Abstract
Introduction
Psoriatic arthritis (PsA) is a common inflammatory disease affecting the peripheral and axial skeleton. History of psoriasis (PSO), either personal or family history, is an important factor in the diagnosis of PsA. We investigated the association between history of PSO and clinical characteristics of PsA.
Methods
PsA patients were consecutive recruited from 2019 to 2020. These patients were subjected to clinical, biochemical, and radiographic examinations, and disease activity was evaluated. Continuous and categorical variables analyses were presented.
Results
All registered patients (296 cases) met the classification criteria of PsA. They were divided into three groups based on the history of psoriasis (PSO), as: (1) 145 patients with PSO themselves (pPsA); (2) 96 patients with family history of PSO (fPsA); (3) 55 patients with family history and coexisting PSO themselves (fPsA/PSO). Compared to fPsA/PSO, the levels of CRP, ESR, uric acid, DAPSA, BASDAI, ASDAS, and BASFI were lower in fPsA, but similar to pPsA. The severity of sacroiliitis tended to be more severe in fPsA/PSO than fPsA (OR2 vs. 3 0.508; 95% CI 0.272 to 0.949, p < 0.05). No significant differences were found in HLA-B-27 and common inflammatory articular and extra-articular manifestations among the three groups. Furthermore, there were no differences in LEI, TJC, SJC, and DAS28CRP. Interestingly, a correlation was found between the ages of individuals with PSO and the onset of arthritis, and the earliest arthritis onset occurred in fPsA/PSO patients (p < 0.001).
Conclusions
Our study demonstrates that currently existing cutaneous lesions in patients themselves are correlated with disease activity and severity of axial joint damage, whereas family history does not have an evident impact on the disease activity of PsA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
History of psoriasis (PSO), either personal or family history, is an important factor in the diagnosis of psoriatic arthritis (PsA). |
We unveiled potential differences in disease patterns, especially axial joints, of patients with or without the history of PSO, either personal or family history. |
Patients with existing skin lesion or a past history of PSO is correlated with the higher disease activity, particularly more severe axial involvement. |
Customized treatments are needed by taking the skin disease, family history of PSO, and potential axial involvements into consideration. |
Introduction
Psoriatic arthritis (PsA), a common inflammatory disease associated with psoriasis (PSO), can affect both the peripheral and axial skeleton [1]. At different stages of disease, clinical manifestation may vary, including arthritis, extra-articular involvements, and cutaneous and nail lesions [2]. There seems to be a correlation between the severity of skin lesion and arthritis [3]. Nail bed inflammation, another type of enthesitis, is often associated with arthritis of small joints, particularly distal interphalangeal (DIP) joints [4, 5]. Gender may also impact the disease activity in PsA patients [6, 7].
Based on the Classification of Psoriatic Arthritis (CASPAR) criteria, a family history of PSO in first-degree relatives, present or past, contributes to the pathogenesis of PsA. Therefore, family history is a factor for establishing a diagnosis of PsA [8]. The incidence of PsA is 19 times higher when first-degree relatives have PSO compared to the general population [9]. However, very few studies have been performed to characterize clinical features and disease activity of PsA patients with or without a family history of PSO. A previous study compared the recurrence risk ratio of PsA patients whose first-degree relatives had PSO [10], but did not investigate the differences in clinical features. A recent study showed that heel pain was statistically associated with history of dactylitis and PsA in first-degree family members [11]. Our study aimed to compare demographic and clinical characteristics of PsA patients in different conditions and to unveil the potential differences in characteristic features and disease activity, especially axial joints, between PsA patients with or without family history of PSO. In addition, we studied the impact of skin lesions in patients themselves on the radiological damage of PsA and the association between the factors mentioned above and disease activity.
Methods
The Aim, Design, and Setting of the Study
A prospective single-center cross-sectional observational study with 296 PsA patients was conducted from January 2019 to December 2020 in the First Affiliated Hospital of Zhengzhou University. All patients were diagnosed for the first time and fully met the CASPAR criteria, and those with other autoimmune rheumatic diseases, infections, malignancies, and serious concomitant diseases were excluded. The Ethics Committee of the First Affiliated Hospital of Zhengzhou University approved this study that was in accordance with the Declaration of Helsinki (KY-2021–00,926), and all patients provided informed consent forms before data collection.
