Abstract
Rheumatoid arthritis (RA) is a chronic autoimmune disease of unexplained causes. Its pathological features include synovial tissue hyperplasia, inflammatory cell infiltration in joint cavity fluid, cartilage bone destruction, and joint deformation. C–C motif chemokine ligand 3 (CCL3) belongs to inflammatory cell chemokine. It is highly expressed in inflammatory immune cells. Increasingly, studies have shown that CCL3 can promote the migration of inflammatory factors to synovial tissue, the destruction of bone and joint, angiogenesis, and participate in the pathogenesis of RA. These symptoms indicate that the expression of CCL3 is highly correlated with RA disease. Therefore, this paper reviews the possible mechanism of CCL3 in the pathogenesis of RA, which may provide some new insights for the diagnosis and treatment of RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Rheumatoid arthritis (RA) is an inflammatory and systemic autoimmune disease characterized by synovial inflammation, synoviocyte expansion, and damage to cartilage and bone. |
variety of chemokines can cause immune activation of RA. |
In particular, C–C motif chemokine ligand 3 (CCL3) is widely involved in the inflammatory response, cartilage destruction and vascular formation of RA. |
Therefore, therapeutic strategies targeting CCL3 and its receptors are being actively developed to treat RA. |
This review considers the possible mechanism of CCL3 in the pathogenesis of RA, which may provide some new insights for the diagnosis and treatment of RA. |
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease with systemic inflammatory changes of unknown etiology that affect all synovial joints, especially the hands and feet. It is the symmetrical, persistent, and progressive process of chronic inflammation [1]. A large-sample epidemiological study found that the global prevalence of RA was about 0.4–1.3%, with women 2–3 times higher than men, and the incidence was in all ages, with the peak of incidence in 40–50 years [2]. Besides, the global point-prevalence of RA is increasing year by year [3]. During the pathogenesis of RA, fibroblastoid synovial cells are the main cells in the synovial layer, which can affect macrophages, neutrophils, T cells, and other inflammatory cells to aggravate the pathogenesis of RA by secreting inflammatory factors, chemokines and metalloproteinases [4]. Lianhua et al. found that Shikonin alleviates collagen-induced arthritis (CIA) mice by inhibiting M1 macrophage polarization [5]. Kang SE shows that the combined inhibition of TNF-α and CXCL10 by the novel BsAb blocks the effect of tumor necrosis factor-α (TNF-α) and CXCL10 induced immune response and improves arthritis in K/BXN serum transfer mice [6]. Therefore, inhibiting the inflammatory response in the synovial microenvironment is the key to the treatment of RA.
In recent years, more and more studies have found that chemokines have the ability to regulate signaling pathways related to leukocyte migration and inflammatory response. Inappropriate chemokine activity leads to an excessive inflammatory response, which is characteristic of autoimmune and inflammatory disease [7]. In particular, C–C motif chemokine ligand 3 (CCL3) have attracted the attention of most researchers. CCL3, also named macrophage inflammatory protein-1α (MIP-1α), belongs to the CC chemokine subfamily. CCL3 receptors are CCR1, CCR5, and CCR9. Under pathophysiological conditions, the expression of CCL3 in vivo is low. However, in the occurrence and development of RA, mature hematopoietic cells, macrophages, lymphocytes, neutrophils and other immune cells can highly express CCL3 in response to inflammatory stimuli, thus participating in the immune inflammatory response of RA [8]. Rossato et al. show that an early peritoneal CC chemokine response is an important feature of arthritis susceptibility [9]. Sun et al. have shown that B cells affect osteoblast differentiation through a high expression of CCL3 and proinflammatory factors which further inhibit bone formation in RA [10]. Based on protein–protein interactions (PPI) showing a network of 43 CVD-related biomarkers, CCL3 was identified as increased in patients with RA and periodontal disease [11]. Those findings provide vivid evidence for the role of CCL3 in the pathogenesis and immune regulation of RA. Therefore, this paper will review the role and mechanism of CCL3 in the pathogenesis of RA, hoping to offer some new directions for the research and treatment of RA.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Chemokines Family
Chemokine Ligand
Chemokines, also known as chemotactic hormones or chemotactic agents, are a small molecule type of cytokine that induces chemotaxis in cells [12]. The structure and function of chemokines have been further clarified. Chemokines have a highly conserved tertiary structure defined by an irregular “N-loop”, a C-terminal helix, and a three-stranded β-sheet connected by loops. So far, more than 40 human chemokines have been found, belonging to the largest family of cytokines. To facilitate communication, since the late 1990s the chemokine international naming organization has mainly been divided into four chemokine subfamilies according to the relative differences of cysteine positions in the structure [13]. There are three other arbitrary amino acids between two cysteines at the N-terminal of the CX3C chemokine subgroup, one other arbitrary amino acid between two cysteines at the N-terminal of the CXC chemokine subgroup, and two cysteines at the N-terminal of CC chemokine subgroup are adjacent to each other. Most chemokines have four conserved cysteine residues. However, the XC chemokine subfamily has only two cysteine residues [14] (Fig. 1). The XC chemokine subfamily contains only XCL1 and XCL2 chemokines. The main function of chemokines is chemotactic immune cells involved in the body's defense and exudation of inflammatory process [15].
