Abstract
Introduction
Osteoporosis (OP) is one of the major comorbidities of rheumatoid arthritis (RA). Recent studies have shown that immune cells modulate bone health and regulate bone remodeling. However, the alterations of lymphocyte subsets in RA patients with OP are unclear. Here, we assessed the absolute numbers and proportions of the subsets in RA sufferers with OP and investigated the clinical significance.
Methods
A total of 777 RA patients and 117 gender- and age-matched healthy controls (HCs) were enrolled in this study. Patients were divided into RA-non-OP and RA-OP group according to their bone mineral density (BMD) and the history of fragility fracture. Peripheral lymphocyte subsets of participants were assessed by flow cytometry.
Results
Among 220 (28.31%) RA-OP patients, there were higher levels of erythrocyte sedimentation rate (ESR) (P = 0.011), C-reactive protein (CRP) (P = 0.028), rheumatoid factor (RF) (P = 0.013) and anti-cyclic citrullinated peptide antibody (ACPA) (P = 0.010), while red blood cells (RBC) (P = 0.039) were lower than those in RA-non-OP group. Compared with those of HCs and RA-non-OP group, the level of circulating Th17 cells in RA-OP patients was significantly increased (P < 0.05), while those of Tregs decreased (P < 0.01), leading to a higher ratio of Th17/Treg (P < 0.01). Notably, the level of B cells in both RA-non-OP and RA-OP group was reduced, this alteration was more obvious in patients with OP (P < 0.05).
Conclusions
Immune disorders characterized by peripheral Th17/Treg imbalance and reduced B cells may contribute directly or indirectly to OP in RA, and this deserves more clinical attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Osteoporosis (OP) is one of the major complications of rheumatoid arthritis (RA), which results in increased bone fragility and fracture risk, leading to the prolongation of the disease course and the reduced quality of life. |
Not only do immune disorders participate in the development of RA disease, but they also modulate bone health and regulate bone remodeling. |
We intended to investigate the levels of peripheral lymphocyte subsets in RA patients complicated with OP and expound the possible immunologic mechanisms of high incidence of osteoporosis in RA patients. |
What was learned from the study? |
Th17/Treg imbalance and reduced B cells were more significant in RA-OP patients than RA-non-OP patients, which provides clinical evidence for early prevention and treatment of OP in RA patients. |
It is necessary to focus on the level of peripheral lymphocyte subsets in future clinical practice. |
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by painful and swollen joints that may lead to irreversible damage [1]. Osteoporosis (OP) is one of the major complications of RA that results from a number of complex pathophysiologic processes such as systemic inflammation and circulating autoantibodies [2]. The frequencies of OP in RA patients are reported to be approximately 30% in the hip and lumbar spine [3]. Alteration of bone architecture results in increased bone fragility and fracture risk, leading to the prolongation of the disease course and reduced quality of life.
Recently, we have reported that a relative increase of T helper 17 cells (Th17) due to the absolute reduction of regulatory T cells (Tregs) causes an imbalance of Th17/Treg in RA patients [4]. Th17 cells have been shown to contribute to the bone destruction in arthritis by up-regulating receptor activators of nuclear factor kappa-Β ligand (RANKL) on synovial fibroblasts as well as inducing local inflammation [5]. In contrast, Tregs inhibit osteoclastogenesis through anti-inflammatory cytokines such as IL-10 and cytotoxic T lymphocyte antigen 4 (CTLA4) signaling [6, 7]. Consequently, T-cell subsets either directly or indirectly regulate bone remodeling by means of releasing various cytokines and conducting abnormal signal pathways. Similarly, B cells are a major source of osteoprotegerin (OPG), an inhibitor of the key osteoclastic factor, which binds to RANKL hampering RANK–RANKL interaction [8]. However, the alterations of lymphocyte subsets in RA patients with OP are unclear.
This study aims to investigate the levels of peripheral lymphocyte subsets in RA patients complicated with OP and expound the immunologic mechanisms of high incidence of osteoporosis in patients with RA, which provides clinical evidence for early prevention and treatment of OP.
