Abstract
Chikungunya virus (CHIKV) infection is caused by an arbovirus prevalent in various parts of the world. The virus can induce autoantibodies and rheumatic diseases, such as rheumatoid arthritis and spondylarthritis. However, until now, no case of Sjögren syndrome (SS) was described associated with CHIKV. In this article, we describe a 49-year-old female with polyarthralgia and a temporary rash on her trunk and arms. Her physical examination showed polyarthritis of her ankles and wrists. Serologies for CHIKV were interpreted as positive with IgM 6.5 (normal range < 0.8) and negative for IgG. Antinuclear antibodies were positive at a titer of 1:640 as well as anti-Ro/SS-A. The diagnosis of subacute CHIKV infection was determined. The Schirmer test, Rose Bengal, and salivary scintigraphy were positive and the diagnosis of SS was confirmed. She was treated with hydroxychloroquine, methotrexate, and a single dose of betamethasone depot. This is the first report on CHIKV associated with SS. Sequence analysis of the CHIKV proteome versus SS autoantigens showed an extensive peptide sharing between the virus and numerous SS autoantigens, thus supporting the hypothesis that autoimmune cross-reactivity might causally link CHIKV to SS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
CHIKV infection is an arbovirus that may induce autoantibodies and rheumatic diseases. |
Herein, the authors describe a clinical case of a 49-year-old woman who had CHIKV and developed SS almost simultaneously. |
Presence of positive antinuclear and anti-Ro/SS-A antibodies support the diagnosis of SS. |
Moreover, molecular analyses highlighting sharing of minimal immune determinants between the virus and the SS autoantigens might suggest a specific link between the infection and the autoimmune disease. Such a hypothesis appears to be supported by the fact that only a few of the peptides shared between CHIKV and SS are also present in pathogens that have been associated with SS such as EBV and hCMV. |
It would be reasonable to interrogate patients with recent SS about travel and infections and vice versa, considering autoimmune conditions in patients infected with CHIKV. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13582220.
Introduction
The Chikungunya virus (CHIKV), a member of the Togaviridae family, Alphavirus genus, was first isolated from humans in 1952, in Tanzania. Brazil had a large outbreak of CHIKV infection between September 2014 and 2017 [1]. Nowadays, Brazil is facing a new outbreak of CHIKV infection. In 25.5–40.2% of the patients who underwent CHIKV infection, a chronic joint condition may evolve [2].
Previous reports have described the presence of rheumatological conditions triggered by CHIKV infection, such as rheumatoid arthritis, spondyloarthritis, and psoriasis [3,4,5,6]. In a similar manner, patients who had previous rheumatological conditions may experience worsening of the symptoms of the disease after CHIKV infection [6]. In a large series of 202 CHIKV patients, there is a description of two (2.6%) cases of SS associated with this viral infection [7].
Herein, we describe a patient that had a confirmed diagnosis of CHIKV and has developed a simultaneous SS. In addition, an analysis for a molecular mimicry between CHIKV and SS autoantigens was performed and we present the results. The CAse REport Statement and Checklist (CARE) from Enhancing the QUAlity and Transparency Of health Research (EQUATOR) Reporting guidelines was followed to describe the present case. The patient consented to participating in this study and also to publish her case. The authors declare that the World Medical Association Declaration of Helsinki were followed.