Structure of four chemokine subtypes. The XC chemokine subfamily has only two cysteine residues. There are three other arbitrary amino acids between two cysteines at the N-terminal of the CX3C chemokine subgroup, one other arbitrary amino acid between two cysteines at the N-terminal of the CXC chemokine subgroup, and two cysteines at the N-terminal of CC chemokine subgroup are adjacent to each other. CXCL C-X-C motif chemokine ligand, CC C-C motif chemokine, CX3CL C-X3-C motif chemokine ligand, XC X-C motif chemokine
Chemokine Receptor
There are currently more than 20 chemokine receptors. According to the difference of binding ligands, they divide into the following four types: CXCR, CCR, CX3CR, and XCR. Chemokine receptors belong to the G-protein-coupled receptor superfamily of seven transmembrane receptors. They share many structural features: similar in size with approximately 350 amino acids. There is homology between different chemokine receptors [16]. Studies have shown that chemokine receptor N-terminal amino acid residues deletion cannot bind ligands. This confirms that the extracellular N-terminal sequence is the recognition site for ligands. The chemokine receptor C-terminal contains Ser/Thr site, which is mainly involved in intracellular signal transduction, and C-terminus phosphorylation may be involved in signal transduction after receptor activation. Ligand-receptor binding of chemokines is a classical two-site model [17]. In the first step, the chemokine core domain binds to the N-terminal recognition site of the chemokine recognition site 1 (CRS1). The core region of chemokines refers to structures other than N-terminal sequences. Then, the N-terminal of chemokine binds to the transmembrane domain of chemokine recognition site 2 (CRS2) (Fig. 2).
The binding of chemokine to its receptor is a two-locus model. In the first step, the chemokine core domain binds to the N-terminal recognition site of the chemokine receptor (CRS1). Then, the N-terminal of chemokine binds to the transmembrane domain of chemokine receptor (CRS2). CRS chemokine receptors
Chemokines Family in Rheumatoid Arthritis
In the process of chemokine ligand–receptor binding, we can see the redundant characteristics of the binding. It determines the specificity of chemokine action with its receptor. Therefore, chemokines have different expressions in synovial fluid of patients with arthritis [18]. In the family of chemokines, several chemokines are involved in the pathogenesis of RA (Table1). Macrophages secrete CXCL1, CXCL5, and CXCL8, which trigger synovial inflammation and fibrosis [19,20,21]. A new study indicates that that CXCL7 levels were increased in RA patient platelet [22]. Neutrophils produce CXCL6 and trigger the production of CXCL4, CXCL9, CXCL10, and CXCL16 from fibroblast-like synovial cells and macrophages [23,24,25]. Furthermore, CXCL12 stimulated osteoclast migration and bone resorption of osteoclasts in RA animal models [26]. Elevated levels of CCL2, CCL3, and CCL5 in RA were closely associated with increased T cell infiltration in joints [27]. Studies have shown that CCL13 is correlated with RA, and CCL13 is highly expressed in chondrocytes [28]. Besides, CCL18 promotes the attraction of T cells to joint inflammation through antigen-presenting cells [29, 30]. XCL1 and XCL2 synergistically up-regulated CD4+/CD28− T cells, thereby inducing osteoclast proliferation [31, 32]. CX3CR1 expressed by monocytes interacts with CX3CL1 produced by macrophages, and this interaction promotes their migration and recruitment into RA synovium [33].
The synovial tissue and synovial fluid of RA contain plentiful chemokines, and their receptors have higher expression in T cells, monocytes, neutrophils, and other immune cells. In general, a variety of chemokines derived from immune cells can cause the immune activation of RA, and it is of great significance to clarify their mechanism of action for the prevention and treatment of RA, especially representative inflammatory chemokines such as CCL3 and CCL2.
Relationship Between the Pathological Mechanism of Rheumatoid Arthritis and CCL3
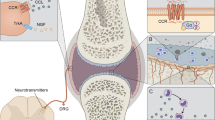
Chintalacharuvu [34] found that CCL3−/− mice exhibited milder inflammation and joint destruction induced in CIA mice. Single-cell transcriptomic profiling showed that CCL3 was elevated in the synovial state of CXCL10+CCL2+ inflammatory macrophages in RA [35]. Hosonuma showed that hepatocyte growth factor from synovial inflammation in RA patients activates monocyte migration to synovial membrane and promotes bone destruction by enhancing CCL3/CX3CL1 production and chemotaxis [36]. Up to now, the mechanism of CCL3 in the pathogenesis of RA has attracted more researchers. Therefore, this paper discusses the pathogenesis of CCL3 in RA (Fig. 3).
Mechanisms of CCL3 in pathologic rheumatoid arthritis. In healthy joint, the synovial membrane is 1–2 layers, and the synovial fluid in the joint cavity protects the articular cartilage, and the cartilage layer is smooth and complete. Synovial hyperplasia (red pannus formation), inflammatory cell infiltration in synovial fluid, and bone surface wear in RA joint. Fibroblast-like synoviocyte (FLS) are the primary cells in the synoviocytes. The cross-talk between FLS and inflammatory immune cells is an important reason to accelerate the progression of RA. Proliferating FLS cells secrete pro-inflammatory cytokines such as CCL3 and TNF-α, which stimulate the recruitment of more T cells and the aggregation of macrophages. These inflammatory immune cells in turn secrete TNF-α and IL-1β to increase the activation of synovial cells. In the microenvironment of joint synovial inflammation, MMPs produced in synovial fluid can directly degrade cartilage tissue. CCL3 and cytokines can also interact with receptor activators of nuclear factor kappa-β ligand (RANKL) to stimulate osteoclast activation, resulting in bone erosion. Due to synovial hyperplasia caused by inflammation, CCL3 and VEGF-A promote angiogenesis by activating the JNK/ERK/P38 signaling pathway. FLS Fibroblast-like synoviocyte, IL-1β secreting interleukin-1β, TNF-α Tumor necrosis factor-α, CCL3 C-C motif chemokine ligand 3, RANKL Receptor activator of nuclear factor-kB Ligand, MMPs Matrix metalloproteinases, Ang-2 Angiopoietin-2, VEGF-A Vascular endothelial growth factor A, JNK JUN N -terminal kinases, ERK extracellular-signal regulated protein kinase, P38 mitogen-activated protein kinases 38, EC Endothelial cells