Methods
Participants
A total of 777 patients with RA (222 males and 555 females, with a mean age of 60.17 ± 10.58 years old) according to the criteria of the American College of Rheumatology [9] were included in the study. At the same time, 117 gender- and age-matched (42 males and 75 females, with a mean age of 59.62 ± 10.21 years old) healthy controls (HCs) were also enrolled. All participators donated their peripheral blood (PB) samples that were used for the absolute number and proportion of lymphocyte subsets by modified flow cytometry. Patients were divided into a RA-non-OP group (n = 557) and a RA-OP group (n = 220) according to their bone mineral density (BMD) of lumbar or bilateral hip and the history of fragility fracture for further comparation of the general clinical data as well as the status of peripheral lymphocyte subsets among these participants [10]. All patients were hospitalized in the Department of Rheumatology, Second Hospital of Shanxi Medical University, from December 2015 to June 2019 and were mainly previously treated with glucocorticoid, DMARDs, biological agents, and traditional Chinese medicine. None of the selected patients had serious heart, liver, kidney, thyroid, or parathyroid diseases, Cushing's syndrome, serious infection, tumor, long-term use of estrogen, androgen, and anticoagulant, gonadectomy, or gastrointestinal surgery. In addition, HCs had no autoimmune diseases, osteoporosis, or obvious organic damage after routine physical examination, immunological tests, and BMD. Our study was approved by the Medical Ethics Committee of the Second Hospital of Shanxi Medical University (2016-KY-007). We obtained the participants’ written informed consent to publish the material.
Flow Cytometric Analysis
Reagent and Consumables
Phorbol myristate acetate (PMA), Ionomycin, fetal bovine serum (FBS) and RPMI 1640 medium were purchased from Sigma-Aldrich, USA. Anti-CD3FITC/CD8PE/CD45PerCP/CD4APC, anti-CD3FITC/CD16 + PECD56PE/CD45PerCP/CD19APC, anti-CD4-FITC, anti-IFN-γ-PE, anti-IL-4-PE, anti-IL-17-PE, anti-CD25-PE, anti-FOXP3-FITC antibodies, absolute count Trucount tubes, and GolgiStop were obtained from Becton–Dickinson (BD), USA.
Analysis of T, B, NK, CD4+T and CD8+T Cells in PB
For analysis of T, B, NK, CD4+T, and CD8+T cells, 50 µl of EDTA-anticoagulated venous blood was reversely pipetting into two Trucount tubes (A and B), respectively, without touching the standard beads in the bottom, which may eliminate the variation produced by adding the beads manually. Then 20 μl of anti-CD3FITC/CD8PE/CD45PerCP/CD4APC antibodies and anti-CD3FITC/CD16+PECD56PE/CD45PerCP/CD19APC antibodies were added into A and B tubes, respectively, followed by vortex stirring and kept at room temperature for 15 min. Finally, cells were collected by flow cytometry and detected by MultiSET software.
Analysis of Th1, Th2, Th17, and CD4Tregs in PB
For analysis of Th1, Th2, and Th17 cells, added 10 μl Ionomycin (final concentration 750 ng/ml), 10 μl phorbol myristate acetate (PMA)(final concentration 30 ng/ml) and 1 μl GolgiStop into 80 μl heparin-anticoagulated venous blood to stimulate cells for 5 h. The cells are divided into tube A and B (A is used for detecting Th1 and Th2 cells and B is for Th17 cells). Then cells were labeled by anti-CD4 antibody, followed by 1 ml freshly prepared Fixation/Permeabilization solution and incubated at 4 °C for 30 min in the darkness. Finally, the anti-IFN-γ-PE (for Th1) and anti-IL-4-PE (for Th2) were added into tube A, and anti-IL-17-PE (for Th17) was added into tube B, which were incubated for 30 min at room temperature. As for CD4Tregs, another 80 μl venous blood was added stimulants mentioned above and the surface labeled with anti-CD4-FITC and anti-CD25-PE, followed by 1 ml Fixation/Permeabilization to rupture the cytomembrane and anti-FOXP3-FITC antibody to stain intracellularly. The relative percentages were analyzed by CellQuest software. The computational formula for the absolute number of each subgroup is the percentage of each subsets × the total number of CD4+T cells (cells/μl).
