Abstract
Introduction
Belimumab is a recombinant human monoclonal antibody that binds to soluble B-lymphocyte stimulator and inhibits its biological activity. Since receiving approvals for the treatment of systemic lupus erythematosus (SLE), several observational studies have investigated the effectiveness of belimumab in the real-world setting. This study reports a systematic review and meta-analysis of the literature to evaluate the real-world effectiveness of belimumab for the treatment of SLE.
Methods
A literature search following PRISMA Guidelines and limited to studies in English was performed (2014−2020) to identify relevant studies reporting effectiveness outcomes of belimumab in patients with SLE. A modified version of the Newcastle–Ottawa Scale was used to assess study quality. Outcomes, including SLE Disease Activity Index (SLEDAI) score, prednisone-equivalent use, and SLE flare were pooled and analyzed using statistical aggregation methods.
Results
The literature search identified 514 articles for initial review. Of these, 17 articles were suitable for data extraction and summary. Baseline characteristics of patients in real-world studies were generally similar to those of relevant clinical trials, including age, sex, disease duration, SLEDAI score, and prednisone-equivalent use. Real-world use of belimumab was associated with reductions in SLEDAI score (mean baseline score to month 6: 10.1–4.4; 57% reduction), prednisone-equivalent dosing (mean baseline dose to month 6: 12.1 mg/day to 6.9 mg/day; 43% reduction), and flare frequency (12 months prior to belimumab to 12 months after belimumab: 1.15–0.39 mean flares per patient per year; 66% reduction). Long-term data (up to 2 years post-treatment initiation) for SLEDAI score and prednisone-equivalent dose indicated that improvements in both outcomes continue over time among patients remaining on therapy.
Conclusions
In the real-world setting, observed outcomes with belimumab for the treatment of SLE are consistent with those reported from randomized clinical trials. Improvements persist long-term for SLEDAI activity and prednisone-equivalent use with belimumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What is already known about this subject? |
Belimumab is a targeted human monoclonal antibody that is approved for use in patients with active systemic lupus erythematosus (SLE) and adult patients with active lupus nephritis. |
Although the efficacy and positive benefit–risk profile of belimumab has been demonstrated in several phase 3 trials, a number of real-world, observational studies have also investigated the effectiveness and safety of belimumab outside of a research setting. |
What does this study add? |
This systematic review and meta-analysis evaluated the real-world effectiveness of belimumab in SLE by using data from 17 relevant observational studies published between 2014 and 2020. |
Use of belimumab in real-world clinical practice was associated with reduced disease activity, corticosteroid use, and frequency of SLE flares; furthermore, long-term data indicated that improvements continue over time among patients remaining on therapy. |
How might this impact clinical practice or future developments? |
These data demonstrate that real-world belimumab use provides favorable outcomes consistent with those observed in randomized controlled trials, with improvements persisting over a 24-month period. |
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disease that can affect many different organ systems and has various clinical presentations that may mimic the signs and symptoms of other conditions [1, 2]. Symptoms can include fatigue, joint pain, fever, shortness of breath, chest pain, and a distinct facial butterfly rash, all of which can periodically worsen in flare episodes [1, 2]. Given the heterogeneous presentation of symptoms, the diagnosis of SLE is difficult, and is typically based on characteristic clinical findings of the joints, skin, kidneys, the central nervous system, and blood [1]. There is no cure for SLE, and treatment goals focus on decreasing disease activity, preventing new flares, and slowing organ damage progression [3].
A variety of medications can be used for SLE treatment, including non-steroidal anti-inflammatories, corticosteroids, anti-malarials, immunosuppressants, and biologic agents [3]. A serological characteristic of SLE is the presence of autoreactive B cells [4]; belimumab is a targeted recombinant human monoclonal antibody that inhibits the action of circulating B-lymphocyte stimulator. Belimumab is approved for use in patients aged ≥ 18 years with active, autoantibody-positive SLE in both intravenous (IV) and subcutaneous (SC) formulations [5]. Approval has also been granted in the USA, EU, Brazil, China, Japan, and Russia for IV use of belimumab in pediatric patients aged ≥ 5 years [5,6,7,8,9,10]. Further approval has been granted in the USA for the treatment of adult active lupus nephritis [11].
