Abstract
Introduction
In addition to the considerable patient and societal burdens, the financial burdens of ankylosing spondylitis (AS) are substantial. Understanding both all-cause and AS-specific direct costs in patients with AS is important if we are to understand the financial impact on patients with AS and payers in the United States. This study assessed both all-cause and AS-specific healthcare utilization and direct costs in US patients with AS using administrative claims data.
Methods
Adults aged ≥ 18 years enrolled in the MarketScan® Commercial and Medicare databases with ≥ 1 inpatient or ≥ 2 non-rule-out outpatient diagnoses of AS between January 1, 2013, and December 31, 2013, were included. Patients had continuous enrollment with medical and pharmacy benefits for ≥ 12 months before and after the index date (first diagnosis). Non-AS controls were matched up to 5:1 to patients with AS on age, geographic region, index calendar year, and sex. All-cause and AS-specific healthcare utilization and direct costs were measured during the follow-up period and reported as per patient per year.
Results
Patients with AS (N = 6679) had significantly higher rates of total all-cause inpatient admission (12% vs 6%), emergency department visits (23% vs 15%), nonhospital-based outpatient visits (100% vs 84%), hospital-based outpatient visits (68% vs 46%), other outpatient services (97% vs 81%), and medication use (97% vs 82%) compared with matched controls (N = 19,951). Patients with AS had approximately tenfold higher median total healthcare costs than matched controls ($24,978 vs $2139 per patient per year), largely driven by increased outpatient and pharmacy costs; P < 0.05 for all comparisons. The median (IQR) total AS-specific healthcare costs were $10,250 ($774, $28,824).
Conclusion
In this analysis of claims data, increased outpatient and pharmacy costs were key contributors to higher all-cause total healthcare costs in US patients with AS.
Funding
Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disorder that mainly affects the sacroiliac joints and axial skeleton and occurs in 0.1–1% of the US general population [1,2,3,4,5]. Vertebral inflammation can result in structural changes of the axial skeleton, including bone growth and fusion [6]. Other clinical manifestations of AS include enthesitis, extra-articular organ involvement, and peripheral arthritis [7,8,9,10]. Functional disability caused by AS can result in work restrictions and an inability to perform daily household activities, and may cause patients to become too disabled to work [11,12,13,14].
In addition to the considerable patient and societal burdens, the financial burdens of AS are substantial and have increased since the introduction of biologic therapies in 2002 for the treatment of AS [15]. A US study conducted before the US Food and Drug Association (FDA) approval of tumor necrosis factor inhibitors (TNFis) to treat AS showed that the mean annual direct healthcare costs (e.g., medications, outpatient visits, hospitalizations, home help) related to AS were $1755 per patient ($2674 for all-cause direct healthcare costs), and indirect costs (e.g., lost productivity in the workplace and disability compensation) were $4945 per patient; the most significant predictor for the high cost was functional disability [16].
In a systematic review of the economic burden of AS, which reviewed studies conducted before and after the approval of TNFis and mostly included studies from outside the United States, indirect healthcare costs related to AS were shown to be higher than direct costs related to AS [17]. In a study of US healthcare claims data conducted after the approval of TNFis to treat AS, the annual mean [standard deviation (SD)] all-cause, direct medical costs were $6514 ($32,982) and the annual mean (SD) prescription drug costs were $11,214 ($14,249) per patient with AS [18]. In that study, biologic use, age, and comorbidities were associated with higher all-cause direct costs [18].
The presence of comorbid diseases associated with AS increases healthcare costs, as these comorbidities require additional treatments and medications and can complicate the management of AS. Several diseases have a higher incidence in patients with AS than in the general population, including inflammatory bowel disease, uveitis, psoriasis, and cardiovascular disease [19,20,21,22,23,24,25]. Other factors that may also influence AS-related costs include severity of disease, treatment response, dosing schedules, and medication adherence and persistence [16, 17].
Most of the recent studies of AS-related costs have been conducted outside of the United States. Direct healthcare costs differ for patients outside the United States in terms of healthcare coverage (e.g., most patients in European countries receive health insurance coverage through public or government-subsidized programs) and varying costs of medications. Understanding both all-cause and AS-specific direct costs in patients with AS is important if we wish to understand the financial impact and burden of disease on patients with AS and payers in the United States. In addition, studies comparing the healthcare utilization and costs in patients with AS compared with healthy matched controls are limited. This retrospective study used the most recent healthcare claims data available to examine and compare healthcare utilization and both all-cause and AS-specific direct costs in patients with AS vs matched controls in the United States.
Methods
Data Sources
This US-based, retrospective, observational study used healthcare claims data from the Truven Health MarketScan Commercial Claims and Encounters (Commercial) database and the MarketScan® Medicare Supplemental (Medicare) database. These databases include complete longitudinal records of inpatient services, outpatient services, long-term care, and prescription drug claims for commercially insured and Medicare-eligible patients covered under a variety of health plans. All study data were de-identified and compliant with protocols of US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, institutional review board approval to conduct this study was not necessary.