CCL3 Promote the Migration of Inflammatory Factors to Synovial Tissue
Inflammatory cytokine infiltration in joint synovium is one of the main features of RA disease [37]. Zhang et al. demonstrated inflammatory cell status in synovial tissues of RA by integrating single-cell transcriptome and mass cytography. For example, in leukocyte-rich RA, CCL3 is mainly upregulated in monocytes. However, in RA and osteoarthritis (OA) synovium without leukocytes, the CCL3 expression level was comparable in B and T cells [38]. Using immunofluorescence technology to further analyze the localization of CCL3 in synovial tissues, the results show that CCL3 was highly expressed in the cytoplasm of CD4+ T cells. When synovial macrophages and fibroblasts are stimulated by pro-inflammatory factors, synovial cells are activated to produce CCL3. More importantly, CCL3 recruits more inflammatory cells to migrate and accumulate in the joint, then inflammatory cells generate more inflammatory factors in the synovial microenvironment, further aggravating the local inflammatory response in RA [39]. In rheumatoid arthritis fibroblast-like synovial cells (RA-FLS), Zhang et al. observed that CCL3 causes the onset of RA through PI3K/AKT signaling pathway. Besides, CCL3 could active CD4+ T cells to mediate synovitis [40]. CCL3/CCR5 may overexpress inflammatory mediators such as cyclooxygenase-2 (COX-2), matrix metalloproteinases-3 (MMP-3), and interleukin-6 (IL-6) in synovial cells through mitogen-activated protein kinase pathway, thus contributing to RA synovitis [41]. In RASF, mRNA sequencing data showed that Mir-147b-3p mimics and ZNF148 siRNA usually upregulate CCL3 and other inflammatory pathways mediate synovitis [42]. All of the above studies indicate that CCL3 is involved in the pathological process of synovitis.
CCL3 Promotes Cartilage Destruction
Cartilage destruction involves an interaction between osteoclast (OC) generation and osteoblast (OB) inhibition. Joint bone destruction is an irreversible process and is the main cause of disability in patients. Studies have shown that many cytokines and immune cells contribute to osteoclast formation [43, 44]. Immune cells in the pathogenesis of RA directly up-regulate the expression of receptor activator of nuclear factor-kB Ligand (rankl), but indirectly increase the expression of rankl through secretion of pro-inflammatory cytokines, and promote the formation of OCs, ultimately leading to cartilage fragmentation [45]. CCL3 is a chemokine of macrophages, which can induce osteoclast aggregation and activity [46]. Various rodent studies in vitro have demonstrated a link between CCL3 production and bone degradation [47, 48]. Studies have shown that CCL3 can promote the differentiation of OC precursors in peripheral blood. It is worth noting that curcumin further inhibits the differentiation of osteoclast precursors and the formation of osteoclasts by inhibiting the production of CCL3. The addition of CCL3 could significantly reverse the OC formation decreased by curcumin [49]. Zhang et al. demonstrated that C/EBPβ and NF-κB are involved in the expression of CCL3 in IL-1β-induced human chondrocytes [50]. In TNF-Tg and CIA mouse models and in human RA samples, Wen Sun et al. showed that B cells in synovial tissue significantly reduced osteoblast differentiation through the high expression of CCL3 [10]. In addition, most studies have confirmed that CCL3 is an inhibitor of OBs [51]. These findings suggest that CCL3 is a major contributor to cartilage matrix degradation in RA. In the CIA model, CCL3−/− mice were protected from bone injury, further demonstrating the important role of CCL3 in osteoclasts [34]. However, CCL3 is indirectly involved in the pathological process of bone resorption in osteoclast. MMPs lead to bone degradation by dissolving the lacunar organic matrix. Studies have shown that the increased expression of CCL3 and enhanced activation of MMPs in human CD14 OC promote the differentiation and reabsorption of OC [52]. Most importantly, other studies have shown that CCL3 increases MMP3 activity in FLS cells in a culture medium in a dose-dependent or time-dependent manner. MMPs can promote the migration of FLS cells to adjacent cartilage and induce cartilage degradation [53]. Therefore, CCL3 may play an important role in RA cartilage degradation through autocrine or paracrine action.
CCL3 Promotes Pannus Formation
Pannus is composed of newly neovascularization, proliferating synovial cells, inflammatory cells, and cellulose, with tumor-like features [54]. The proliferation of synovial vessels may be one of the reasons why RA is difficult to cure, a feature similar to vascular proliferation in malignant tumors. Angiogenesis is a vital factor in the formation and maintenance of RA pannus. On the one hand, the formation of neovascularization will increase the surface area of vascular endothelium, which makes it easier for inflammatory cells to seep into the synovium, thus aggravating inflammation. On the other hand, some mediators that regulate angiogenesis are also involved in the pathogenic mechanism of RA [55]. Arthritis often causes local hypoxia due to active metabolism in the joints. In the local hypoxic microenvironment of the joint during RA, synovial fibroblasts secrete CXCL12. It combines with its receptor CXCR4 to participate in vascular remodeling and promote the migration of various endothelial cells, thereby promoting capillary formation. The new vessels recruit more immune inflammatory cells into synovial tissue with the help of inflammatory chemokines such as CCL3 [56]. In addition, Liao et al.’s studies have shown that CCL3 inhibits mir-374b expression by activating the JNK/ERK/P38 signaling pathway, thereby promoting VEGF-A expression and angiogenesis in human osteosarcoma cells [57]. After being stimulated by some proinflammatory cytokines, synovial fibroblasts in RA joint tissue secrete CCL3 and CCL28, which are also involved in vascular proliferation in RA. Their promotion of vascular proliferation is mainly indirectly by macrophages and fibroblasts in synovial tissue to secrete pro-angiogenic factors [58,59,60]. In a mouse CIA model, Krausz et al. found that the synergistic effect of angiopoietin-2 (Ang-2) and TNF-α on Tie2 signaling in macrophages enhances the expression of CCL3 and IL-6 while inhibiting the production of thrombospondin-2 (TSP-2), widely coordinating angiogenesis in RA [61]. Therefore, blocking angiogenesis will become a new treatment for RA.