Basic demographic data, blood tests, clinical features, and radiographic patterns were collected. Family history of PSO was carefully taken. If the answer was affirmative, the affected family member was consulted again and their relationship with the patients was documented. These patients were divided into the following groups: (1) pPsA referred to those with personal history of PSO only, (2) fPsA referred to those with family history of PSO only, (3) fPsA/PSO referred to those who had family history as well as existing PSO.
The Evaluations of Laboratory and Radiographic Findings
The results from laboratory tests were recorded and these included complete blood count, tests for hepatic and renal functions, blood glucose and lipid panel, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and HLA-B typing. In addition, the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), two indicators for the magnitude of inflammatory response and immune function, were calculated [12, 13].
Radiographic examination of spine and sacroiliac joints was performed at baseline. Magnetic resonance imaging (MRI) was done only when possible axial involvement could not be confirmed by X-rays. The results of the relevant examinations were collected, including the evaluations of other joints and musculoskeletal system by radiographs, computed tomography (CT), MRI, and ultrasound. Radiographic findings such as spinal osteophytes, degeneration in intervertebral discs/endplates, inflammatory syndesmophytes, and the scores of sacroiliac joint abnormalities were evaluated by at least two radiologists with expertise in the musculoskeletal system and reached a consensus based on modified New York (mNY) criteria [14]. Bone mass density (BMD) was measured and a T-score less than – 2.5 was defined as osteoporosis, and a score between – 2.5 and – 1 as osteopenia [15].
The Assessments of Clinical Features
Current manifestation and past history of PsA patients were collected by an experienced rheumatologist. Basic information included inflammatory joint disease and extra-articular involvements including dactylitis, enthesitis, uveitis, etc. Family history, the onset ages of cutaneous lesions, and arthritis were also collected. The spine or buttocks pain was described as either current or past. Current pain was designated as persistent pain in the back, lower back, and buttocks areas ≤ 3 months, while past pain was those with pain in these regions over 3 months, but the pain did not occur in the last 3 months before establishing a PsA diagnosis.
At the time of enrolment, disease activity was assessed using the Disease Activity Score (DAS28) [16], Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [17], and Disease Activity index for Psoriatic Arthritis (DAPSA) [18]. Ankylosing Spondylitis Disease Activity Score (ASDAS) [19] and Bath Ankylosing Spondylitis Functional Index (BASFI) [20] were used to evaluate axial disease activity and function. Psoriasis Area and Severity Index (PASI) was used to assess the severity of PSO [21]. In addition, 66 swollen (SJC) and 68 tender joints (TJC) were counted for peripheral arthritis [22], and Leeds Enthesitis index (LEI) was used for the assessment of enthesitis [23].
Statistical Analyses
All statistical analyses were performed using IBM SPSS Statistics 26.0. Descriptive statistics were used to analyze the demographics, clinical characteristics, and imaging lesions. The t test, Mann–Whitney U test, and Kruskal–Wallis test were used to detect the differences in continuous variables and Pearson’s Chi-square (χ2), correction for continuity, or Fisher’s exact tests for qualitative variables. Odds ratios (OR) with 95% confidence intervals (95% CI) were calculated by logistic regression analysis to evaluate the risk factors and correlations between groups. P values ≤ 0.05 were considered statistically significant.
Results
Out of 371 patients who visited our hospital between the years 2019 and 2020, 145 pPsA, 96 fPsA, and 55 fPsA/PSO patients were included in the study. The following patients were excluded: 23 patients not complying with axial assessment, 13 patients with uncertain family history of PSO, and 39 patients with other autoimmune rheumatic diseases or serious concomitant diseases.
Sociodemographic, Laboratory, and Clinical Assessments Characteristics
Sociodemographic Characteristics
Compared to both pPsA and fPsA, fPsA/PSO patients usually had an earlier onset of arthritis (average age: 34.8 years), and the onset ages of PSO were also younger in fPsA/PSO patients than pPsA (P = 0.015). No difference was found in durations of arthritis and PSO between fPsA/PSO and pPsA, whereas fPsA patients had a significant shorter duration of arthritis (P = 0.038) (Table 1). Notably, a female predominance was found in fPsA compared to fPsA/PSO patients (P = 0.002).
Laboratory and Clinical Assessment Outcomes
Most of the routine laboratory results, including complete blood count, biochemicals, PLR and NLR, were similar among pPsA, fPsA, and fPsA/PSO patients. Compared to fPsA/PSO patients, CRP, ESR, and UA were significantly lower in fPsA patients (PCRP = 0.002, PESR = 0.013, PUA = 0.001). However, there was no statistical difference between pPsA and fPsA/PSO (PCRP = 0.323, PESR = 0.489, PUA = 0.651).