Case Report
A 49-year-old Caucasian female was previously diagnosed with systemic hypertension, hypothyroidism, and lactose intolerance. She had no previous musculoskeletal complaints. She noted transient skin lesions (rash) on her trunk and arms. Her physical examination was remarkable for polyarthritis of her ankles (Fig. 1) and wrists. Laboratory tests demonstrated a hemoglobin of 14.5 g/dl, white blood cells of 11,990 cells/ml, without atypical lymphocytes, platelets 267,000/ml, erythrocyte sedimentation rate of 54 mm/1st hour, C-reactive protein of 0.7 mg/l [normal range (nr) < 1 mg/l], AST 17 U/L (nr < 35 U/l), ALT 24 U/ml (nr < 35 U/l), creatine kinase 33 U/ml uIU/ml (nr < 35 U/l), thyrotrophic stimulating hormone 0.77 uIU/ml (nr 0.38–5.33 uIU/ml), and negative anti-thyroglobulin and anti-peroxidase antibodies. Serologies for CHIKV were positive with IgM 6.5 (normal range < 0.8) and negative for IgG. No antibodies to Zika, dengue, hepatitis A, B, and C, herpes simplex 1 and 2, HIV, HTLV I and II, toxoplasmosis, mononucleosis, syphilis were detected. Interestingly, antinuclear antibodies (ANA) were positive in a titer of 1:640 as well as anti-Ro/SS-A. The diagnosis of a subacute CHIKV infection was determined and a single dose of betamethasone depot was administered. No other antibodies were found including anti-dsDNA, anti-Sm, anti-U1RNP, anti-La, anti-CCP, rheumatoid factor, anti-Jo-1, anti-Scl70, lupus anticoagulant, IgG and IgM anti-anticardiolipin, IgG and IgM anti-beta-2-glycoprotein I, anti-neutrophil cytoplasm antibodies (ANCA); IgG and IgA anti-endomysium, anti-gliadin and anti-tissue transglutaminase; antiglutamate decarboxylase, anti-insulin, anti-parietal cell, anti-liver/kidney microsomal (LKM1), and anti-mitochondrial. Serum electrophoresis was interpreted as normal, without hypergammaglobulinemia and the total complement levels were normal (CH100—139 mg/dl). She denied xerostomia, xerophthalmia, parotiditis, and dry skin nor personal and family history for autoimmune diseases. ANA and anti-Ro antibodies were redone and confirmed the previous results. Schirmer test demonstrated 5 mm in left eye and 18 mm in right eye. Rose Bengal test showed a positive score of 1 in her right eye and 2–3 in her left one. A salivary scintigraphy revealed a moderate reduction of function of the left and normal result at the right gland, and the unstimulated salivary flow rate was < 0.1 ml per minute. The diagnosis of SS was determined based on the objective tests for sicca syndrome involving one eye (positive Schirmer test) (1 point), mouth (altered salivary flow rate) (1 point), and positive anti-Ro/SS-A (3 points) with a total of 5 points, fulfilling the classification criteria for this disease [8]. The patient refused a minor salivary gland biopsy. Hydroxychloroquine 400 mg/day was prescribed. Her ankle arthritis persisted and methotrexate 15 mg/week plus folic acid 5 mg/week were initiated and improvement was noted. She has a follow-up of 9 months and an arthritis remission was seen.
Moreover, searching for a molecular mechanism that might causally relate the concomitant emergence of CHIKV and SS, molecular mimicry between CHIKV and SS autoantigens was investigated. Specifically, SS autoantigens were obtained from UniProt database (https://www.uniprot.org/) using “Sjögren” as keyword. Only reviewed entries were analyzed. Then, the SS autoantigen primary sequences were dissected into pentapeptides overlapped by four amino acid residues. Each pentapeptide was analyzed for occurrences in the proteome of CHIKV, strain NCBITaxId371094, by using Pir Peptide Match program (https://research.bioinformatics.udel.edu/peptidematch/index.jsp) [16]. The specificity of the peptide overlaps between CHIKV and the human SS autoantigens was also analyzed. That is, infectious pathogens that have been associated with SS—namely: Coxsackievirus B4 (CVB4), Epstein–Barr virus (EBV), Hepatitis B virus (HBV), and Human cytomegalovirus (hCMV) [18]—were investigated for the presence of peptides shared between CHIKV and SS autoantigens.
Table 1 shows a significant peptide sharing between CHIKV and SS autoantigens [9,10,11,12,13,14,15,16,17] that lends support to the hypothesis that a CHIKV-induced autoimmune cross-reactivity might lead to the development of SS. In addition, Table 1 shows that the CHIKV versus SS autoantigens appears to be highly CHIKV-specific since only a few peptides shared between CHIKV and SS are also present in other infectious pathogens that have been associated with SS [18].
Discussion
This article is a report of a patient with CHIKV infection, who had a simultaneous diagnosis of SS associated with CHIKV infection, and we performed a molecular mimicry analysis between SSs autoantigens and CHIKV.
