Abstract
Introduction
Psoriatic arthritis (PsA) is a chronic, progressive disease that places a significant burden on patients and healthcare systems. The SUSTAIN study collected real-world evidence on long-term effectiveness, impact on quality of life, and safety of ustekinumab treatment for PsA.
Methods
SUSTAIN was a prospective, non-interventional study conducted in Germany. Patients with active PsA received ustekinumab for 160 weeks in routine clinical care, with assessments at baseline, week 4, and every 12 weeks thereafter. This analysis focuses on patients who remained in SUSTAIN until week 160.
Results
Of 337 patients enrolled, 129 were documented at week 160, of which 123 (95.3%) had received previous PsA medication, including biologics. Decreases from baseline to week 4 were observed for tender joint count (TJC, 8.0 to 5.8) and swollen joint count (SJC, 4.5 to 3.1); these decreases continued to week 28 and were maintained to week 160 (1.0 and 0.4, respectively). Similarly, skin assessments in patients with PsA and psoriasis revealed improvement at week 4, which continued to week 28, with a sustained effect until week 160. Similar patterns of response were observed for patient-assessed pain, sleep quality, and health scores. Improvements in TJC, SJC, Psoriasis Area and Severity Index, and affected body surface area were observed irrespective of the number of prior biologic therapies used. Minimal disease activity was achieved by 36 (31.9%) patients at week 28, and by 38 (33.6%) at week 52. Ustekinumab-related adverse events (AEs) and serious AEs were reported in 61 (47.3%) and 4 (3.1%) patients, respectively. At week 160, 100% of patients assessed ustekinumab tolerability as good or very good.
Conclusions
In a real-world setting, patients with active PsA who received ustekinumab until 160 weeks (3 years), including those who received prior biologic therapies, had a rapid onset of effect and sustained response to treatment, with high tolerability.
Trial registration
PEI NIS No. 290.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Psoriatic arthritis (PsA) is a heterogeneous, systemic inflammatory disease with many musculoskeletal and dermatological manifestations, which places a significant burden on both patients and healthcare systems. |
The SUSTAIN study was designed to increase understanding of the long-term effectiveness, impact on quality of life, and safety of ustekinumab for the treatment of PsA in routine clinical use in a real-world setting, including in patients who are not necessarily included in randomized controlled trials. |
What was learned from the study? |
Ustekinumab was rapid-acting and effective for up to 160 weeks (3 years) in patients with active PsA who responded to ustekinumab treatment in a real-world setting. |
Ustekinumab was well tolerated, and was effective regardless of prior biologic use, highlighting it as a valuable therapeutic option in routine clinical practice. |
Real-world evidence on the effectiveness and safety of ustekinumab resembles the compound profiles observed in phase 3 clinical studies. |
Introduction
Psoriatic arthritis (PsA) is a heterogeneous, systemic inflammatory disease with many musculoskeletal manifestations, including peripheral and axial joint involvement, nail disease, arthritis, spondylitis, enthesitis, and dactylitis [1, 2]. If untreated or inadequately treated, it can cause permanent joint damage and deformities [3]. PsA can be associated with extra-articular manifestations, namely psoriasis, uveitis, and inflammatory bowel diseases, and comorbidities, including cardiovascular disease, metabolic syndrome, and obesity [2, 4]. As such, many patients with PsA have a significantly impaired physical and mental quality of life (QoL) compared with the general population [5]. As a chronic, progressive disease, PsA places a significant burden on both patients and healthcare systems [6].