Bone Mineral Density Measure
Dual-energy X-ray absorptiometry (DXA) was used to evaluate the bone mineral density of lumbar spine from L2 to L4 and the either hip (total hip and femoral neck) (osteopenia was defined as a bone mineral density t-score from − 1 to − 2.5, and osteoporosis was defined as a t-score below − 2.5, and normal mass above − 1). All procedures were performed according to the manufacturer’s standardized protocols.
Statistical Analysis
The collected data were examined using SPSS 23.0. All values were represented as the mean ± standard deviation (\(\overline{x} \pm s\)). In the first stage, the clinical characteristics like age, blood routine, hepatic and renal function, ESR and CRP of the RA-non-OP and RA-OP groups were compared using independent-samples t test, whereas parameters like gender and autoantibodies using Chi-square test. In the second stage, the one-way analysis of variance was used for the calculations of the absolute number and proportion of peripheral lymphocyte subsets among HC, RA-non-OP, and RA-OP group. All P values reported herein are two-tailed and P value < 0.05 was taken as statistically significant.
Results
Comparison of Clinical Characteristics Between RA-Non-OP and RA-OP Groups
In RA patients complicated with OP, 56 (25.45%) had old fractures, including 30 spinal, six rib, five pubis and ischium, three femoral neck, three ankle, two phalangeal, two tibiofibular, one radius, one wrist, one ulnar, one the femoral head and one intrafemoral epicondylar fractures. There was no significant difference in gender or age between 557 RA-non-OP patients (401 females and 156 males with a mean age of 60.20 ± 10.58 years old) and 220 RA-OP sufferers (154 females and 66 males with a mean age of 60.10 ± 10.60 years old) (P > 0.05) (Table 1). Compared with RA-non-OP, the patients with OP had a higher ESR (51.35 ± 37.35 vs. 43.87 ± 33.80 mm/h, P = 0.011) and CRP (32.63 ± 61.58 vs. 24.69 ± 33.86 mg/l, P = 0.028) (Fig. 1). Moreover, OP patients had higher autoantibodies such as RF (P = 0.013) and ACPA (P = 0.010) (Fig. 2). Besides, patients suffered OP have lower levels of RBC (4.07 ± 0.58 vs. 4.17 ± 0.54 × 1012/l, P = 0.039) (Table 1). There were no significant differences in other recorded autoantibodies, the count of white blood cell and platelet as well as hepatorenal function between RA-non-OP and RA-OP group (Table 1).
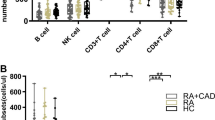
Differences in Absolute Numbers of Peripheral Lymphocyte Subsets Among HC, RA-non-OP, and RA-OP Patients
As shown in Fig. 3, there were no statistical differences in the absolute numbers of T, CD8+ T, Th1 and Th2 cells among HC, RA-non-OP, and RA-OP groups (P > 0.05). Compared with HC, the absolute number of B cells in RA-non-OP patients was slightly decreased and Th17 increased, but the differences were not statistically significant (P > 0.05). Interestingly, the values of B cells were further decreased (178.75 ± 134.70 vs. 198.78 ± 121.79 cells/µl; P = 0.038) and Th17 increased (8.40 ± 7.73 vs. 7.33 ± 5.69 cells/µl; P = 0.031) in RA-OP group (Fig. 3). In addition, there were distinct decreases in the number of NK (255.13 ± 178.81 vs. 304.42 ± 144.91 cells/µl, P = 0.004) and Treg cells (30.42 ± 18.95 vs. 34.25 ± 16.22 cells/µl, P = 0.036) in the RA-non-OP sufferers compared to HCs. Notably, the decreases were more obvious in patients with OP (236.27 ± 149.88 vs. 304.42 ± 144.91 cells/µl, P < 0.001; 26.44 ± 16.28 vs. 34.25 ± 16.22, P < 0.001), of which Treg was statistically significant compared to RA-non-OP patients (26.44 ± 16.28 vs. 30.42 ± 18.95, P = 0.006) (Fig. 3 and Supplementary Material).