In the randomized clinical trial setting, several phase 3 trials (including BLISS-52 [NCT00424476], BLISS-76 [NCT00410384], BLISS-SC [NCT01484496], and the North East Asian study [NCT01345253]) have been conducted, demonstrating a positive benefit–risk profile with belimumab, including improved SLE Responder Index (SRI) rates and a reduced risk of flares [12,13,14,15]. Although such studies provide evidence of efficacy, the strict eligibility criteria of randomized trials can limit their generalizability to everyday clinical practice, as trial populations may differ from patients in a real-world setting [16]. Since receiving approval for use, a number of observational studies have investigated the real-world effectiveness of belimumab to assess the impact of belimumab outside of the research setting, providing further information on safety, effectiveness, and economic performance [16]. The objective of this systematic review and meta-analysis was to identify effectiveness (e.g., disease activity, standard of care treatment, and quality of life) data reported in published real-world (non-clinical trial) studies of belimumab in SLE, and estimate the overall effectiveness of belimumab in the real-world context.
Methods
Study Design
To identify articles published between 2014 and 2020 that report effectiveness outcomes of belimumab for the treatment of patients with SLE, a systematic literature review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [17]. Published observational (prospective or retrospective) cohort studies on this topic were identified via simultaneous searches of the MEDLINE and EMBASE databases through ProQuest, using the following database-specific terms: emb("systemic lupus erythematosus – drug therapy – belimumab") (LIMITS: Document type: Article + Review, English language) NOT (emb("phase 2 clinical trial") OR emb("phase 3 clinical trial") OR emb("phase 1 clinical trial"). This study is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
The searches were limited to include only those articles and reviews written in English, and to exclude conference abstracts, or articles that had no abstract. Two reviewers independently assessed the identified publications, and specific data of interest were abstracted from the studies that met the inclusion criteria; these are detailed in Table 1. Manual searches of the references cited in published systematic reviews were also conducted, and meta-analyses used to identify relevant studies that were not captured in the database search. Data extraction was performed by one researcher and then reviewed by a second for accuracy. Data for each outcome were collected at baseline and then at any available follow-up points from the respective study publications. The variables extracted from each study comprised study characteristics, patient characteristics, and study outcomes (Supplementary Material, Table S1). This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
The SLE Disease Activity Index (SLEDAI) measures disease activity across the 10 days prior and uses a weighted (by type of manifestation) checklist of 24 variables, comprising clinical and laboratory items [18]. A total SLEDAI score is then calculated, ranging from 0 to 105. The modified SLE Disease Activity Index—Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA-SLEDAI) and SLEDAI-2K were introduced to expand the scope of the SLEDAI [19, 20]. The SELENA-SLEDAI flare index comprises SELENA-SLEDAI score, changes in disease activity, medication changes, hospitalizations, and physician’s global assessment of disease [19].
A modified version of the Newcastle–Ottawa Scale was used to assess the quality of the observational studies included in the review. The scale was modified to remove the ‘comparability’ domain and ‘selection of the non-exposed cohort’ item, as this literature review was non-comparative; thus, the maximum score of the unaltered scale was reduced from 9 to 6. The Agency of Healthcare Research and Quality conversion of these scores was modified to the following: ‘Good’ (2 or 3 in selection domain AND 2 or 3 in outcome domain), ‘Fair’ (1 in selection domain AND 2 or 3 in outcome domain), and ‘Poor’ (0 in selection domain OR 0 or 1 in outcome domain).
Statistical Analyses
SLE disease activity (as assessed by SELENA-SLEDAI or SLEDAI-2 K [considered equivalent for the purpose of the analysis]), prednisone-equivalent oral corticosteroid use, and incidence of flares were the only outcomes of interest that provided sufficient data for quantitative pooling. All outcomes were reported for belimumab only (i.e., they were not comparative effectiveness measures) as a single numeric value plus standard error (SE).
Statistical aggregation methods used in the present study included inverse variance weighting and the DerSimonian and Laird method for fixed and random effects pooling, respectively, at 6 months or the closest available timepoint. In studies where standard deviation (SD) was not reported, it was imputed using the sample-size weighted average of the available SDs. Median data were used as a proxy for the mean where reported. Heterogeneity among outcomes was calculated using the Q statistic and associated I2 values, with values > 50% indicative of heterogeneity among studies. Sensitivity analyses were not conducted based on heterogeneity results, as the identified observational studies provided insufficient information to adequately assess the potential sources of heterogeneity. In studies reporting prednisone-equivalent dosage and SLEDAI outcomes at multiple time points up to 24 months, the time-varying effects in these outcomes between 6 and 24 months were assessed via linear regression using the OpenBUGS software program, using a Bayesian analysis framework with vague prior information.