The primary aim of PsA treatment is to provide prolonged treatment effects with minimal joint damage due to disease progression, and to improve patient QoL [2, 7]. Current recommendations support a treat-to-target approach, where patients are closely monitored and treatment is modified with the goal of reaching remission or low disease activity, while also considering the different disease domains involved [7,8,9,10]. Currently available treatments include: traditional or conventional disease-modifying antirheumatic drugs (DMARDs; e.g., methotrexate [MTX], sulfasalazine, cyclosporine, and leflunomide), biologic therapies such as tumor necrosis factor (TNF) inhibitors (e.g., adalimumab, certolizumab, etanercept, golimumab, and infliximab), interleukin (IL)-17 inhibitors (e.g., secukinumab and ixekizumab), an IL-12/23 inhibitor (ustekinumab), IL-23 inhibitors (e.g., guselkumab and risankizumab), and new targeted oral agents including a phosphodiesterase-4 inhibitor and a Janus kinase/signal transducer and activator of transcription (STAT) inhibitor [7]. Patients with PsA tend to receive many of these treatments during the course of their disease, if they develop an insufficient response to a given treatment or are intolerant to it [7].
Ustekinumab (Stelara®) is a human monoclonal antibody that binds the p40 subunit common to both IL-12 and IL-23, blocking downstream signaling pathways [11], as well as T-helper (Th)-17 differentiation and inflammation. It was approved for the treatment of active PsA in adult patients—alone or in combination with MTX—on the basis of the findings of two placebo-controlled phase 3 trials, PSUMMIT-1 and PSUMMIT-2 [12,13,14,15]. Ustekinumab is currently the only biologic therapy approved for PsA that targets the IL-12/IL-23 pathways [7].
The SUSTAIN (Sustainability of effectiveness, safety and patient-reported outcomes for UStekinumab in the Treatment of Active psoriatic arthritis IN a real-life cohort) study was initiated to collect real-world evidence on the long-term efficacy and safety of ustekinumab treatment of PsA in routine clinical use. In this analysis from the SUSTAIN study, we assess the efficacy and tolerability of ustekinumab treatment for 160 weeks (3 years).
Methods
Study Design
SUSTAIN was a prospective, multicenter, non-interventional study (NIS) conducted at 75 sites in Germany according to standards set in the Declaration of Helsinki, based on the German Drug Law (Arzneimittelgesetz (AMG)]. Data were collected for 160 weeks, with study visits and assessments at weeks 0 and 4 and every 12 weeks thereafter.
Patients
Patients (≥ 18 years old) were eligible for enrollment into SUSTAIN if they had a confirmed diagnosis of active PsA and a previous inadequate response to a DMARD. All patients were prescribed ustekinumab on the basis of the treating physician’s decision as per routine clinical practice, and according to the approved label. Contraindications mentioned in the prescribing information had to be heeded. The planned population size was approximately 400 patients; if patients discontinued ustekinumab treatment before the final week (week 160), the NIS documentation was concluded. Patients were excluded from the study if they participated in another clinical trial or were treated with another biological agent for PsA during the observation period. All patients provided written informed consent, and the study was approved by the ethics commission of the Bavarian State Chambers of Physicians (Bayerische Landesärztekammer).
Study Assessments
Key assessments included:
-
Demographic and disease characteristics, including prior PsA medication history.
-
Tender and swollen joint counts (TJC and SJC), including subgroups of small peripheral (all hand and foot joints, excluding wrists and ankles) and large joints (ankles, wrists, elbows, knees, hips and shoulder joints). TJC was calculated using 80 joints, and SJC was calculated using 78 joints.
-
Skin symptoms affected by psoriasis, evaluated using the Psoriasis Area and Severity Index (PASI) and body surface area (BSA). PASI ranges from 0 (no activity) to 72 (worst activity), and BSA affected ranges from 0% (no psoriasis) to 100% (worst psoriasis).
-
Patient evaluation of pain intensity and sleep quality using a 100 mm visual analog scale (VAS).
-
Patients’ health, evaluated on the basis of the Health Assessment Questionnaire Disability Index (HAQ-DI). The HAQ-DI ranges from 0 (no impairment) to 3 (maximum impairment).
-
Enthesitis, evaluated using a PsA-modified Maastricht Ankylosing Spondylitis Enthesitis Score (MASES). MASES ranges from 0 (no enthesitis) to 15 (worst enthesitis).