CCL3 Promotes Rheumatoid Arthritis Secondary to Other Diseases
RA is an immunological, systemic disease with clinical manifestations involving joints and other systemic systems. For example, interstitial lung diseases are the most common, which can eventually progress to pulmonary fibrosis [62]. Through radiation lung injury irradiation on a CCL3−/− mouse model, CCR1 −/− mouse model, and wild mice, it was found that the degree of pulmonary fibrosis in normal mice was significantly higher than that in CCL3−/− mice and CCR1−/− mice. CCL3 can initiate NF-kB signaling to participate in the pathogenesis of pulmonary interstitial lesions. Therefore, selective blocking of the CCL3/CCR1 signaling axis can effectively relieve pulmonary fibrosis secondary to RA [63]. TGF-β1 is a typical cytokine-stimulating epithelial–mesenchymal transformation. It may stimulate CCL3 expression in lung NK cells and is associated with increased TGF-β1 in RA patients. Inhibition of TGF-β signaling can down-regulate pulmonary CCL3 to regulate interstitial pneumonia [64]. These studies show that CCL3 may be involved in the progression of lung diseases and is most closely related to the formation of pulmonary fibrosis.
CCL3 in Other Autoimmune Diseases
CCL3 and its receptors are a major part of the immune system. Studies have found that CCL3 can induce the migration and infiltration of chemotactic immune cells to target organs, and eventually participate in the immune process of target organs [65]. In recent years, extensive studies have observed that CCL3 is up-regulated in a variety of autoimmune diseases, which can affect the susceptibility and development of diseases [66]. It has been shown that CCL3 mediate a variety of auto-inflammatory immune diseases (Table 2).
CCL3 levels were associated with systemic lupus erythematosus (SLE) activity. Elevated levels of CCL3 also have been detected in the serum of patients with SLE. CCL3, through recruiting polymorphonuclear cells, participates in the SLE responses [67]. The expression of CCL3 and IL-7 in skin of systemic sclerosis (SSc) patients are significantly increased. CCL3 is involved in the recruitment of adhesion molecules of monocytes and helper T lymphocytes, which indirectly leads to SSc fibrosis [68]. Hashimoto's thyroiditis (HT), as one of the autoimmune diseases, has an increasing incidence year by year. The expression of CCL3 was up-regulated in the thyroid tissues of HT patients. MRP14 participates in lymphocyte infiltration in HT by regulating the expression of CCL3 [69]. In an animal model of ulcerative colitis (UC), increasing serum concentration of CCL3 intensify immune-mediated colitis. CCL3 was highly expressed in lamina propria inflammatory cells and intestinal epithelial cells. Elevated concentrations of CCL3 in peripheral blood may enhance the intestinal Th1 response, thereby aggravating ulcerative colitis [70]. Chemokines were used in the field of autoimmune hepatitis (AIH) research from 1972 to 2014. CCL3 promotes fibrosis in mouse models by facilitating immune cell migration and activation of hepatic stellate cells [71]. In addition, RNA sequencing of serum samples of myasthenia gravis (MG) showed that CCL3 was involved in Th0 cell to Th1 cell differentiation. Therefore, the abnormal increase of CCL3 is related to the inflammatory response of MG patients. Other studies have suggested that CCL3 involvement in immune cell migration and apoptosis may be a molecular feature of MG disease [72].
Prospects of CCL3 in the Treatment of Rheumatoid Arthritis
As the etiology and pathogenesis of RA are not completely clear, there is still no cure for RA. Once RA is diagnosed, it should be treated with medication as soon as possible. Several drugs commonly used to treat RA are mainly botanicals, non-steroidal anti-inflammatory drugs, disease-modifying anti-rheumatic drugs (DMARD), biological agents, and chemokine inhibitors under development (Table 3). Common botanical drugs include Tripterygium wilfordii, total glucosides of paeony, and sinomenine [73,74,75]. Nonsteroidal anti-inflammatory drugs such as diclofenac, naphthalbumethone, meloxicam, and ketoprofen are commonly used to relieve RA pain and inflammation [76]. However, of these botanicals, most lack evidence of clinical safety and efficacy. DMARDs take between 1 and 6 months of dosing to achieve significant improvement in clinical symptoms and a degree of delay in the progression of RA lesions. Traditional DMARDs include methotrexate, leflunomide, cyclosporine, and hydroxychloroquine [77]. TNF-α inhibitors, IL-6R antibodies, JAK inhibitors, T-cell co-stimulation modulator, and selective B-cell depleting agents to targets have emerged. Tocilizumab, etanercept, tofacitinib, abatacept, and rituximab are considered to be the most effective biological agents for RA [78,79,80,81,82]. The targeted biological agents above play a role by blocking a specific cell surface molecule or inflammatory cytokines. The use of these drugs is a breakthrough in the treatment of RA. However, biological agents are expensive, or their toxicity increases, causing local rashes and signs of infection.