Differences in Proportions of Peripheral Lymphocyte Subsets Among HC, RA-non-OP, and RA-OP Groups
There were no statistical differences in the proportions of NK, CD8+ T cells among HC, RA-non-OP, and RA-OP groups (P > 0.05) (Fig. 4). Compared with RA-non-OP group, RA-OP patients had a lower percentage of B cells (10.82 ± 5.96 vs. 11.85 ± 5.49%, P = 0.018) but higher Th17 cells (1.34 ± 1.42 vs. 1.10 ± 0.78%, P = 0.003) (Fig. 4). Also, there was a distinct decrease in the proportion of Treg cells (4.44 ± 2.09 vs. 6.56 ± 5.90%, P < 0.001) in RA-non-OP group compared to HCs and the alteration was more significant in RA-OP patients (4.02 ± 2.11 vs. 6.56 ± 5.90%, P < 0.001) (Fig. 4 and Supplementary Material).
Further reduction of Treg cells and rise of Th17 cells led to an increased ratio of Th17/Treg in RA-non-OP (0.31 ± 0.36 vs. 0.20 ± 0.15, P = 0.044) and RA-OP group (0.46 ± 0.92 vs. 0.20 ± 0.15, P < 0.001). It is also clear that the Th17/Treg ratio in RA-OP group was statistically higher than that in RA-non-OP group (0.46 ± 0.92 vs. 0.31 ± 0.36, P = 0.001) (Fig. 5 and Supplementary Material).
Discussion
RA is the representation of destructive arthritis with bone loss at sites of articular and peri-articular inflammation. Three forms of bone loss have been identified in patients with inflammatory rheumatic diseases: localized bone loss with erosion, periarticular osteopenia, and generalized bone loss [11]. Secondary systemic osteopenia or osteoporosis involving the axial and appendicular skeleton remotes from local inflammation of synovium, which is an important complication in rheumatosis [12]. The prevalence of osteoporosis in RA patients is increased about twofold compared with the general population and is responsible for a risk of both vertebral and non-vertebral fractures [13]. Our study revealed that 33.15% of RA patients suffered OP and 25.45% of them had a history of spinal or appendicular bone fracture, which seriously affected their quality of life and increased health care costs. Therefore, it is particularly urgent to explore the possible mechanism of secondary osteoporosis and prevent or intervene in the early stage of the primary disease.
The research showed that RA patients with OP had higher levels of inflammatory indicators and autoantibodies such as RF and ACPA as well as lower numbers of RBC. Inflammatory parameters like ESR and CRP were used to reveal the activity of the disease. There are several studies showing that the decrease in the periarticular and axial bone mass is correlated with disease activity in RA patients [14, 15]. In fact, patients with positive either RF and/or ACPA in blood have more severe clinical features and aggressive damage and even increase the mortality rate [16]. The immune complexes formed by ACPA and RF enhance inflammatory and destructive response. Recent evidence sustained the hypothesis of a significant influence produced by ACPA on osteoclasts that was activated depending on IL-8, which demonstrated an extraordinary susceptibility of periarticular bone loss in RA patients who were ACPA positive [17]. In addition, the detrimental role of ACPA on bone loss was even several years before the onset of arthritis, even at a systemic level [18]. ACPA was associated with systemic bone loss, with a titer-dependence on BMD [19]. In RA patients, impaired iron homeostasis and suppressive effects on erythropoiesis due to proinflammatory cytokines such as IL-6 and TNF-α may lead to oligocythemia [20]. And low RBC levels reduce the amount of oxygen in the blood, which in turn increase oxidative stress and cell acidification, affecting bone formation and remodeling [21]. Furthermore, the decrease of RBC causes the decline of muscle strength, which weakens traction and stimulation on bone [22]. In consequence, ESR, CRP, RF, ACPA, and RBC can be used for early detection and as targets in the prevention from OP in RA sufferers.