Results
Characteristics of the Included Studies
Systematic review of the literature from MEDLINE/EMBASE records identified 514 articles, of which 39 full-text articles were suitable for review. Publications (n = 23) were excluded from the analyses for a variety of reasons, with the most common being a lack of belimumab specificity (n = 7) (Fig. 1). Two publications reported US database studies that did not evaluate SLE outcomes of interest, and another article was excluded because it described prevalent belimumab use (i.e., treatment was not newly initiated). Two studies were identified via the manual search. A total of 17 articles were selected as suitable for data extraction and summary (Fig. 1), comprising a mixture of prospective and retrospective studies.
The included studies were conducted in Brazil, Canada, Europe, and the USA, and the majority were restricted to adult patients with SLE (mean age, 37–47 years; Table 2). Where reported, disease duration and baseline SLE severity were similar between studies. Most of the studies included patients who were using oral corticosteroids at baseline, with a mean daily dose range (where reported) of prednisone-equivalent 9–30 mg/day. All studies evaluated belimumab 10 mg/kg IV, consistent with recommended dosage levels [5]. Newcastle–Ottawa Scale assessments determined that all studies identified for inclusion were of ‘good’ quality.
Outcomes
SLEDAI
Of the 13 studies reporting SLEDAI score data up to 6 months, the majority saw improvement in SLEDAI between 3 and 6 months (Fig. 2a). Mean baseline SLEDAI score was 10.1 (range, 8.0–12.7). Mean SLEDAI at 6 months was approximately 4.4, a reduction from baseline of 57%. Significant heterogeneity was observed among the 6-month SLEDAI data, with mean SLEDAI scores ranging from 1.1 to 6.0, with relatively large SDs (range, 1.2–4.1).
Those studies (n = 6, comprising a total of 888 patients) reporting longer-term mean SLEDAI score showed an apparent, but small, decline in disease activity after 6 months [21,22,23,24,25,26]. A linear regression model estimated that the monthly reduction in mean (standard error [SE]) SLEDAI was 0.08 (0.01; fixed-effect model) and 0.09 (0.05; random-effect model), which corresponds to a SLEDAI decrease of 1.44 over months 6–24.
Prednisone-equivalent use
Mean baseline prednisone-equivalent dose was 12.1 mg/day (range, 8.8–30.0 mg/day). Among the 15 publications reporting such data, observed reductions in prednisone-equivalent use occurred within the first 6 months and converged towards a mean dosage of 6.9 mg/day (mean dose range 5.3 to 9.3 mg/day) at 6 months (Fig. 2b). This represents a 43% reduction in mean prednisone-equivalent dose over the first 6 months. However, there was variation among studies with relatively large SEs being reported for prednisone-equivalent use at 6 months (range, 2.5–7.4 mg/day). There was a reduction in the proportion of prednisone-equivalent users between baseline and 6 months among the three studies that reported this measure (range of 2.1–72.3% reduction) [23, 27, 28].
Among studies reporting longer-term outcomes (n = 6, comprising a total of 888 patients [21,22,23,24,25,26]), there was a demonstrable but small monthly decline in mean prednisone-equivalent dosing after 6 months. A linear regression model estimated the monthly reduction in mean (SE) prednisone-equivalent dose to be 0.14 (0.02) mg/day with the fixed-effect model, and 0.15 (0.05) mg/day with the random-effect model. While these findings are not necessarily a clinically significant change per day, this represents a 2.6 mg/day reduction across the 18-month period between months 6 and 24 (an additional 38% reduction in mean dose from the estimated 6-month value of 6.9 mg/day).
Flare incidence
The proportion of patients with a flare incidence within 12 months of treatment initiation ranged from 10% [29] to 61% [21] across the ten studies reporting such data; however, the definition of flare was not consistent between studies. Three studies reported flare incidence in the 12- and 24-month periods before and after belimumab initiation using the SELENA-SLEDAI flare index (Table 3) [25, 26, 30]. The mean flare incidence per patient, per year in the 12 months prior to belimumab treatment was 1.15, while after 12 months of treatment the mean incidence decreased to 0.39, a reduction of 66%. Over the first 24 months after belimumab initiation, the overall mean number of flares per patient, per year was 0.30 for the fixed-effect model, and 0.31 for the random-effect model.