-
Dactylitis, evaluated using the Dactylitis Severity Scale, where each of 20 digits was determined by the investigator to have no (0) to severe (3) dactylitis; total score 0–60).
-
Achievement of minimal disease activity (MDA), defined as meeting five out of the seven following criteria: TJC ≤ 1; SJC ≤ 1; PASI ≤ 1; or BSA ≤ 3%; patient pain VAS ≤ 15; patient global disease activity VAS ≤ 20; HAQ-DI ≤ 0.5; or tender entheseal points (MASES) ≤ 1.
Safety and tolerability assessments included the frequency of adverse events (AEs) and serious adverse events (SAEs), which were recorded and coded according to the Medical Dictionary for Regulatory Activities (MedDRA) v23.1 terminology. Patient-assessed tolerability categories were defined as very good, good, moderate, and insufficient.
Statistical Analysis
Data presented in this manuscript are from the patient population documented at week 160 (analysis population) unless otherwise stated. Data were analyzed using descriptive statistics, depending on the type of characteristic measured. Numerical data that were measured multiple times throughout the study were analyzed by visit (week) and were provided as absolute and relative changes from baseline. Missing values were not imputed. Kaplan–Meier estimators were used to assess the rate of patients achieving MDA and the time until MDA was achieved. TJC and SJC were also analyzed in patients with axial involvement (diagnosed by the treating physician on the basis of their routine clinical practice [imaging was not required]) or dactylitis. TJC, SJC, PASI, BSA, and MASES were also analyzed by number of prior biological therapies received (0, 1, 2, or > 2).
Results
Study Population
In total, 337 patients were enrolled into SUSTAIN between February 2015 and March 2017, for a planned observation period of 160 weeks. There were 146 (43.3%) patient discontinuations before week 160, which includes 104 (30.9%) patients who discontinued treatment owing to a lack or loss of efficacy or worsening of PsA/psoriasis, and 13 (3.9%) patients who withdrew because of AEs. Overall, 129 (38.3%) patients treated with ustekinumab were documented at week 160; of these, 125 (37.1%) patients were still receiving treatment at this timepoint (i.e., 4 patients discontinued treatment at the week 160 visit).
Baseline Characteristics
Baseline disease characteristics of the 129 patients documented at week 160 are summarized in Table 1 (for baseline demographics, see Supplementary Table 1). At baseline, 94 (72.9%) and 61 (47.3%) patients had peripheral small and large joints that were affected by PsA, respectively, and 14 (10.9%) patients had axial involvement. In addition, 25 (19.4%) patients had active dactylitis, 19 (14.7%) had enthesitis, and 4 (3.1%) had uveitis.
Baseline Concomitant Diseases and Prior Therapies for PsA
Overall, 109/129 (84.5%) patients had a concomitant disease at baseline, the most common being vascular disorders (n = 75, 58.1%), metabolism and nutrition disorders (n = 59, 45.7%), and musculoskeletal and connective tissue disorders (n = 52, 40.3%) (Table 2).
Before enrollment, 123/129 (95.3%) patients had received prior therapies for PsA (Table 3). The most common were MTX (n = 102, 79.1%) and ibuprofen (n = 41, 31.8%). In total, 56 (43.4%) patients had received one or more TNF inhibitor, including adalimumab (n = 39, 30.2%), etanercept (n = 32, 24.8%), golimumab (n = 18, 14.0%), certolizumab (n = 6, 4.7%) and infliximab (n = 4, 3.1%).
Patients received prior PsA therapies for a mean of 6.8 (standard deviation [SD] 7.6) years. The reasons for discontinuation of prior therapies are presented in Supplementary Table 2; of note, a lack of tolerability led to discontinuation of MTX for 41 (50.6%) patients, while lack of efficacy was the main reason for discontinuation of biologic therapies.