Meanwhile, we found that an imbalance in the immune system in RA may further result in the occurrence of OP by comparing the peripheral lymphocyte subsets among healthy controls, RA-non-OP group and RA-OP group. Osteoblasts and osteoclasts play a major role in bone remodeling and any imbalance between them causes various metabolic bone diseases [7]. However, for the past few years many researchers have confirmed that immune cells can interact with osteoclasts and osteoblasts to regulate bone formation and resorption and that receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) act as a bridge between immune system and bone system [6].
That Tregs play a significant role in the maintenance of immunological self-tolerance and modulation of immune responses has been verified in numerous studies, especially in autoimmune diseases such as RA [23]. Our study did point out that significantly decreased quantity and proportion of Tregs were identified in the RA patients compared to control subjects and even less in RA sufferers complicated with OP. Treg cells inhibit the production of osteoclasts by preventing the production of RANKL and M-CSF, leading to an increase in bone mass [24]. Studies have shown that the main mechanisms through which Treg cells affect bone formation including direct cell–cell contact and anti-inflammatory cytokine mechanisms [25]. Tregs regulate osteoclastogenesis by secreting inhibitory cytokines such as IL-10 and IL-35 and additionally via CTLA4 signaling, which binds with CD80/CD86 expressed on mononuclear osteoclast cells thereby resulting in an activation of indoleamine-2,3-dioxygenase (IDO) that increase the degradation of tryptophan into kynurenine and further accelerating the apoptosis of osteoclast precursor cells [25, 26]. Binding of TGF-β to its receptor on the cells’ surface accelerates osteoblasts generating through the Smad protein and simultaneously induce mesenchymal stem to differentiate into osteoblasts [27, 28]. Treg cells upregulate Wnt10b production by CD8+T cells during the intermittent supplementation with the butyrate, which can activate Wnt signaling that promotes proliferation and differentiation of osteoblasts and inhibits their programmed death [29].
We also found that Th17 cells and the ratio of Th17/Treg in RA-OP patients were significantly increased compared to RA-non-OP group, which suggested that Th17 cells may be also responsible for initiating and stimulating bone resorption. In fact, much clinical evidence showed the number of Th17 cells in the blood and surrounding tissues of OP patients is several folds higher than that in the OP-free population [30]. To summarize, Th17 cells influence bone metabolism in two ways. On the one hand, the surface of Th17 cells express high levels of RANKL, which binds to RANK expressed on osteoclast precursor cells, promoting the differentiation of precursor cells into osteoclasts to accelerate bone absorption. On the other hand, IL-17 secreted by Th17 cells directly enhances the expression of RANKL in osteoclastogenesis-supporting cells such as osteoblasts and synovial fibroblasts and also promotes macrophages to generate plenty of inflammatory cytokines such as IL-1, IL-6, and TNF-α, which indirectly accelerates the expression of RANKL in supporting cells, potentiating the binding of RANKL to RANK on the surface of osteoclast precursor cells [7, 31]. Considering these influence of Tregs and Th17 cells on bone remodeling, we draw a conclusion that Th17 cells accelerate bone resorption, whereas Tregs inhibit. Thus, regulating the balance of immune environment, especially the level of Th17 and Treg cells may be one of the new approaches for the treatment of the systematic osteonosus, and even early and timely intervention in RA disease can prevent further development of OP.
Moreover, our data confirmed that the continuous decline of B cells may be also involved in the occurrence of OP in RA sufferers. B lymphocytes are primary of the hematopoietic niche of the bone marrow and osteoblastic lineage cells support the differentiation of both HSCs and B cells in the niche [32]. Simultaneously, B cells and their products also affect other cells and are involved in the development of OP. Interestingly, peripheral B cells have been reported to inhibit osteoclast formation by secretion of TGF-β [33]. In an animal model of periodontitis, the decline of B cells aggravates bone loss, suggesting that B cells can restrict bone resorption under certain conditions [34]. B cells are the major source of OPG, which binds RANKL and prevents the activation of RANK and thus its deficiency leads to enhanced osteoclastogenesis. In addition, OPG, interfering the differentiation of OCPs to OC, may serve to buffer changes in the rate of bone resorption associated with excess levels of osteoblastic RANKL [35].