In recent years, more and more studies have been conducted on inhibiting the expression of CCL3 and its receptor in RA to alleviate the progression of the disease, providing new ideas for the treatment of RA. CCL3 infiltrates synovial tissue by recruiting inflammatory cells such as monocytes and T cells. In addition, serum CCL2 and CCL3 expression levels were lower in RA patients who responded to IL-1 receptor antagonist therapy [83]. Similarly, CXCL10, CCL2, and CCL3 levels were significantly reduced when treated with TNF-α antagonists [84]. The expression of CCL3 is affected by the use of synthetic and biologic antirheumatic drugs. Therefore, targeting CCL3 or its receptor (CCR1/5/9) may improve its therapeutic potential in RA and other autoimmune diseases. These strategies were tested in a large sample of RA models and limited human RA clinical trials, in which blocking chemokines has shown promising results. In the CIA model, targeting CCL3 with monoclonal antibodies strongly inhibited joint injury and bone erosion in RA. However, antibodies against CCL3 have not been tested in human RA trials [85]. Manczak et al.’s studies have shown that neutralizing CCL3 antibodies can alleviate chronic joint inflammation [86]. CCR1 is an effective therapeutic target because it mediates TNF‐α and IL‐10 expression in inflammatory diseases. For example, the CCR1 antagonist BX471 can be used to treat a variety of inflammatory diseases, including RA [87, 88]. Previous studies have shown that CCR5 antagonists, Aplaviroc and Vicriviroc, have been used to treat HIV infection. Vercirnon, a CCR9 antagonist, can be used to treat Crohn’s disease [89, 90]. Due to the lack of specificity of chemokine receptors, that is, one receptor can bind to multiple ligands, and one ligand can have multiple receptors. So different ligands can act as agonists or antagonists when they bind to receptors. Chemokines and their receptors play a complex role, which is related to the migration and activation of leukocytes in different periods. These obstacles may slow the development of effective compounds in human clinical trials progress [91]. Although most chemokine blockers have shown some failures in RA human trials, targeted chemokines will continue to be studied [92, 93].
Conclusions
In summary, CCL3 promotes the migration of inflammatory factors to synovial tissue, migration of osteoclasts, destruction of bone joints, and production of vascular opacities. These results suggest that the source and biological characteristics of CCL3 and its receptor affect the pathological process of RA. The application of anti-CCL3 antibody can delay the onset of RA and reduce inflammation, which opens up a new way for the prevention and treatment of RA. It appears that targeting CCL3 or its receptor has not been successful in treating the clinical disease of RA. This may be due to the interaction between immune and non-immune cells in RA synovial tissue. Limited studies on the interaction of CCL3 with RA suggest that it is inappropriate to use the chemokine CCL3 as a diagnostic or therapeutic target. However, using CCL3 inhibitors in conjunction with other conventional anti-rheumatoid therapies may be beneficial, and future studies should focus more on combination therapy. However, more research is needed in this area.
References
Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170(1):Itc1–16.
Almutairi KB, Nossent JC, Preen DB, Keen HI, Inderjeeth CA. The prevalence of rheumatoid arthritis: a systematic review of population-based studies. J Rheumatol. 2021;48(5):669–76.
Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863–77.
Qin Y, Cai ML, Jin HZ, Huang W, Zhu C, Bozec A, Chen Z, et al. Age-associated B cells contribute to the pathogenesis of rheumatoid arthritis by inducing activation of fibroblast-like synoviocytes via TNF-α-mediated ERK1/2 and JAK-STAT1 pathways. Ann Rheum Dis. 2022;81(11):1504–14.
Lianhua HE, Huijie L, Qingxia Q, Juan HE, Jian C, Yiping HU, Qingwen W, et al. Shikonin alleviates collagen-induced arthritis mice by inhibiting M1 macrophage polarization. J Tradit Chin Med. 2022;42(6):932–9.
Kang SE, Park JK, Yoo HJ, Kang HS, Park YW, Park BC, Song YW, et al. Efficacy of novel bispecific antibody targeting TNF-α/CXCL10 in the treatment of experimental arthritis. Transl Res. 2021;232:75–87.
Mughees M, Kaushal JB, Sharma G, Wajid S, Batra SK, Siddiqui JA. Chemokines and cytokines: axis and allies in prostate cancer pathogenesis. Semin Cancer Biol. 2022;86(Pt 3):497–512.
Baba T, Tanabe Y, Yoshikawa S, Yamanishi Y, Morishita S, Komatsu N, Mukaida N, et al. MIP-1α/CCL3-expressing basophil-lineage cells drive the leukemic hematopoiesis of chronic myeloid leukemia in mice. Blood. 2016;127(21):2607–17.
Rossato C, Albuquerque LL, Katz ISS, Borrego A, Cabrera WHK, Spadafora-Ferreira M, Jensen JR, et al. Early peritoneal CC chemokine production correlates with divergent inflammatory phenotypes and susceptibility to experimental arthritis in mice. J Immunol Res. 2019;2019:2641098.
Sun W, Meednu N, Rosenberg A, Rangel-Moreno J, Wang V, Glanzman J, Xing L, et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat Commun. 2018;9(1):5127.
Panezai J, Ghaffar A, Altamash M, Åberg M, Van Dyke TE, Larsson A, Engström PE. Periodontal disease augments cardiovascular disease risk biomarkers in rheumatoid arthritis. Biomedicines. 2022;10(3):714.
Cui LY, Chu SF, Chen NH. The role of chemokines and chemokine receptors in multiple sclerosis. Int Immunopharmacol. 2020;83: 106314.
Bachelerie F, Graham GJ, Locati M, Mantovani A, Murphy PM, Nibbs R, Thelen M, et al. An atypical addition to the chemokine receptor nomenclature: IUPHAR Review 15. Br J Pharmacol. 2015;172(16):3945–9.
Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–16.
Legler DF, Thelen M. Chemokines: Chemistry, biochemistry and biological function. Chimia. 2016;70(12):856–9.
Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. Febs j. 2018;285(16):2944–71.
Sonawani A, Kharche S, Dasgupta D, Sengupta D. Insights into the dynamic interactions at chemokine-receptor interfaces and mechanistic models of chemokine binding. J Struct Biol. 2022;214(3): 107877.
Miyabe Y, Miyabe C, Iwai Y, Luster AD. Targeting the chemokine system in rheumatoid arthritis and vasculitis. JMA journal. 2020;3(3):182–92.