Ustekinumab Exposure
The 45 mg dose was given to 89 (69.5%) patients, while the 90 mg dose was given to 39 (30.5%) patients; data were missing for 1 patient. The mean weight-adjusted ustekinumab dose was 0.7 (SD 0.2) mg/kg body weight. Concurrent MTX was taken by 69 (53.5%) patients, while an alternative DMARD was taken by 9 (7.0%) patients.
Joint Counts
Mean decreases from baseline to week 4 were observed for both TJC (8.0 to 5.8; Fig. 1a) and SJC (4.5 to 3.1; Fig. 1a); these decreases continued up to around week 28 and were sustained over the remainder of ustekinumab treatment documentation (reaching 1.0 and 0.4 for TJC and SJC at week 160, respectively). Improvements were also observed in both small peripheral and large joints as early as week 4 (Figs. 1c–f). This rapid and sustained improvement in TJC and SJC was also demonstrated in patients with axial involvement or with dactylitis (Supplementary Fig. 1).
Mean TJC and SJC decreased from baseline throughout the study irrespective of MTX use. The mean absolute decrease in TJC was similar in MTX-treated patients and those who did not receive MTX (Supplementary Fig. 2a). However, patients who did not receive MTX had a consistently greater mean reduction in SJC than MTX-treated patients (Supplementary Fig. 2b).
Skin Parameters
Both PASI and BSA affected by psoriasis decreased from baseline after 4 weeks of ustekinumab treatment (mean absolute change of −3.2% and −2.6%, respectively) and continued to decrease up to week 28 (−6.4% and −11.4%, respectively) (Fig. 2a, b). These improvements were maintained up to week 160, where the mean absolute change from baseline was –7.2% for PASI and –13.6% for BSA affected.
Patient-Reported Outcomes
In line with the pattern of response observed with joint counts and skin parameters, a decrease from baseline in patient-assessed pain score was demonstrated by week 4 (mean absolute change of −10.0), with further improvements to week 28 (mean absolute change of −19.1). These improvements were maintained to week 160 of ustekinumab treatment (mean absolute change of −19.8) (Fig. 3). Similar improvements in patient-assessed sleep quality and HAQ-DI scores were also observed (Supplementary Fig. 3).
Minimal Disease Activity Assessments
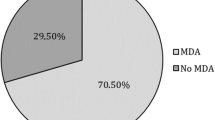
MDA was assessed at weeks 28 and 52 in patients who did not have MDA at baseline (n = 113/129). Following ustekinumab treatment, MDA was achieved by 36 (31.9%) patients at week 28, and by 38 (33.6%) patients at week 52 (Table 4). The rate of patients achieving each individual MDA criterion is also presented in Table 4. Overall, 67.1% of patients had SJC ≤ 1 and 62.1% of patients attained PASI ≤ 1 or BSA ≤ 3% at week 28; the equivalent proportions at week 52 were 69.9% and 81.0%, respectively.
The rate of patients achieving each individual MDA criterion in relation to overall MDA status (whether achieved or not achieved) is summarized in Supplementary Table 3. In patients without MDA, SJC ≤ 1 and PASI ≤ 1 or BSA ≤ 3% were still achieved by 62.7% and 67.2% of patients, respectively, at week 28, and 71.4% and 77.8% of patients, respectively, at week 52. For patients who successfully achieved MDA at least once during the study, the median time until MDA achievement was 42 weeks [95% confidence interval (CI) 30.0–65.9], with a median duration of 33 weeks (95% CI 23.9–71.7). Worst-case analysis resulted in a median MDA duration of 12 weeks (95% CI 0.1–26.1).
MDA achievement at weeks 28 (32.2% versus 31.5%, respectively) and 52 (30.5% versus 37.0%, respectively) was similar in patients who received concomitant MTX treatment and those who did not (Supplementary Table 4).
Ustekinumab Effectiveness Following Prior Biologic Therapies
Ustekinumab, as assessed by mean change from baseline to week 160 in TJC, SJC, enthesitis, BSA, and PASI, and MASES was effective irrespective of the number of prior biologic therapies received (Fig. 4).
