43(1):66–72.
Pianarosa E, Chomistek K, Hsiao R, et al. Global rural and remote patients with rheumatoid arthritis: a systematic review. Arthritis Care Res. (Hoboken). 2020.
Canning J, Siebert S, Jani BD, et al. Examining the relationship between rheumatoid arthritis, multimorbidity, and adverse health-related outcomes: a systematic review. Arthritis Care Res (Hoboken). 2022;74(9):1500–12.
McQueenie R, Nicholl BI, Jani BD, et al. Patterns of multimorbidity and their effects on adverse outcomes in rheumatoid arthritis: a study of 5658 UK Biobank participants. BMJ Open. 2020;10(11): e038829.
Morton FR, Jani BD, Mair FS, et al. Association between risk, duration and cause of hospitalisations in people with rheumatoid arthritis and multimorbidity in the UK Biobank and Scottish Early Rheumatoid Arthritis (SERA) cohorts: Longitudinal observational study. Semin Arthritis Rheum. 2023;58: 152130.
Crowson CS, Gunderson TM, Dykhoff HJ, et al. Comprehensive assessment of multimorbidity burden in a population-based cohort of patients with rheumatoid arthritis. RMD Open. 2022;8(1): e002022.
Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford). 2009;48(10):1309–13.
Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17(1):212.
Schieir O, Tosevski C, Glazier RH, Hogg-Johnson S, Badley EM. Incident myocardial infarction associated with major types of arthritis in the general population: a systematic review and meta-analysis. Ann Rheum Dis. 2017;76(8):1396–404.
Bournia VK, Fragoulis GE, Mitrou P, et al. All-cause mortality in systemic rheumatic diseases under treatment compared with the general population, 2015–2019. RMD Open. 2021;7(3): e001694.
Targońska-Stępniak B, Biskup M, Biskup W, Majdan M. Gender differences in cardiovascular risk profile in rheumatoid arthritis patients with low disease activity. Biomed Res Int. 2019;2019:3265847.
Abásolo L, Júdez E, Descalzo MA, González-Alvaro I, Jover JA, Carmona L. Cancer in rheumatoid arthritis: occurrence, mortality, and associated factors in a South European population. Semin Arthritis Rheum. 2008;37(6):388–97.
Ji J, Liu X, Sundquist K, Sundquist J. Survival of cancer in patients with rheumatoid arthritis: a follow-up study in Sweden of patients hospitalized with rheumatoid arthritis 1 year before diagnosis of cancer. Rheumatology (Oxford). 2011;50(8):1513–8.
Atzeni F, Rodríguez-Carrio J, Popa CD, Nurmohamed MT, Szűcs G, Szekanecz Z. Cardiovascular effects of approved drugs for rheumatoid arthritis. Nat Rev Rheumatol. 2021;17(5):270–90.
Rat AC, Boissier MC. Rheumatoid arthritis: direct and indirect costs. Jt Bone Spine. 2004;71(6):518–24.
Almutairi K, Inderjeeth C, Preen DB, Keen H, Nossent J. The prevalence of rheumatoid arthritis in Western Australia. BMC Rheumatol. 2022;6(1):93.
Harrison C, Henderson J, Miller G, Britt H. The prevalence of diagnosed chronic conditions and multimorbidity in Australia: a method for estimating population prevalence from general practice patient encounter data. PLoS ONE. 2017;12(3): e0172935.
Mehta J. Juvenile Idiopathic Arthritis (JIA)—Pediatrics—MSD Manual Professional Edition. https://www.msdmanuals.com/en-au/professional/pediatrics/juvenile-idiopathic-arthritis/juvenile-idiopathic-arthritis-jia?query=Juvenile%20Idiopathic%20Arthritis%20(JIA). Accessed 15 Mar 2022.
Australian Institute of Health and Welfare. A snapshot of juvenile arthritis. https://www.aihw.gov.au/getmedia/125411fa-7c84-4753-aec1-0a0d76a63a2d/14900.pdf.aspx?inline=true#:~:text=Etanercept%20and%20adalimumab%20are%20biologic,and%20adalimumab%20since%20November%202010. Accessed 15 Mar 2022.
Acknowledgements
The authors thank the data custodians of Hospital Morbidity Data Collection and staff at the Western Australian Data Linkage Branch for their assistance in provision of data. Special thanks to the University of Western Australia for supporting Dr Khalid Almutairi with an Australian Government Research Training Program PhD Scholarship during his doctoral study.
Funding
Dr Khalid Almutairi was supported by an Australian Government Research Training Program PhD Scholarship at the University of Western Australia and the Australian Rheumatology Association WA Research Fellowship Award. Prof. Johannes Nossent was supported by an unrestricted grant by The Arthritis Foundation of WA to WARDER development. No funding was received for publication of this article.
Author Contributions
All authors (Khalid Almutairi, Charles Inderjeeth, David Preen, Helen Keen, Johannes Nossent) made substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data; and the drafting of the manuscript or revising it critically for important intellectual content. The first draft of the manuscript was written by Khalid Almutairi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript to be published and agreed to take accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures
The authors (Khalid Almutairi, Charles Inderjeeth, David Preen, Helen Keen, Johannes Nossent) have nothing to disclose.
Compliance with Ethics Guidelines
Ethic approval for this study was granted by the WA Department of Health Human Research Ethics Committee (approval number 2016/24) and the Human Research Ethics Committee of the University of Western Australia (approval number RA/4/20/4070). The study was completed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Data Availability
All data generated or analysed are available in this article or the supplementary file.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Almutairi, K.B., Inderjeeth, C.A., Preen, D.B. et al. Mortality Trends Among Patients with Rheumatoid Arthritis in Western Australia. Rheumatol Ther 10, 1021–1037 (2023). https://doi.org/10.1007/s40744-023-00562-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00562-0