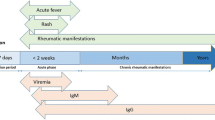
CHIKV infection has three phases: an acute one within the first 2 weeks, characterized by fever, headache, myalgia, conjunctivitis, arthralgia, and exanthematous rash; a subacute phase (from 15 days to 3 months), and a chronic phase (more than 3 months) characterized by arthritis. In these two last phases, fever is not present and musculoskeletal manifestations are the prominent complex. In fact, a recent meta-analysis showed a frequency of chronic CHIKV that spans from 25.5% to 40.2% in the studied cases [2].
Risk factors for switching of an acute CHIKV to a chronic phase are older age, female gender, prominent arthritis at the acute phase, previous joint lesions, and previous comorbidities, like obesity and diabetes [2]. Our patient was a female and had previous comorbidities (systemic hypertension and hypothyroidism but persistent negative anti-thyroid antibodies), without any autoimmune disease. Therefore, she was at risk that her disease would evolve to a chronic phase and have manifestations of CHIKV infection.
Autoimmune disease pathogenesis is linked to genetic predisposition associated with environmental factors, hormone misbalance, low vitamin D level, smoking, and several other factors, including infections [19]. These interactions have been referred to as the “mosaic of autoimmunity” [19]. Furthermore, numerous epidemiological and clinical studies have linked infections to SS, especially EBV, but also retroviruses, HCMV, hepatitis B virus, hepatitis C virus, Coxsackie virus, and human T-lymphotropic virus type 1, to the pathogenesis of SS [18].
Previous studies have demonstrated IgG antibodies against EBV in SS sera patients (OR 4; 95% CI 1.82–8.83, p = 0.001). Anti-Ro/SSA and anti La/SSB antibodies were associated with IgG anti-EBV antibodies [18]. Furthermore, the viruses may play a role as superantigens in mediating interactions with T-cells and B-cells leading to autoimmunity in a genetically susceptible person [19]. As reported in Table 1, the molecular mimicry between the virus and numerous SS autoantigens might possibly be the pathological mechanism linking CHIKV infection and SS, and indeed the peptide commonality between the virus and the SS-related autoantigens is remarkable, by amounting to 53 pentapeptides.
Based on these facts, it would be reasonable to interrogate patients with recent SS about travel and infections as part of their assessment and vice versa, considering non-rheumatoid arthritis autoimmune conditions in patients infected with CHIKV.
The patient had a discordance between xerophthalmia and ocular tests. She denied any ocular symptoms, but had positive ocular tests for dry eyes. This fact is observed in some SS cases, it is not uncommon. One possible explanation is that her SS is in a very early stage of the disease (less than 2 months). Probably, with the follow-up of the patient, she may develop xerophthalmia symptoms.
Conclusions
In conclusion, this is a description of a patient with CHIKV infection, associated with an almost simultaneously emergence of SS. In addition, molecular mimicry analyses highlighting sharing of minimal immune determinants between the virus and the SS autoantigens might suggest a direct link between the CHIKV and SS.
References
Rodrigues Faria N, Lourenço J, Marques de Cerqueira E, Maia de Lima M, Pybus O, Carlos Junior Alcantara L. Epidemiology of Chikungunya Virus in Bahia, Brazil, 2014–2015. PLoS Curr. 2016. https://doi.org/10.1371/currents.outbreaks.c97507e3e48efb946401755d468c28b2.
Rodríguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzón S, et al. Prevalence of post-chikungunya infection chronic inflammatory arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2016;68:1849–58.
Amaral JK, Bilsborrow JB, Schoen RT. Chronic chikungunya arthritis and rheumatoid arthritis: what they have in common. Am J Med. 2020;133(3):e91–7.
Kumar R, Sharma MK, Jain SK, Yadav SK, Singhal AK. Cutaneous manifestations of chikungunya fever: observations from an outbreak at a Tertiary Care Hospital in Southeast Rajasthan, India. Indian Dermatol Online J. 2017;8(5):336–42.
Blettery M, Brunier L, Polomat K, et al. Brief report: management of chronic post-chikungunya rheumatic disease: the Martinican experience. Arthritis Rheumatol. 2016;68(11):2817–24.