Mean absolute change from baseline in (a) TJC, (b) SJC, (c) PASI, (d) BSA, and (e) enthesitis, from baseline to week 160. Data show the 95% CI. Patient numbers are as follows: no biologic, n = 72; one biologic, n = 27; two biologics, n = 16; more than two biologics, n = 14. BSA body surface area, CI confidence interval, MASES Maastricht Ankylosing Spondylitis Enthesitis Score, PASI Psoriasis Activity and Severity Index, SJC swollen joint count, TJC tender joint count
Safety
Ustekinumab treatment-related AEs and SAEs were reported in 61 (47.3%) patients and 4 (3.1%) patients, respectively (Table 5), and AEs occurring in ≥ 3% of patients are presented in Table 6. Off-label use of ustekinumab (28 [21.7%] patients), which includes the use of ustekinumab at a higher dose than recommended by the summary of product characteristics, contributed to the overall number of treatment-related AEs. At week 160, 100% of patients who provided a response (n = 117) assessed the tolerability of ustekinumab as good or very good.
Discussion
Given the significant disease burden that PsA places on both patients and healthcare systems, patients require a treatment that provides long-term efficacy in order to minimize joint damage, improves QoL, and has a favorable safety profile. This 160-week analysis from the prospective, multicenter, non-interventional SUSTAIN study demonstrates that ustekinumab can provide a rapid and sustained therapeutic benefit, with high tolerability, for patients with PsA in routine clinical care.
As patients who discontinued treatment were not included in our analysis, the results of treatment efficacy and safety are limited to those patients who benefited from ustekinumab treatment. Overall, 129 (38.3%) patients remained in the study and were documented at the final week 160 visit. Notably, only a low proportion of discontinuations in the overall population were caused by lack of tolerability to ustekinumab, with only 13/337 (3.9%) patients withdrawing because of AEs. In comparison, the percentage of patients (among those documented at week 160) who discontinued biologic therapies (such as adalimumab, certolizumab, etanercept, golimumab, and infliximab) prior to enrollment in the SUSTAIN study owing to lack of tolerability was much higher, ranging from 16.1% to 28.2%.
As the SUSTAIN patient population was not preselected, it is representative of patients in the real-world setting. This is highlighted by the large proportion of the 129 patients in the analysis population who had concomitant diseases at baseline (n = 109; 84.5%), and that most (n = 123; 95.3%) had received prior therapy for PsA, including a large proportion who received one or more TNF inhibitor (n = 56; 43.4%). Owing to the real-world nature of SUSTAIN, physicians conducted examinations and documented comorbidities according to their individual preference and their usual clinical routine, which means that some parameters may have been recorded infrequently and/or inconsistently. This might explain the low baseline incidence of psoriasis (n = 7; 5.4%); rather than documenting psoriasis as a concomitant disease, it is possible that the investigating physicians considered it as a cutaneous manifestation of PsA instead, and so the information was not captured. Nevertheless, 81 and 101 patients had PASI and BSA assessments, respectively, documented at baseline.
Improvements in TJC and SJC, including in both small peripheral and large joints, were observed at the initial week 4 visit, which demonstrates that ustekinumab had a rapid treatment effect in this patient population. The improvements continued to around week 28 and were then sustained up to week 160, which suggests that ustekinumab also has a positive, long-term impact on the joints in patients with PsA. However, it should be noted that the assessment of joint counts is an objective disease measure that, while useful in controlled clinical trials, may not necessarily reflect patients’ and physicians’ views of treatment success in daily practice. Indeed, a post hoc analysis of SUSTAIN suggested that objective arthritis disease measures (such as joint counts, C-reactive protein, and American College of Rheumatology criteria) may underestimate the benefit of treatment of patients with PsA in real-world clinical care (data on file). The analysis demonstrated that patients’ and physicians’ evaluation of the therapeutic success of ustekinumab exceeded the benefit suggested by conventional measures of treatment response.