The disease activity measured with DAPSA was lower in fPsA patients (P = 0.046), with the mean DAPSA score being 18.41 ± 7.17. The assessment with TJC, SJC, and DAS28 did not reveal significant differences among patients in three groups with respect to the severity of peripheral arthritis. PASI and LEI for the severity of PSO and enthesitis did not reveal statistical differences as well (PPASI = 0.209; P1 vs. 3 = 0.834; P2 vs. 3 = 0.269) (Table 1).
Patient-Reported Outcomes
Clinical Features and Assessments of Axial Involvement
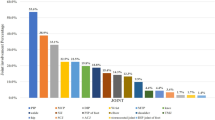
Inflammatory axial symptoms were present in 59/145 (40.7%) pPsA, 47/196 (49.0%) fPsA, and 24/55 (43.6%) fPsA/PSO patients. No significant difference was detected among the three groups with regards to inflammatory axial symptoms (OR1 vs. 3 0.886, 95% CI 0.473 to 1.660; OR2 vs. 3 1.239, 95% CI 0.636 to 2.413). Back and buttock pain was the most common initial symptom (Fig. 1A). Similarly, HLA-B*27 alleles were detected in 54/145 (37.2%) pPsA, 39/96 (40.6%) fPsA, and 25/55 (45.5%) fPsA/PSO patients, but no significant difference was identified in these patients. Of note, axial metrology assessed with BASDAI, ASDAS, and BASFI were significantly lower in fPsA than fPsA/PSO (PBASDAI = 0.031; PASDAS = 0.002; PBASFI = 0.004) (Table 2).
Comparison between family history of psoriasis, individual psoriasis, and articular and extra-articular manifestations. A No difference was detected in three groups in terms of initial symptoms caused by joint involvements. Pain in the back and buttocks area was the most common initial symptoms. B No differences were observed with regards to most inflammatory axial symptoms and extra-articular manifestations among pPsA, fPsA, and fPsA/PSO. MCP metacarpophalangeal joint, PIP proximal interphalangeal joint, DIP distal interphalangeal joint, MTP metatarsophalangeal joint
Radiographic Comparison of Axial Involvement
The characteristic feature of joint involvement in PsA patients is bilateral sacroiliitis, which was present in 71/87 (81.61%) of pPsA patients, 39/51 (76.47%) of fPsA patients and 37/36 (75.00%) of fPsA/PSO patients, with no statistical significance. However, fPsA/PSO patients seemed to have higher grades of sacroiliitis than fPsA patients (OR 0.508; 95% CI 0.272–0.949, P = 0.037), but no difference in vertebral syndesmophytes (P = 0.266). Whole spinal radiography did not reveal a significant difference among pPsA, fPsA, and fPsA/PSO patients with regards to spondylitis in cervical and/or lumbar regions. Notably, compared with fPsA/PSO, both pPsA and fPsA patients had more severe decreases of BMD, particularly the incidence of osteoporosis (OR1 vs. 3 0.203, 95% CI 0.134–0.306, P < 0.001; OR2 vs. 3 0.211, 95% CI 0.129–0.345, P < 0.001) (Table 2).
Inflammatory Peripheral Articular and Extra-Articular Manifestations
In addition to back or buttocks pain, knee pain (23.4%) was also a common initial symptom in pPsA cases. No difference was found in the initial symptoms in other joint inflammation, including digital joints, ankles, and shoulders (Fig. 1A). Similar results were obtained in peripheral arthritis. Further, no significant difference was observed in extra-articular manifestations such as dactylitis, enthesitis, and uveitis among these groups (Fig. 1B).
Discussion
While the identification of clinical features of PsA with different skin lesions has been well characterized [3], few studies have been conducted to compare clinical manifestations and disease activity in PsA patients with or without a history of PSO, either personal or family history. Our study aimed to unveil potential differences in disease patterns, especially axial joints, of these patients, which may lend novel insights to the pathogenesis and improve the treatment efficacy of PsA. We confirm that it is current or past cutaneous lesions, not family history of PSO, that is correlated with the activity of PsA and the severity of axial joint damage.