This study has some limitations. Firstly, all participants were from the same research center rather than multiple centers. In addition, previous drug use of most patients cannot be accurately quantified because of complex disease course. And further prospective studies about how drugs affect immune cells and bone metabolism should be done. Moreover, post-menopausal status is also one of the causes of OP in RA women. These data were not collected previously and will be an important area of research in future work. Last but not least, the specific mechanism and signal pathways of immune cells regulating bone remodeling should be further explored in animal and cell experiments. Despite these limitations, our findings that immune disorders characterized by peripheral Th17/Treg imbalance and reduced B cells may contribute to OP in RA stand for themselves.
Conclusions
RA patients have a high incidence of OP, which was related to disease activity, autoantibodies, and RBC. Notably, immune disorder caused by imbalance of Th17/Tregs and/or insufficient of B cells might also contribute to OP in RA patients. It is necessary to pay attention to the level of peripheral lymphocyte subsets to avoid the aggravation of immune imbalance and they may be identified as signs indicating disease progression.
References
Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170(1):ITC1–16.
Adami G, Saag KG. Osteoporosis pathophysiology, epidemiology, and screening in rheumatoid arthritis. Curr Rheumatol Rep. 2019;21(7):34.
Hauser B, et al. Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(10):1759–66.
Niu HQ, Li ZH, Zhao WP, et al. Sirolimus selectively increases circulating Treg cell numbers and restores the Th17/Treg balance in rheumatoid arthritis patients with low disease activity or in DAS28 remission who previously received conventional disease-modifying anti-rheumatic drugs. Clin Exp Rheumatol. 2020;38(1):58–66.
Komatsu N, Takayanagi H. Immune-bone interplay in the structural damage in rheumatoid arthritis. Clin Exp Immunol. 2018;194(1):1–8.
Zhu L, Hua F, Ding W, et al. The correlation between the Th17/Treg cell balance and bone health. Immun Ageing. 2020;17:30.
Dar HY, Azam Z, Anupam R, et al. Osteoimmunology: the nexus between bone and immune system. Front Biosci (Landmark Ed). 2018;23:464–92.
Xu S, Zhang Y, Liu B, et al. Activation of mTORC1 in B lymphocytes promotes osteoclast formation via regulation of β-catenin and RANKL/OPG. J Bone Miner Res. 2016;31(7):1320–33.
Aletaha D, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Siris ES, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int. 2014;25(5):1439–43.
Coury F, Peyruchaud O, Machuca-Gayet I. Osteoimmunology of bone loss in inflammatory rheumatic diseases. Front Immunol. 2019;10:679.
Llorente I, García-Castaeda N, Valero C, et al. Osteoporosis in rheumatoid arthritis: dangerous liaisons. Front Med. 2020;7:601618.
Raterman HG, Lems WF. Pharmacological management of osteoporosis in rheumatoid arthritis patients: a review of the literature and practical guide. Drugs Aging. 2019;36(12):1061–72.
Sharma M, Dhakad U, Wakhlu A, et al. Lean mass and disease activity are the best predictors of bone mineral loss in the premenopausal women with rheumatoid arthritis. Indian J Endocrinol Metab. 2018;22(2):236–43.
Sivas F, et al. The relation between joint erosion and generalized osteoporosis and disease activity in patients with rheumatoid arthritis. Rheumatol Int. 2006;26(10):896–9.
Katchamart W, Koolvisoot A, Aromdee E, et al. Associations of rheumatoid factor and anti-citrullinated peptide antibody with disease progression and treatment outcomes in patients with rheumatoid arthritis. Rheumatol Int. 2015;35(10):1693–9.
Krishnamurthy A, Joshua V, Haj Hensvold A, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. 2016;75(4):721–9.
Kleyer A, Finzel S, Rech J, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73(5):854–60.