To provide a more holistic view of ustekinumab treatment in patients with active PsA, various other clinical assessments were also conducted in the SUSTAIN study. A similar pattern of response to that of TJC and SJC was observed with the dermatological assessments (PASI and BSA affected by psoriasis), as well as with QoL and health assessments (patient-assessed levels of pain and sleep quality, and HAQ-DI). Initial beneficial treatment effects were observed in all parameters at week 4, with continued improvements to around week 28, followed by sustained effects up to week 160. These continuous measures of efficacy are further supported by the assessment of MDA, which is a valid and widely used measure of PsA disease activity [16, 17]. In the SUSTAIN study, MDA was achieved in 31.9% and 33.6% of patients receiving ustekinumab at weeks 28 and 52, respectively. These rates are comparable to a recent analysis from the prospective, observational PsABio study of patients with PsA, where 104/385 patients (27.0%) achieved MDA after 6 months of ustekinumab treatment [18].
Many patients with PsA receive several different therapies during the disease course, and although evidence is limited, a higher number of prior biologic therapies—particularly TNF inhibitors—appears to be associated with reduced biologic therapy success [13, 19, 20]. For example, in the PSUMMIT-2 study of ustekinumab, patients who received at least one previous TNF inhibitor had a lower likelihood of treatment success than those who were TNF inhibitor-naïve [13]. In the SUSTAIN study, the analysis population had received prior PsA therapy for an average of 6.8 years; 123 (95.3%) had received at least one prior PsA therapy and 56 (43.4%) had received at least one TNF inhibitor. Nevertheless, ustekinumab demonstrated clinical benefits (as assessed by TJC, SJC, PASI, BSA, and MASES) in both patients with no prior biologic use and those who had received one, two, or more than two prior biologics.
The safety profile of ustekinumab in this analysis of SUSTAIN was generally similar to that observed in earlier clinical trials and real-world studies, and no new safety signals were identified [12, 21, 22]. At week 160, 100% of patients who responded rated the tolerability of ustekinumab as good or very good. Taken together with the improvements in patient-assessed pain, sleep quality, and health scores, these data suggest a high tolerability for ustekinumab in patients with PsA in the real-world setting.
The limitations of SUSTAIN include the lack of a comparator study arm, and because it is a German study, the results may not be generalizable to patients in other countries. In addition, patients who discontinued treatment were not included in this analysis, meaning the results of treatment efficacy and safety are limited to those patients who benefited from ustekinumab treatment. As such, it is possible that treatment effectiveness may be slightly overestimated. Finally and as previously discussed, physicians documented comorbidities according to their individual preference, which means that some parameters may have been recorded infrequently and/or inconsistently.
Conclusions
This analysis of the SUSTAIN study demonstrates that, in routine clinical practice, ustekinumab can provide rapid and sustained (long-term) treatment efficacy for up to 160 weeks (3 years), as well as high tolerability, in patients with active PsA who respond to ustekinumab treatment. Of note, ustekinumab was efficacious irrespective of prior biologic use. As the primary aim of any physician treating PsA is to prescribe a well-tolerated therapy, with fast onset and sustained efficacy that also improves QoL, we propose that ustekinumab offers a valuable therapeutic option.
References
Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med (Lond). 2017;17(1):65–70.
Ocampo DV, Gladman D. Psoriatic arthritis. F1000Res. 2019;8.
Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol. 2003;4(7):441–7.
Ogdie A, Schwartzman S, Husni ME. Recognizing and managing comorbidities in psoriatic arthritis. Curr Opin Rheumatol. 2015;27(2):118–26.
Husted JA, Gladman DD, Farewell VT, Long JA, Cook RJ. Validating the SF-36 health survey questionnaire in patients with psoriatic arthritis. J Rheumatol. 1997;24(3):511–7.
Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T. 2010;35(12):680–9.
Ogdie A, Coates LC, Gladman DD. Treatment guidelines in psoriatic arthritis. Rheumatology (Oxford). 2020;59(Suppl 1):i37–46.
Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–71.