Additional outcomes
Clinical Response
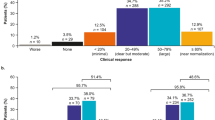
Six studies provided clinical response data [23, 24, 28, 31,32,33]. Of the studies reporting physician assessment of clinical improvement, 78–88% reported at least a 20% improvement at 6 months [23, 28]. The proportion of patients achieving ≥ 50% improvement in clinical response ranged from 42% [28] to 51% [31].
Global Assessment Score
Of the five studies that reported global assessment score, only two used the same measure, Schwarting [28] and Touma [32], both using a scale from 0 to 100. Patients in the Schwarting study (n = 8) reported a mean reduction in global assessment of disease at 6 months of 50, whereas patients in the Touma study (n = 22) reported a mean change of only 0.5 [28, 32]. Physicians participating in the Schwarting study reported a mean reduction in global assessment at 6 months of 24.8 [28], whereas physicians in the Touma study reported a mean reduction of 18.4 [32].
Quality of Life
Quality-of-life measures were provided by only two studies. Of these, Parodis [34] reported increases over 24 months in the 36-item Short Form Health Survey (SF-36) physical component score and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue instruments, but little change in the SF-36 mental component score and the EuroQoL 5 Dimension instruments. Scheinberg [8] reported a mean (SD) FACIT-Fatigue score at baseline of 37.6 (3.8), which showed improvement to 48.8 (3.3) at 6 months.
Disease severity
Disease severity outcomes were reported in three studies. Two of these reported improvements in Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) score; this decreased by 9 months and remained low through to 24 months [25, 26]. Collins (2016) reported improvements in physician’s assessment of disease severity: at baseline, 2.2, 77.6, and 20.2% of patients were assessed as having mild, moderate, or severe disease, respectively, which improved to 65.0, 33.1, and 1.9% at 24 months, respectively.
Discussion
Consistent with the efficacy findings of randomized controlled trials, the real-world results of key clinical outcomes demonstrate the benefits of treating SLE with belimumab. Reductions in SLEDAI score, prednisone-equivalent dosing, and flare frequency were all apparent within 6–12 months of belimumab treatment across the 17 real-world observational studies evaluated in this analysis.
The baseline patient characteristics extracted from the observational studies were similar to those of patients in the phase 3 belimumab IV clinical trials [12,13,14]. In these clinical trial populations, nearly all patients were female (range of 93–97% across the three studies) with a mean age range of 32–40 years [12,13,14]. The present review identified a mean age range of 37–47 years across the observational studies, while females accounted for over 80% of the population in 15 of the 17 studies included. Mean disease duration was generally lower in the clinical trials than in the observational studies (5–7.9 years for clinical trials, vs. 9–15.7 years for the observational studies). Mean SELENA-SLEDAI scores within clinical trials ranged between 9.5 and 10, versus 8 and 12.7 for the observational studies. The baseline prednisone dose of 9–30 mg/day for the observational studies was also similar to the values reported in clinical trials [12,13,14]. The observational studies included a more diverse patient population than those selected for randomized clinical trial inclusion, and this can result in heterogeneity among studies. However, this variation is representative of the real-world patient cohort and is more generalizable to the population with SLE who receive belimumab in clinical practice.
In phase 3 randomized clinical trials investigating the efficacy of belimumab, significantly higher SRI rates were reported with belimumab than placebo at week 52 [12,13,14]. The observational studies included here also saw a reduction in mean SELENA-SLEDAI score from 10.1 to 4.4 after 6 months, fulfilling the ≥ 4-point SELENA-SLEDAI reduction required for an SRI response [35]. This suggests that belimumab can achieve clinically meaningful reductions in disease activity in a real-world setting, as measured by the SELENA-SLEDAI.
A reduction or discontinuation of corticosteroid dose has been demonstrated with belimumab in clinical trials, which is likely of benefit to the patient given that there is little randomized control trial evidence to support a positive effect of corticosteroids on suppression of inflammation in SLE [36]. Corticosteroid use is also not without harm; high doses of corticosteroids in patients with SLE are associated with an increased risk of mortality and organ damage [37, 38]. After 6 months of belimumab treatment, the real-world observational data showed a reduction from mean prednisone-equivalent baseline dose of 12.1 mg/day to an average dose of approximately 7 mg/day; this is below the common clinical ‘low dose’ threshold of 7.5 mg/day, and supports the ‘steroid-sparing’ effect of belimumab treatment that was identified in phase 3 clinical trials [12].