A large number of loci have been identified in genomic association studies of PsA in the past decade such as HLA-B*27:05:02 allele for dactylitis and HLA-C*06:02:01 for asymmetrical sacroiliitis [24]. Deeper genetic differences may also exist in families with PSO or PsA [25]. Thus, we hypothesized that the alleles involved in axial joint damage may differ in PsA patients with a family history of PSO. Our results demonstrated that the incidence of positive HLA-B*27 alleles is similar in patients, irrespective of history of PSO. Somewhat surprisingly, fPsA/PSO patients had earlier onset of PSO and arthritis. A previous study has demonstrated that higher frequency of HLA-C*06 allele is associated with early onset of PSO [26]. However, the HLA-C typing was not sequenced and a potential allele associated with earlier onset of arthritis remains elusive.
Additionally, our results are, to some extent, different from previous epidemiological data from Western countries showing that the male-to-female ratio was about 1:1 [27, 28]. We found a female predominance (57.3%) in fPsA. Geographic and genetic factors may lead to such a discrepancy. Further, the incidence of dactylitis (110/296, 37.2%) in our study was slightly lower than a previous report [29]. One explanation is that some patients with gout or pseudogout were misdiagnosed as PsA [30, 31]. We postulate that genetic factors may be another cause of lower prevalence of dactylitis in Chinese PsA patients [32]. Similarly, because of the low prevalence and genetic differences in PsA patients, significant variations do exist regarding the occurrence of uveitis [33,34,35].
A history of PSO, whether individual or familial, is an important factor in the diagnosis of PsA. Although it has been proven that family history of PsA has an impact on skin phenotypes [25], very little is known about the role of family history in disease activity and clinical features in PsA patients. Thus, we further investigated the relationship between axial associated characteristics of PsA and history of PSO, as well as certain genetic analyses and articular symptoms. We demonstrate that currently existing skin lesion, not family history of PSO, is correlated with the disease activity of pPsA and fPsA/PSO patients, particularly axial involvements. Our study did reveal similar disease patterns of patients in three groups with regards to inflammatory arthritis, and extra-articular manifestation such as the prevalence of different parts of arthritis and dactylitis. However, compared to fPsA cases, fPsA/PSO patients seem to have higher disease activity as evidenced by higher levels of CRP and ESR, and more severe sacroiliitis. These findings suggest a correlation of PSO with disease activity and joint damage, and the shared features of inflammation in these pathological changes may be referred to as “psoriatic disease”. While the underlying causes of the discrepancy in radiographic damage are unknown, the female predominance in fPsA suggests a role of sex hormones. Occupational and environmental factors may also have a role; for example, male patients are more likely to engage in heavy labor [36]. However, prospective information on the association of physical activity with joint damage were not available for all patients. Previous studies show that high disease activity and an elevated CRP level are risk factors for radiographic progression of PsA [37, 38], which is consistent with our studies. Furthermore, pain and function-related subjective disease scores such as BASDAI, ASDAS, and BASFI for axial metrology, are also higher in pPsA and fPsA/PSO patients than fPsA patients. Similarly, the assessment with DAPSA reveals higher disease activity in pPsA and fPsA/PSO patients. Notably, no statistical significance was observed in two markers of systemic inflammation, NLR and PLR. They can be used to evaluate the magnitude of inflammatory response. A previous study has shown a correlation between the DAS28 score and NLR/PLR [13]. However, in our study, the assessments with TJC, SJC, and DAS28 did not prove a significant difference in the severity of peripheral arthritis. In addition, no differences were found in the number of enthesitis as evaluated with LEI in three groups. Collectively, it is cutaneous lesions, instead of family history, that affects disease activity, particularly axial joints. It was worth noting that both longer course and later onset of axial disease may predict spinal involvements (sacroiliac joints or spine) in PsA patients [39, 40]. Our study did show a longer duration of arthritis in both pPsA and fPsA/PSO patients. Further, over half of the patients in our study had axial involvements, although only 130/296 (43.9%) patients have current axial symptoms. However, 56/296 (18.9%) patients had axial symptoms in the past, not at the time of enrolment, suggesting that axial involvement is a slowly progressive process, and that cutaneous lesions may, via a yet-unknown mechanism, affect the pace of axial destruction. Unexpectedly, bilateral sacroiliitis was dominant in axial involvement, whereas most of these are of different grades on either side and only the higher grades were recorded. In agreement with previous studies, the level of UA is higher in pPsA and fPsA/PSO patients, indicating a link between skin pathology and UA metabolism [41, 42]. Moreover, our results imply that fPsA/PSO tend to have less bone loss, possibly due to earlier onset and diagnosis of arthritis.