Singh JA, Guyatt G, Ogdie A, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5–32.
Smolen JS, Braun J, Dougados M, et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis. 2014;73(1):6–16.
Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011;3(6):535–45.
McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780–9.
Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990–9.
European Medicines Agency. Stelara: ustekinumab https://www.ema.europa.eu/en/medicines/human/EPAR/stelara. Accessed 03 Nov, 2021.
Janssen Biotech Inc. Highlights of prescribing information: Stelara® (ustekinumab) injection, for subcutaneous or intravenous use. In.; 2012.
Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(1):48–53.
Coates LC, Strand V, Wilson H, et al. Measurement properties of the minimal disease activity criteria for psoriatic arthritis. RMD Open. 2019;5(2): e001002.
Smolen JS, Siebert S, Korotaeva TV, et al. Effectiveness of IL-12/23 inhibition (ustekinumab) versus tumour necrosis factor inhibition in psoriatic arthritis: observational PsABio study results. Ann Rheum Dis. 2021;80(11):1419–28.
Iannone F, Santo L, Bucci R, et al. Drug survival and effectiveness of ustekinumab in patients with psoriatic arthritis. Real-life data from the biologic Apulian registry (BIOPURE). Clin Rheumatol. 2018;37(3):667–75.
Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(1):29–37.
Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178(2):509–19.
Reich K, Mrowietz U, Radtke MA, et al. Drug safety of systemic treatments for psoriasis: results from The German Psoriasis Registry PsoBest. Arch Dermatol Res. 2015;307(10):875–83.
Acknowledgements
The authors thank the teams at the individual study sites for continuous support of SUSTAIN, and Winicker-Norimed GmbH, as contract research organization, for successfully conducting the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The SUSTAIN study was sponsored and funded by Janssen-Cilag GmbH (Germany). External authors did not receive funding (except for participation as a study site). Janssen-Cilag GmbH (Germany) funded the journal’s Rapid Service Fee.
Medical Writing and Editorial Assistance
Medical writing support, funded by Janssen-Cilag GmbH (Germany), was provided by Joe Pickering, PhD, from Mudskipper Business Limited.
Author Contributions
J.W.: conception/design of the study, data analysis and interpretation, drafting the manuscript, critical revision of the manuscript, final approval of manuscript; N.D.: data analysis and interpretation, compilation and review of manuscript; M.R.: data interpretation, compilation and review of manuscript; V.T.: review of manuscript; M.S.: conceptualization of the SUSTAIN study, contribution to study setup and review of manuscript; F.H.: review of manuscript; H.S.: review of manuscript; M.Si.: review of manuscript; F.B.: study design, data generation, review of manuscript.
Disclosures
Jörg Wendler was an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Chugai, Janssen-Cilag GmbH, Novartis, Roche Pharma; Nils Damann, Marit Röcken, and Verena Teicher are employees of Janssen-Cilag GmbH and hold stocks of Johnson & Johnson; Maximilian Schuier is an employee of Johnson & Johnson, holding stocks thereof; Frank Hamann, Holger Schwenke, and Maren Sieburg declare no competing interests; Frank Behrens has received research grants and consultancy/speaker fees from Janssen.
Compliance with Ethics Guidelines
All patients provided written informed consent, and the study was approved by the ethics commission of the Bavarian State Chambers of Physicians (Bayerische Landesärztekammer). Written informed consent was obtained from all participants. The study was carried out in accordance with the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
SUSTAIN: Sustainability of effectiveness, safety and patient-reported outcomes for UStekinumab in the Treatment of Active psoriatic arthritis IN a real-life cohort.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wendler, J., Damann, N., Röcken, M. et al. Ustekinumab Is Rapid-Acting and Is an Effective Long-Term Treatment for Patients with Active Psoriatic Arthritis: Real-World Evidence from the Non-interventional SUSTAIN Study. Rheumatol Ther 9, 1435–1450 (2022). https://doi.org/10.1007/s40744-022-00484-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00484-3