Longer-term data (6–24 months) reported for SLEDAI score and for prednisone-equivalent dose indicate that reductions were maintained through to 24 months among patients remaining on therapy, although the magnitude of the monthly reduction was smaller than during the first 6 months. This is consistent with the findings of long-term open-label continuation studies, which reported sustained reduced corticosteroid use with belimumab [39, 40].
The majority of patients with SLE experience symptom exacerbations, or flares, throughout the course of their life, which may impact on both short- and long-term clinical outcomes; thus, the prevention of flares is an important target in the management of the disease [41]. In randomized clinical trials, belimumab was found to reduce the incidence and severity of flares; in BLISS-52, the risk of flare was significantly reduced and median time to first disease flare was significantly increased with belimumab versus placebo [12]. The observational studies evaluated here had varying definitions of flare, making a direct comparison of the results difficult. However, a meta-analysis was performed on data from three studies which reported standardized SELENA-SLEDAI flare index data to determine the overall mean number of flares per patient, per year, over the first 12 and 24 months following belimumab treatment. Mean flare rates for the fixed-effects model were 39 and 30% for the first 12 and 24 months, respectively; for the random-effects model, these values were 38 and 31%, respectively. BLISS-52 and BLISS-76 randomized clinical trials reported varying results for the SELENA-SLEDAI flare index, ranging from 15.7% at 52 weeks, and 71% from 24 to 76 weeks, respectively, so this small sample size meta-analysis is within the range of these findings [12, 13].
Additional evidence of improved outcomes associated with belimumab were reported in only a few real-world studies, ranging from just two with mention of Quality of Life and Global Assessment Score, to three studies reporting disease severity, and six studies detailing clinical response. Therefore, data for these measures were not meta-analyzed; however, the results summarized from this small sample showed either no worsening, or a general improvement following belimumab treatment, which is consistent with the clinical trial results [12,13,14].
A strength of this study was the quality of the included studies; the Newcastle–Ottawa Scale indicated that the quality of all of the studies included in this analysis was ‘good’. As is common with syntheses of observational data, only limited information exists to evaluate comparability of the identified studies. Statistical heterogeneity was present among nearly all outcomes analyzed, but the potential sources of this heterogeneity could not be explored, as only partial information was available. The sample size of the 17 studies ranged between 10 and 501 patients; this variation reduces the ability to compare individual studies, and may have contributed to the heterogeneity observed between studies. The present study identified trials published up to 2020, excluding potentially relevant studies published later. One publication from July 2020 reported results from the 24-month Argentina OBSErve study of 81 patients with SLE showing that belimumab treatment leads to clinical improvement and reductions in corticosteroid dose, consistent with the findings presented here [42]. Similar results were also reported in a recently published post hoc pooled analysis of real-world data from six OBSErve studies [43], most of which were included in our evaluation.
Conclusions
In the real-world setting, outcomes with belimumab for the treatment of SLE are consistent with those observed in randomized clinical trials. Belimumab is associated with improvements in SLEDAI score, reductions in daily prednisone-equivalent dose, and flare incidence. Reductions in prednisone-equivalent use and SLEDAI activity with belimumab were maintained over a 24-month period.
References
Kuhn A, Bonsmann G, Anders HJ, Herzer P, Tenbrock K, Schneider M. The diagnosis and treatment of systemic lupus erythematosus. Dtsch Arztebl Int. 2015;112(25):423–32.
Mayo Clinic Staff. Lupus: Symptoms and causes 2021, Jan 27 [Available from: https://www.mayoclinic.org/diseases-conditions/lupus/symptoms-causes/syc-20365789.
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736.
Blair HA, Duggan ST. Belimumab: a review in systemic lupus erythematosus. Drugs. 2018;78(3):355–66.
GlaxoSmithKline. Benlysta (belimumab) prescribing information. 2021. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Benlysta/pdf/BENLYSTA-PI-MG-IFU-COMBINED.PDF.
GlaxoSmithKline. Benlysta summary of product characteristics. 2020. Available at https://www.ema.europa.eu/en/documents/product-information/benlysta-epar-product-information_en.pdf.