We believe our results are reliable because of accurate radiographic scoring and complete clinical and radiographic data. All clinical features, quantitative radiographic scoring, and morphological analyses were jointly evaluated by experienced rheumatologists and radiologists in order to identify subtle distinctions of disease activity and axial joints among different patient groups.
There are some limitations in our study. First, not a single evaluation method is available for comprehensive assessments of disease activity and functional and morphological changes. Second, family history is not always easy to obtain, as some patients cannot provide complete and accurate information for their family members. Third, owing to costs, inconsistencies in imaging methods and lack of genetic analyses make it impossible to further analyze the differences in the results. Nevertheless, the accuracy of our methodology has been proven [43]. Taking all into consideration, we think that a multi-center, longitudinal study including more patients, particularly, fPsA/PSO patients, and more comprehensive study of genetic factors, may improve our understanding of the role of family history in PsA pathogenesis and progression.
Conclusions
Our study demonstrates that pPsA has a similar disease burden as fPsA/PSO, whereas fPsA patients seem not so active. These results suggest that current or past cutaneous lesions, instead of family history of PSO, are correlated with the activity of PsA and the severity of axial joint damage. Careful personal and familial history taking may not only help decide the most appropriate treatments for different PsA patients but also predict the involvement of axial joint and improve prognosis.
References
Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. The Lancet. 2018;391(10136):2273–84.
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–70.
Mease PJ, Etzel CJ, Huster WJ, et al. Understanding the association between skin involvement and joint activity in patients with psoriatic arthritis: experience from the Corrona Registry. RMD Open. 2019;5(1): e000867.
Antony AS, Allard A, Rambojun A, et al. Psoriatic nail dystrophy is associated with erosive disease in the distal interphalangeal joints in psoriatic arthritis: a retrospective cohort study. J Rheumatol. 2019;46(9):1097–102.
Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis–clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43(6):790–4.
Kenar G, Yarkan H, Zengin B, et al. Gender does not make a difference in “composite psoriatic disease activity index (CPDAI)” in patients with psoriatic arthritis. Rheumatol Int. 2018;38(11):2069–76.
Eder L, Thavaneswaran A, Chandran V, Gladman DD. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann Rheum Dis. 2013;72(4):578–82.
Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73.
Rahman P, Elder JT. Genetic epidemiology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64 Suppl 2(ii37–9; discussion ii40–1.
Chandran V, Schentag CT, Brockbank JE, et al. Familial aggregation of psoriatic arthritis. Ann Rheum Dis. 2009;68(5):664–7.
Morales Ivorra I, Juárez López P, López de Recalde M, Carvalho PD, Rodriguez Moreno J. Heel pain in psoriatic arthropathy: analysis of a series of 291 patients. Reumatología Clínica (English Edition). 2018;14(5):290–3.
Asahina A, Kubo N, Umezawa Y, et al. Neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and mean platelet volume in Japanese patients with psoriasis and psoriatic arthritis: response to therapy with biologics. J Dermatol. 2017;44(10):1112–21.
Uslu AU, Kucuk A, Sahin A, et al. Two new inflammatory markers associated with Disease Activity Score-28 in patients with rheumatoid arthritis: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int J Rheum Dis. 2015;18(7):731–5.
van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–8.
Compston JE, McClung MR, Leslie WD. Osteoporosis. The Lancet. 2019;393(10169):364–76.
Prevoo ML, van 't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8.
Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21(12):2286–91.
Schoels M, Aletaha D, Funovits J, et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis. 2010;69(8):1441–7.
Lukas C, Landewe R, Sieper J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(1):18–24.
Fernandez-Sueiro JL, Willisch A, Pertega-Diaz S, et al. Evaluation of ankylosing spondylitis spinal mobility measurements in the assessment of spinal involvement in psoriatic arthritis. Arthrit Rheum-Arthr. 2009;61(3):386–92.
Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–44.
Fransen J, Antoni C, Mease PJ, et al. Performance of response criteria for assessing peripheral arthritis in patients with psoriatic arthritis: analysis of data from randomised controlled trials of two tumour necrosis factor inhibitors. Ann Rheum Dis. 2006;65(10):1373–8.
Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008;59(5):686–91.