Tanay A. Chikungunya virus and autoimmunity. Curr Opin Rheumatol. 2017;29(4):389–93.
Duran AS, Beron A, Duran SS, et al. SAT0568 Rheumatological manifestations in a series of patients with chikungunya fever. Ann Rheum Dis. 2017;76:991.
Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69(1):35–45.
Seelig HP, Schranz P, Schröter H, Wiemann C, Renz M. Macrogolgin—a new 376 kD Golgi complex outer membrane protein as target of antibodies in patients with rheumatic diseases and HIV infections. J Autoimmun. 1994;7(1):67–91. https://doi.org/10.1006/jaut.1994.1006.
Griffith KJ, Chan EK, Lung CC, et al. Molecular cloning of a novel 97-kd Golgi complex autoantigen associated with Sjögren’s syndrome. Arthritis Rheum. 1997;40(9):1693–702. https://doi.org/10.1002/art.1780400920.
Osborn DPS, Pond HL, Mazaheri N, et al. Mutations in INPP5K cause a form of congenital muscular dystrophy overlapping Marinesco-Sjögren syndrome and dystroglycanopathy. Am J Hum Genet. 2017;100(3):537–45. https://doi.org/10.1016/j.ajhg.2017.01.019.
Uchida K, Akita Y, Matsuo K, et al. Identification of specific autoantigens in Sjögren’s syndrome by SEREX. Immunology. 2005;116(1):53–63. https://doi.org/10.1111/j.1365-2567.2005.02197.x.
Liang C, Xiong K, Szulwach KE, et al. Sjogren syndrome antigen B (SSB)/La promotes global microRNA expression by binding microRNA precursors through stem-loop recognition. J Biol Chem. 2013;288(1):723–36. https://doi.org/10.1074/jbc.M112.401323.
Yu DF, Chen Y, Han JM, et al. MUC19 expression in human ocular surface and lacrimal gland and its alteration in Sjögren syndrome patients. Exp Eye Res. 2008;86(2):403–11. https://doi.org/10.1016/j.exer.2007.11.013.
Anttonen AK, Mahjneh I, Hämäläinen RH, et al. The gene disrupted in Marinesco-Sjögren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet. 2005;37(12):1309–11. https://doi.org/10.1038/ng1677.
Eystathioy T, Chan EK, Takeuchi K, et al. Clinical and serological associations of autoantibodies to GW bodies and a novel cytoplasmic autoantigen GW182. J Mol Med (Berl). 2003;81(12):811–8. https://doi.org/10.1007/s00109-003-0495-y.
Chen C, Li Z, Huang H, Suzek BE, Wu CH. A fast peptide match service for UniProt knowledgebase. Bioinformatics. 2013. https://doi.org/10.1093/bioinformatics/btt484.
Kivity S, Arango MT, Ehrenfeld M, et al. Infection and autoimmunity in Sjogren’s syndrome: a clinical study and comprehensive review. J Autoimmun. 2014;51:17–22.
Shoenfeld Y, Gilburd B, Abu-Shakra M, Amital H, Barzilai O, Berkun Y, et al. The mosaic of autoimmunity: genetic factors involved in autoimmune diseases. Isr Med Assoc J. 2008;10:3.
Acknowledgements
We thank the participants of the study.
Funding
The work is supported by a grant from the government of the Russian Federation (contract 14.W03.31.0009 of 13/02/2017) for state support of scientific research conducted under the supervision of leading scientists. No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Jozélio Freire de Carvalho, Darja Kanduc, Felipe Freire da Silva, Amir Tanay, Alberta Lucchese, and Yehuda Shoenfeld have nothing to disclose.
Compliance with Ethics Guidelines
The authors declare that the World Medical Association Declaration of Helsinki were followed. The patient gave us an informed consent to participate in this study and also to publish her case.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
de Carvalho, J.F., Kanduc, D., da Silva, F.F. et al. Sjögren’s Syndrome Associated with Chikungunya Infection: A Case Report. Rheumatol Ther 8, 631–637 (2021). https://doi.org/10.1007/s40744-021-00281-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-021-00281-4