Hou SM, Chen PC, Lin CM, Fang ML, Chi MC, Liu JF. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res Ther. 2020;22(1):251.
Tejera-Segura B, López-Mejías R, de Vera-González A, Delgado-González A, González-Gay MA, Ferraz-Amaro I. Implication of CXCL5 (epithelial neutrophil-activating peptide 78) in the development of insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(3):373–9.
Salim PH, Xavier RM. Influence of genetic polymorphisms (IL-10/CXCL8/CXCR2/NFκB) on the susceptibility of autoimmune rheumatic diseases. Rev Bras Reumatol. 2014;54(4):301–10.
Guerrero S, Sánchez-Tirado E, Agüí L, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM. Simultaneous determination of CXCL7 chemokine and MMP3 metalloproteinase as biomarkers for rheumatoid arthritis. Talanta. 2021;234: 122705.
Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S. How do chemokines navigate neutrophils to the target site: Dissecting the structural mechanisms and signaling pathways. Cell Signal. 2019;54:69–80.
Yu X, Song Z, Rao L, Tu Q, Zhou J, Yin Y, Chen D. Synergistic induction of CCL5, CXCL9 and CXCL10 by IFN-γ and NLRs ligands on human fibroblast-like synoviocytes-A potential immunopathological mechanism for joint inflammation in rheumatoid arthritis. Int Immunopharmacol. 2020;82: 106356.
Isozaki T, Arbab AS, Haas CS, Amin MA, Arendt MD, Koch AE, Ruth JH. Evidence that CXCL16 is a potent mediator of angiogenesis and is involved in endothelial progenitor cell chemotaxis : studies in mice with K/BxN serum-induced arthritis. Arthritis Rheum. 2013;65(7):1736–46.
Peng L, Zhu N, Mao J, Huang L, Yang Y, Zhou Z, Wu B, et al. Expression levels of CXCR4 and CXCL12 in patients with rheumatoid arthritis and its correlation with disease activity. Exp Ther Med. 2020;20(3):1925–34.
Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2(2):102–7.
Yamaguchi A, Nozawa K, Fujishiro M, Kawasaki M, Suzuki F, Takamori K, Sekigawa I, et al. CC motif chemokine ligand 13 is associated with rheumatoid arthritis pathogenesis. Mod Rheumatol. 2013;23(5):856–63.
Momohara S, Okamoto H, Iwamoto T, Mizumura T, Ikari K, Kawaguchi Y, Tomatsu T, et al. High CCL18/PARC expression in articular cartilage and synovial tissue of patients with rheumatoid arthritis. J Rheumatol. 2007;34(2):266–71.
Islam SA, Ling MF, Leung J, Shreffler WG, Luster AD. Identification of human CCR8 as a CCL18 receptor. J Exp Med. 2013;210(10):1889–98.
Wang CR, Liu MF, Huang YH, Chen HC. Up-regulation of XCR1 expression in rheumatoid joints. Rheumatology (Oxford). 2004;43(5):569–73.
Fessler J, Husic R, Schwetz V, Lerchbaum E, Aberer F, Fasching P, Dejaco C, et al. Senescent T-Cells promote bone loss in rheumatoid arthritis. Front Immunol. 2018;9:95.
Nanki T, Imai T, Kawai S. Fractalkine/CX3CL1 in rheumatoid arthritis. Mod Rheumatol. 2017;27(3):392–7.
Chintalacharuvu SR, Wang JX, Giaconia JM, Venkataraman C. An essential role for CCL3 in the development of collagen antibody-induced arthritis. Immunol Lett. 2005;100(2):202–4.
Zhang F, Mears JR, Shakib L, Beynor JI, Shanaj S, Korsunsky I, Raychaudhuri S, et al. IFN-γ and TNF-α drive a CXCL10+ CCL2+ macrophage phenotype expanded in severe COVID-19 lungs and inflammatory diseases with tissue inflammation. Genome Med. 2021;13(1):64.
Hosonuma M, Sakai N, Furuya H, Kurotaki Y, Sato Y, Handa K, Isozaki T, et al. Inhibition of hepatocyte growth factor/c-Met signalling abrogates joint destruction by suppressing monocyte migration in rheumatoid arthritis. Rheumatology (Oxford). 2021;60(1):408–19.
Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol. 2021;22(1):10–8.
Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, Raychaudhuri S, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. 2019;20(7):928–42.
Croia C, Bursi R, Sutera D, Petrelli F, Alunno A, Puxeddu I. One year in review 2019: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(3):347–57.
Zhang G, Liu HB, Zhou L, Cui XQ, Fan XH. CCL3 participates in the development of rheumatoid arthritis by activating AKT. Eur Rev Med Pharmacol Sci. 2018;22(20):6625–32.
Alaaeddine N, Hilal G, Baddoura R, Antoniou J, Di Battista JA. CCL20 stimulates proinflammatory mediator synthesis in human fibroblast-like synoviocytes through a MAP kinase-dependent process with transcriptional and posttranscriptional control. J Rheumatol. 2011;38(9):1858–65.
Jiang C, Wu X, Li X, Li M, Zhang W, Tao P, Lu S, et al. Loss of microRNA-147 function alleviates synovial inflammation through ZNF148 in rheumatoid and experimental arthritis. Eur J Immunol. 2021;51(8):2062–73.
Ostrowska M, Maśliński W, Prochorec-Sobieszek M, Nieciecki M, Sudoł-Szopińska I. Cartilage and bone damage in rheumatoid arthritis. Reumatologia. 2018;56(2):111–20.
Takeshita A, Nishida K, Yoshida A, Nasu Y, Nakahara R, Kaneda D, Ozaki T, et al. RANKL expression in chondrocytes and its promotion by lymphotoxin-α in the course of cartilage destruction during rheumatoid arthritis. PLoS One. 2021;16(7): e0254268.