Haroon M, Winchester R, Giles JT, Heffernan E, FitzGerald O. Certain class I HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann Rheum Dis. 2016;75(1):155–62.
Solmaz D, Bakirci S, Kimyon G, et al. Impact of having family history of psoriasis or psoriatic arthritis on psoriatic disease. Arthritis Care Res (Hoboken). 2020;72(1):63–8.
Gudjonsson JE, Karason A, Antonsdottir AA, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118(2):362–5.
Pina Vegas L, Sbidian E, Penso L, Claudepierre P. Epidemiologic study of patients with psoriatic arthritis in a real-world analysis: a cohort study of the French health insurance database. Rheumatology (Oxford). 2021;60(3):1243–51.
Madland TM, Apalset EM, Johannessen AE, Rossebo B, Brun JG. Prevalence, disease manifestations, and treatment of psoriatic arthritis in western Norway. J Rheumatol. 2005;32(10):1918–22.
Dubash S, Alabas OA, Michelena X, et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021.
Andracco R, Zampogna G, Parodi M, Paparo F, Cimmino MA. Dactylitis in gout. Ann Rheum Dis. 2010;69(1):316.
Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214(108390.
Yang Q, Qu L, Tian H, et al. Prevalence and characteristics of psoriatic arthritis in Chinese patients with psoriasis. J Eur Acad Dermatol Venereol. 2011;25(12):1409–14.
Pittam B, Gupta S, Harrison NL, et al. Prevalence of extra-articular manifestations in psoriatic arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2020;59(9):2199–206.
Charlton R, Green A, Shaddick G, et al. Risk of uveitis and inflammatory bowel disease in people with psoriatic arthritis: a population-based cohort study. Ann Rheum Dis. 2018;77(2):277–80.
Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151(11):1200–5.
Ward MM, Reveille JD, Learch TJ, Davis JC Jr, Weisman MH. Occupational physical activities and long-term functional and radiographic outcomes in patients with ankylosing spondylitis. Arthritis Rheum. 2008;59(6):822–32.
Gladman DD, Mease PJ, Choy EH, et al. Risk factors for radiographic progression in psoriatic arthritis: subanalysis of the randomized controlled trial ADEPT. Arthritis Res Ther. 2010;12(3):R113.
Chandran V, Tolusso DC, Cook RJ, Gladman DD. Risk factors for axial inflammatory arthritis in patients with psoriatic arthritis. J Rheumatol. 2010;37(4):809–15.
Hanly JG, Russell ML, Gladman DD. Psoriatic spondyloarthropathy: a long term prospective study. Ann Rheum Dis. 1988;47(5):386–93.
Jenkinson T, Armas J, Evison G, et al. The cervical spine in psoriatic arthritis: a clinical and radiological study. Br J Rheumatol. 1994;33(3):255–9.
Tsuruta N, Imafuku S, Narisawa Y. Hyperuricemia is an independent risk factor for psoriatic arthritis in psoriatic patients. J Dermatol. 2017;44(12):1349–52.
Gisondi P, Targher G, Cagalli A, Girolomoni G. Hyperuricemia in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2014;70(1):127–30.
Rahman P, Beaton M, Schentag CT, Gladman DD. Accuracy of self-reported family history in psoriatic arthritis. J Rheumatol. 2000;27(3):824–5.
Acknowledgements
Funding
Sponsorship for this study and the Rapid Service Fee were funded by the National Natural Science Foundation of China (U1704177 & 81871811).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors' Contributions
All authors contributed to the study conception and design, and revised the article critically for important intellectual content. All authors approved the final version to be published. Shan-Shan Li and Na Du: Data acquisition, statistical analysis and manuscript preparation, Shi-Hao He and Xu Liang: Data acquisition and manuscript revision. Tian-Fang Li: Study design, result interpretation and manuscript finalization.
Disclosures
Shan-Shan Li, Na Du, Shi-Hao He, Xu Liang, and Tian-Fang Li have nothing to disclose. They declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
The Ethics Committee of the First Affiliated Hospital of Zhengzhou University approved this study that was in accordance with the Helsinki Declaration of 1964, and its later amendments (KY-2021–00,926). All patients provided written informed consent.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, SS., Du, N., He, SH. et al. Exploring the Association Between History of Psoriasis (PSO) and Disease Activity in Patients with Psoriatic Arthritis (PsA). Rheumatol Ther 9, 1079–1090 (2022). https://doi.org/10.1007/s40744-022-00455-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00455-8