Orsolini G, Caimmi C, Viapiana O, et al. Titer-dependent effect of anti-citrullinated protein antibodies on systemic bone mass in rheumatoid arthritis patients. Calcif Tissue Int. 2017;101(1):17–23.
Fraenkel PG. Anemia of inflammation: a review. Med Clin North Am. 2017;101(2):285–96.
Miyamoto T. [Hypoxemia and osteoporosis-possible roles of HIF1α on respiratory disease-related osteoporosis]. Clin Calcium. 2016;26(10):1429–35.
Ferrari M, Manea L, Anton K, et al. Anemia and hemoglobin serum levels are associated with exercise capacity and quality of life in chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:58.
Safari F, et al. CRISPR and personalized Treg therapy: new insights into the treatment of rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2018;40(3):201–11.
Okamoto K, Nakashima T, Shinohara M, et al. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 2017;97(4):1295–349.
Zaiss MM, Axmann R, Zwerina J, et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56(12):4104–12.
Bozec A, et al. T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
Runyan CE, Liu Z, Schnaper HW. Phosphatidylinositol 3-kinase and Rab5 GTPase inversely regulate the Smad anchor for receptor activation (SARA) protein independently of transforming growth factor-β1. J Biol Chem. 2012;287(43):35815–24.
Zhao L, Jiang S, Hantash BM. Transforming growth factor beta1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng A. 2010;16(2):725–33.
Tyagi AM, Yu M, Darby TM, et al. The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity. 2018;49(6):1116–31.
Srivastava RK, Dar HY, Mishra PK. Immunoporosis: immunology of osteoporosis-role of T cells. Front Immunol. 2018;9:657.
Ono T, Takayanagi H. Osteoimmunology in bone fracture healing. Curr Osteoporos Rep. 2017;15(4):367–75.
Bianco P. Minireview: the stem cell next door: skeletal and hematopoietic stem cell “niches” in bone. Endocrinology. 2011;152(8):2957–62.
Weitzmann MN, Cenci S, Haug J, et al. B lymphocytes inhibit human osteoclastogenesis by secretion of TGFbeta. J Cell Biochem. 2000;78(2):318–24.
Zouali M. The emerging roles of B cells as partners and targets in periodontitis. Autoimmunity. 2017;50(1):61–70.
Li Y, Toraldo G, Li A, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–48.
Acknowledgements
Funding
This project was supported by the National Natural Science Foundation of China (No. 82001740 and 81871295) and Graduate Education Innovation Program of Shanxi Province (2021Y357). The journal’s Rapid Service Fee was funded by the National Natural Science Foundation of China.
Author Contributions
Study design and manuscript writing: TC and SZ. Data extraction, quality assessment, analysis and interpretation of data: JW, JQ, MC, HN, GL and JL. All authors were involved in drafting the article or revising the important intellectual content critically and all authors approved the final version to be published. X-FL takes responsibility for the integrity of the data and the accuracy of the analysis.
Prior Presentation
Part of these data were presented as an abstract at the ACR/ARP Annual Meeting, held in Atlanta on November 8–13, 2019. In this manuscript, we present more information about the topic beyond previous abstract, including increases in the number of participants and clinical data like autoantibodies, blood routine as well as hepatic and renal function. We consider that this added information will clarify the immunologic mechanisms of high incidence of OP in patients with RA more completely.
Disclosures
Ting Cheng, Sheng-Xiao Zhang, Jia Wang, Jun Qiao, Min-Jing Chang, Hong-Qing Niu, Guang-Ying Liu and Xiao-Feng Li have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Medical Ethics Committee of the Second Hospital of Shanxi Medical University (2016-KY-007). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. The authors declare that they have obtained consent from the participants and we thank the participants of the study.
Data Availability
All data generated or analyzed during this study are included in this published article as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cheng, T., Zhang, SX., Wang, J. et al. Abnormalities of Peripheral Lymphocyte Subsets in Rheumatoid Arthritis Patients Complicated with Osteoporosis. Rheumatol Ther 9, 1049–1059 (2022). https://doi.org/10.1007/s40744-022-00452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00452-x