Pharmaceuticals and Medical Devices Agency (PMDA). Benlysta report on deliberation results. Available at https://www.pmda.go.jp/files/000235894.pdf.
Scheinberg M, Golmia R. Real life experience on the effect of Belimumab in patients with active systemic lupus. Springerplus. 2014;3:758.
GlaxoSmithKline. The world’s first biologic therapy for patients with SLE, Benlysta receives NMPA approval for children with lupus aged five years and above. 2020. Available at https://www.gsk-china.com/en-gb/media/press-releases/2020/the-world-s-first-biologic-therapy-for-patients-with-sle-benlysta-receives-nmpa-approval-for-children-with-lupus-aged-five-years-and-above/.
State Register of Medicines. Belimumab Registration Certificate. 2021. Available at http://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=bcda2f72-d35a-4662-adb3-f06a3a9e4e7c&t=.
GlaxoSmithKline. Benlysta prescribing information update. 2020. Available at https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Benlysta/pdf/BENLYSTA-PI-MG-IFU.PDF#nameddest=MG.
Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–31.
Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–30.
Chen D, Lao M, Zhang J, Zhan Y, Li W, Cai X, et al. Fetal and maternal outcomes of planned pregnancy in patients with systemic lupus erythematosus: a retrospective multicenter study. J Immunol Res. 2018;2018:2413637.
Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a 52-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69(5):1016–27.
Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–74.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–40.
Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–8.
Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
Andreoli L, Reggia R, Pea L, Frassi M, Zanola A, Cartella S, et al. Belimumab for the treatment of refractory systemic lupus erythematosus: real-life experience in the first year of use in 18 Italian patients. Isr Med Assoc J. 2014;16(10):651–3.
Anjo C, Mascaro JM Jr, Espinosa G, Cervera R. Effectiveness and safety of belimumab in patients with systemic lupus erythematosus in a real-world setting. Scand J Rheumatol. 2019;48(6):469–73.
Collins CE, Dall’Era M, Kan H, Macahilig C, Molta C, Koscielny V, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med. 2016;3(1): e000118.
Fanouriakis A, Adamichou C, Koutsoviti S, Panopoulos S, Staveri C, Klagou A, et al. Low disease activity-irrespective of serologic status at baseline-associated with reduction of corticosteroid dose and number of flares in patients with systemic lupus erythematosus treated with belimumab: a real-life observational study. Semin Arthritis Rheum. 2018;48(3):467–74.
Iaccarino L, Andreoli L, Bocci EB, Bortoluzzi A, Ceccarelli F, Conti F, et al. Clinical predictors of response and discontinuation of belimumab in patients with systemic lupus erythematosus in real life setting. Results of a large, multicentric, nationwide study. J Autoimmun. 2018;86:1–8.
Iaccarino L, Bettio S, Reggia R, Zen M, Frassi M, Andreoli L, et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthrit Care Res (Hobok). 2017;69(1):115–23.
Sthoeger Z, Lorber M, Tal Y, Toubi E, Amital H, Kivity S, et al. Anti-BLyS treatment of 36 Israeli systemic lupus erythematosus patients. Isr Med Assoc J. 2017;19(1):44–8.
Schwarting A, Schroeder JO, Alexander T, Schmalzing M, Fiehn C, Specker C, et al. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: results from the OBSErve Germany study. Rheumatol Ther. 2016;3(2):271–90.
Scheinberg M, de Melo FF, Bueno AN, Costa CM, de Azevedo Bahr ML, Reis ER. Belimumab for the treatment of corticosteroid-dependent systemic lupus erythematosus: from clinical trials to real-life experience after 1 year of use in 48 Brazilian patients. Clin Rheumatol. 2016;35(7):1719–23.
Gatto M, Saccon F, Zen M, Regola F, Fredi M, Andreoli L, et al. Early disease and low baseline damage as predictors of response to belimumab in patients with systemic lupus erythematosus in a real-life setting. Arthrit Rheumatol. 2020;72(8):1314–24.
Hui-Yuen JS, Reddy A, Taylor J, Li X, Eichenfield AH, Bermudez LM, et al. Safety and efficacy of belimumab to treat systemic lupus erythematosus in academic clinical practices. J Rheumatol. 2015;42(12):2288–95.
Touma Z, Sayani A, Pineau CA, Fortin I, Matsos M, Ecker GA, et al. Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada Study. Rheumatol Int. 2017;37(6):865–73.