Yu X, Huang Y, Collin-Osdoby P, Osdoby P. CCR1 chemokines promote the chemotactic recruitment, RANKL development, and motility of osteoclasts and are induced by inflammatory cytokines in osteoblasts. J Bone Miner Res. 2004;19(12):2065–77.
Sucur A, Jajic Z, Artukovic M, Matijasevic MI, Anic B, Flegar D, Grcevic D, et al. Chemokine signals are crucial for enhanced homing and differentiation of circulating osteoclast progenitor cells. Arthritis Res Ther. 2017;19(1):142.
Watanabe T, Kukita T, Kukita A, Wada N, Toh K, Nagata K, Iijima T, et al. Direct stimulation of osteoclastogenesis by MIP-1alpha: evidence obtained from studies using RAW264 cell clone highly responsive to RANKL. J Endocrinol. 2004;180(1):193–201.
Tsubaki M, Kato C, Manno M, Ogaki M, Satou T, Itoh T, Nishida S, et al. Macrophage inflammatory protein-1alpha (MIP-1alpha) enhances a receptor activator of nuclear factor kappaB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol Cell Biochem. 2007;304(1–2):53–60.
Liang Z, Xue Y, Wang T, Xie Q, Lin J, Wang Y. Curcumin inhibits the migration of osteoclast precursors and osteoclastogenesis by repressing CCL3 production. BMC Complement Med Ther. 2020;20(1):234.
Zhang Z, Bryan JL, DeLassus E, Chang LW, Liao W, Sandell LJ. CCAAT/enhancer-binding protein β and NF-κB mediate high level expression of chemokine genes CCL3 and CCL4 by human chondrocytes in response to IL-1β. J Biol Chem. 2010;285(43):33092–103.
Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti MT, Veiby P, Raje N, et al. A novel role for CCL3 (MIP-1α) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011;25(7):1174–81.
Collins FL, Williams JO, Bloom AC, Singh RK, Jordan L, Stone MD, Williams AS, et al. CCL3 and MMP-9 are induced by TL1A during death receptor 3 (TNFRSF25)-dependent osteoclast function and systemic bone loss. Bone. 2017;97:94–104.
Westacott CI, Sharif M. Cytokines in osteoarthritis: mediators or markers of joint destruction? Semin Arthritis Rheum. 1996;25(4):254–72.
Lin J, Sun AR, Li J, Yuan T, Cheng W, Ke L, Zhang P, et al. A three-dimensional co-culture model for rheumatoid arthritis pannus tissue. Front Bioeng Biotechnol. 2021;9: 764212.
Tas SW, Maracle CX, Balogh E, Szekanecz Z. Targeting of proangiogenic signalling pathways in chronic inflammation. Nat Rev Rheumatol. 2016;12(2):111–22.
Kanbe K, Chiba J, Inoue Y, Taguchi M, Yabuki A. SDF-1 and CXCR4 in synovium are associated with disease activity and bone and joint destruction in patients with rheumatoid arthritis treated with golimumab. Mod Rheumatol. 2016;26(1):46–50.
Liao YY, Tsai HC, Chou PY, Wang SW, Chen HT, Lin YM, Fong YC, et al. CCL3 promotes angiogenesis by dysregulation of miR-374b/ VEGF-A axis in human osteosarcoma cells. Oncotarget. 2016;7(4):4310–25.
Yu X, Zhao R, Lin S, Bai X, Zhang L, Yuan S, Sun L. CXCL16 induces angiogenesis in autocrine signaling pathway involving hypoxia-inducible factor 1α in human umbilical vein endothelial cells. Oncol Rep. 2016;35(3):1557–65.
Chen Z, Kim SJ, Essani AB, Volin MV, Vila OM, Swedler W, Shahrara S, et al. Characterising the expression and function of CCL28 and its corresponding receptor, CCR10, in RA pathogenesis. Ann Rheum Dis. 2015;74(10):1898–906.
Kodama T, Koma YI, Arai N, Kido A, Urakawa N, Nishio M, Yokozaki H, et al. CCL3-CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab Investig J Tech Methods Pathol. 2020;100(9):1140–57.
Krausz S, Garcia S, Ambarus CA, de Launay D, Foster M, Naiman B, Reedquist KA, et al. Angiopoietin-2 promotes inflammatory activation of human macrophages and is essential for murine experimental arthritis. Ann Rheum Dis. 2012;71(8):1402–10.
England BR, Hershberger D. Management issues in rheumatoid arthritis-associated interstitial lung disease. Curr Opin Rheumatol. 2020;32(3):255–63.
Small W Jr, James JL, Moore TD, Fintel DJ, Lutz ST, Movsas B, Berk LB, et al. Utility of the ACE inhibitor captopril in mitigating radiation-associated pulmonary toxicity in lung cancer: results from NRG oncology RTOG 0123. Am J Clin Oncol. 2018;41(4):396–401.
Segawa S, Goto D, Yoshiga Y, Sugihara M, Hayashi T, Chino Y, Sumida T, et al. Inhibition of transforming growth factor-beta signalling attenuates interleukin (IL)-18 plus IL-2-induced interstitial lung disease in mice. Clin Exp Immunol. 2010;160(3):394–402.
Oliveira CR, Vieira RP. Anti-inflammatory activity of Miodesin™: modulation of inflammatory markers and epigenetic evidence. Oxid Med Cell Longev. 2020;2020:6874260.
De la Fuente Lopez M, Landskron G, Parada D, Dubois-Camacho K, Simian D, Martinez M, Hermoso RM, et al. The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumour Biol: J Int Soc Oncodevelopmental Biol Med. 2018;40(11):1010428318810059.
Ghafouri-Fard S, Shahir M, Taheri M, Salimi A. A review on the role of chemokines in the pathogenesis of systemic lupus erythematosus. Cytokine. 2021;146: 155640.
Gonçalves RSG, Pereira MC, Dantas AT, Almeida AR, Rego M, Lima EA, Pitta M, et al. CCL3, IL-7, IL-13 and IFNγ transcripts are increased in skin’s biopsy of systemic sclerosis. Exp Dermatol. 2019;28(10):1172–5.
Xiao H, Liang J, Liu S, Zhang Q, Xie F, Kong X, Liu T, et al. Proteomics and organoid culture reveal the underlying pathogenesis of Hashimoto’s thyroiditis. Front Immunol. 2021;12: 784975.
Pender SL, Chance V, Whiting CV, Buckley M, Edwards M, Pettipher R, MacDonald TT. Systemic administration of the chemokine macrophage inflammatory protein 1alpha exacerbates inflammatory bowel disease in a mouse model. Gut. 2005;54(8):1114–20.
Czaja AJ. Review article: chemokines as orchestrators of autoimmune hepatitis and potential therapeutic targets. Aliment Pharmacol Ther. 2014;40(3):261–79.
Park KH, Jung J, Lee JH, Hong YH. Blood transcriptome profiling in myasthenia gravis patients to assess disease activity: a pilot RNA-seq study. Exp Neurobiol. 2016;25(1):40–7.
Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long H, Wang Y. Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and monocyte/macrophage subsets. Front Immunol. 2018;9:2228.
Zhang Y, Mao X, Li W, Chen W, Wang X, Ma Z, Lin N. Tripterygium wilfordii: an inspiring resource for rheumatoid arthritis treatment. Med Res Rev. 2021;41(3):1337–74.
Tang C, Ye L, Hu Z, Wang W, Kuang T, Fan G, Yang M, et al. Efficacy and safety of total glucosides of paeony for rheumatoid arthritis: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020;99(39): e22224.
Yu C, Zhang X, Sun X, Long C, Sun F, Liu J, Teng L, et al. Ketoprofen and MicroRNA-124 Co-loaded poly (lactic-co-glycolic acid) microspheres inhibit progression of adjuvant-induced arthritis in rats. Int J Pharm. 2018;552(1–2):148–53.
Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky DS, Naredo E, van Vollenhoven R, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016;75(8):1428–37.
Zhao S, Mysler E, Moots RJ. Etanercept for the treatment of rheumatoid arthritis. Immunotherapy. 2018;10(6):433–45.
Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizumab (Actemra). Hum Vaccin Immunother. 2017;13(9):1972–88.
Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (Oxford). 2019;58(Suppl 1):i43–54.
Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology (Oxford). 2019;58(Suppl 1):i34–42.
Gottenberg JE, Morel J, Perrodeau E, Bardin T, Combe B, Dougados M, Mariette X, et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. BMJ (Clin Res Ed). 2019;364: l67.
Bao J, Liu W, Bao YX. Recombinant human interleukin receptor antagonist influences serum chemokines in patients with rheumatoid arthritis. Cent Eur J Immunol. 2014;39(2):170–3.
Wollmann BM, Syversen SW, Vistnes M, Lie E, Mehus LL, Molden E. Associations between cytokine levels and CYP3A4 phenotype in patients with rheumatoid arthritis. Drug Metab Dispos. 2018;46(10):1384–9.
Jordan LA, Erlandsson MC, Fenner BF, Davies R, Harvey AK, Choy EH, Williams AS, et al. Inhibition of CCL3 abrogated precursor cell fusion and bone erosions in human osteoclast cultures and murine collagen-induced arthritis. Rheumatology (Oxford). 2018;57(11):2042–52.
Manczak M, Jiang S, Orzechowska B, Adamus G. Crucial role of CCL3/MIP-1alpha in the recurrence of autoimmune anterior uveitis induced with myelin basic protein in Lewis rats. J Autoimmun. 2002;18(4):259–70.
Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8(1):23–33.
Oo YH, Shetty S, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. Dig Dis. 2010;28(1):31–44.
Prathipati PK, Mandal S, Destache CJ. A review of CCR5 antibodies against HIV: current and future aspects. Ther Deliv. 2019;10(2):107–12.
Trivedi PJ, Adams DH. Chemokines and chemokine receptors as therapeutic targets in inflammatory bowel disease; pitfalls and promise. J Crohn’s Colitis. 2018;12(suppl2):S641-s652.
Meitei HT, Jadhav N, Lal G. CCR6-CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun Rev. 2021;20(7): 102846.
Hanna A, Frangogiannis NG. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drugs Ther. 2020;34(6):849–63.
Castellani ML, Bhattacharya K, Tagen M, Kempuraj D, Perrella A, De Lutiis M, Neri G, et al. Anti-chemokine therapy for inflammatory diseases. Int J Immunopathol Pharmacol. 2007;20(3):447–53.
Acknowledgements
Funding
This study and its publication, including the journal’s Rapid Service Fee, were supported by grants from the National Natural Science Foundation of China (no. 82002269) and the Anhui Provincial Science and Technology Major Project (no. 8212929035).
Author Contributions
Ying-Li Yang: conceptualization, methodology, formal analysis and investigation, writing—original draft preparation; Xiao-Feng Li: conceptualization, methodology, investigation, writing—original draft preparation; Biao Song: methodology, investigation; Sha Wu: formal analysis and investigation; Yuan-Yuan Wu: methodology, investigation; Cheng Huang: writing—review and editing, supervision; Jun Li: writing—review and editing, supervision, funding acquisition.
Disclosures
Ying-Li Yang, Xiao-Feng Li, Biao Song, Sha Wu, Yuan-Yuan Wu, Cheng Huang and Jun Li declare that they have no competing interests.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yang, YL., Li, XF., Song, B. et al. The Role of CCL3 in the Pathogenesis of Rheumatoid Arthritis. Rheumatol Ther 10, 793–808 (2023). https://doi.org/10.1007/s40744-023-00554-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00554-0