Yoneva T, Zdravkova Y, Kalinova D, Ivanova-Todorova E, Kyurkchiev D, Rashkov R. Efficacy and safety of belimumab in patients with systemic lupus erythematosus—a 1-year clinical experience. Revmatol (Bulgaria). 2014;22((3–4)):38–50.
Parodis I, Lopez Benavides AH, Zickert A, Pettersson S, Moller S, Welin Henriksson E, et al. The impact of belimumab and rituximab on health-related quality of life in patients with systemic lupus erythematosus. Arthrit Care Res (Hobok). 2019;71(6):811–21.
Furie RA, Petri MA, Wallace DJ, Ginzler EM, Merrill JT, Stohl W, et al. Novel evidence-based systemic lupus erythematosus responder index. Arthrit Rheum. 2009;61(9):1143–51.
Apostolopoulos D, Morand EF. It hasn’t gone away: the problem of glucocorticoid use in lupus remains. Rheumatology. 2016;56(suppl_1):i114–i22.
Al Sawah S, Zhang X, Zhu B, Magder LS, Foster SA, Iikuni N, et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins Lupus Cohort. Lupus Sci Med. 2015;2(1): e000066.
Sheane BJ, Gladman DD, Su J, Urowitz MB. Disease outcomes in glucocorticosteroid-naive patients with systemic lupus erythematosus. Arthrit Care Res (Hobok). 2017;69(2):252–6.
Wallace DJ, Ginzler EM, Merrill JT, Furie RA, Stohl W, Chatham WW, et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthrit Rheumatol. 2019;71(7):1125–34.
Furie RA, Wallace DJ, Aranow C, Fettiplace J, Wilson B, Mistry P, et al. Long-term safety and efficacy of belimumab in patients with systemic lupus erythematosus: a continuation of a 76-week Phase III parent study in the US. Arthritis Rheumatol. 2018;70(6):868–77.
Adamichou C, Bertsias G. Flares in systemic lupus erythematosus: diagnosis, risk factors and preventive strategies. Mediterr J Rheumatol. 2017;28(1):4–12.
Babini A, Cappuccio AM, Caprarulo C, Casado G, Eimon A, Figueredo H, et al. Evaluation of belimumab treatment in patients with systemic lupus erythematosus in a clinical practice setting: Results from a 24-month OBSErve study in Argentina. Lupus. 2020;29(11):1385–96.
Collins CE, Cortes-Hernández J, Garcia MA, von Kempis J, Schwarting A, Touma Z, et al. Real-world effectiveness of belimumab in the treatment of systemic lupus erythematosus: pooled analysis of multi-country data from the OBSErve studies. Rheumatol Ther. 2020;7(4):949–65.
Prete M, Leone P, Frassanito MA, Desantis V, Marasco C, Cicco S, et al. Belimumab restores Treg/Th17 balance in patients with refractory systemic lupus erythematosus. Lupus. 2018;27(12):1926–35.
von Kempis J, Duetsch S, Reuschling N, Villiger R, Villiger PM, Vallelian F, et al. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Med Wkly. 2019;149: w20022.
Acknowledgements
The authors thank Benjamin Wu for study assistance and support.
Funding
This study (GSK study 213063) and the journal’s Rapid Service fee were funded by GlaxoSmithKline.
Medical Writing Assistance
Medical writing support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing, and referencing) was provided by Liam Campbell, PhD, of Fishawack Indicia Ltd., part of Fishawack Health, and was funded by GlaxoSmithKline (GSK).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the conception and design of the study. Sonya J. Snedecor and Sakina Nanji contributed to the acquisition of data. All authors contributed to the data analysis or interpretation.
Disclosures
Sonya J. Snedecor and Sakina Nanji are employees of OPEN Health, who received funding from GSK to conduct this study. Shirley P. Huang was an employee of GSK at the time of the study and held stocks/shares in GSK. Emily Lloyd and Christopher F. Bell are employees of GSK and hold stocks/shares in GSK.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files. Information on GSK’s data sharing commitments and requesting access can be found at www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Huang, S.P., Snedecor, S.J., Nanji, S. et al. Real-World Effectiveness of Belimumab in Systemic Lupus Erythematosus: A Systematic Literature Review. Rheumatol Ther 9, 975–991 (2022). https://doi.org/10.1007/s40744-022-00454-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00454-9