Patient Selection
This study included patients aged ≥ 18 years with ≥ 1 inpatient or ≥ 2 non-rule-out outpatient medical claims for AS [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code 720.0] > 30 days but ≤ 365 days apart between January 1, 2012, and December 31, 2014. Patients with AS were required to have ≥ 1 diagnosis code for AS during 2013; the year 2013 was chosen because of the availability of pharmacy and medical claims data at the time of the analysis. The index date was defined as the date of the first AS diagnosis claim. Diagnoses on claims for diagnostic or rule-out procedures (e.g., laboratory, pathology, radiology services) were excluded.
Continuous enrollment with medical and pharmacy benefits for at least 12 months before the index date (baseline period) and 12 months after the index date (follow-up period) was required. Patients without AS (general population) were required to have no diagnosis of AS anytime between January 1, 2007, and June 30, 2015. These control patients were matched to patients with AS at a ratio of up to 5:1 by age, geographic location, index calendar year, and sex. Matched controls were assigned the same index dates as their matched patients with AS. All patients were followed up for at least 12 months until the earliest occurrence of inpatient death, the end of continuous enrollment, or the end of data availability (June 30, 2015). The 12-month follow-up period was chosen in order to present the data over a fixed 12-month period for clarity and ease of understanding.
Study Variables
Patient demographic characteristics were recorded at the index date and included age, geographic area, insurance type, and sex. Comorbidities of interest were identified using ICD-9-CM codes in a medical claim; however, claims for diagnostic or rule-out procedures such as laboratory, pathology, or radiology services were excluded to avoid incorrectly identifying patients as having a comorbidity based on the testing rather than the test results. The following comorbidities were recorded during the baseline and follow-up periods: cardiovascular conditions (angina, atherosclerosis, cerebrovascular disease/stroke, coronary artery disease, hypertension, myocardial infarction, peripheral vascular disease, venous thromboembolism), inflammatory bowel disease (Crohn’s disease, ulcerative colitis), gastrointestinal ulcers (esophageal, gastric, duodenal, peptic, gastrojejunal ulcers), malignant neoplasms (malignant solid tumors, hematologic malignancies, neuroendocrine tumors), asthma, depression, diabetes mellitus, dyslipidemia, multiple sclerosis, osteoporosis, Parkinson disease, psoriasis, sleep apnea, spinal fracture, and uveitis.
Outcome Measures
All-cause and AS-specific healthcare utilization and associated direct costs were recorded during the follow-up period and reported as per patient per year. The following categories of healthcare utilization were reported: presence and number of inpatient hospitalizations; emergency department (ED) visits, nonhospital-based outpatient visits, hospital-based outpatient visits (includes the same type of visits as nonhospital-based outpatient visits but in a medical office within a hospital), and other outpatient services (e.g., skilled nursing facility, home health services, physical therapy, laboratory tests, imaging, outpatient procedures, injections); outpatient pharmacy claims; and counts of unique medications at the therapeutic class level including nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), TNFis approved for the treatment of AS by the FDA, and comorbidity-specific medications (e.g., antidiabetic, antihypertensive, lipid-lowering, and antidepressant therapies).
Costs were the total reimbursed amount, including patients’ copays, deductible, and coinsurance. All costs were inflated to 2015 dollars, using the medical component of the Consumer Price Index. The following categories of direct healthcare costs were reported: total healthcare costs, inpatient, outpatient (ED, nonhospital-based outpatient, hospital-based outpatient, and other outpatient services), and total outpatient pharmacy costs [included National Drug Code (NDC) claims only]. AS-specific healthcare resource utilization and associated costs were defined as inpatient or outpatient (ED, nonhospital-based outpatient, hospital-based outpatient, and other outpatient services) claims with the diagnosis code for AS (ICD-9-CM 720.0) and AS medications (included NDC and the Healthcare Common Procedure Coding System claims). The outpatient diagnosis could be in any position, but the inpatient diagnosis was required to be a primary discharge diagnosis, and the whole hospitalization stay was considered AS specific.
Statistical Analysis
Bivariate descriptive analyses were conducted on all study variables comparing patients with and without AS. Categorical variables were presented as counts and percentages, and mean (SD) and median [interquartile range (IQR)] healthcare utilizations and direct costs were reported. The statistical significance of cohort differences in bivariate descriptive statistics used χ2 tests for categorical variables, and t tests were used for differences in continuous variables. The threshold for statistical significance was set a priori to a P value of 0.05. All analyses were conducted using SAS version 9.4 (SAS Institute Inc.).
Results
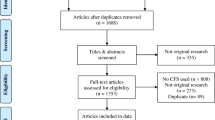
Between January 1, 2012, and December 31, 2014, a total of 46,265 patients had ≥ 1 inpatient claim or ≥ 2 outpatient claims for AS > 30 days and ≤ 365 days apart in the MarketScan Commercial and Medicare databases. Of those, 6834 met all criteria for the AS cohort, and 6679 were matched with 19,951 patients without AS (Fig. 1).
Patients with AS had a mean (SD) age of 50.8 (13.6) years, and matched controls had a mean age of 51.7 (13.4) years (Table 1). The majority of the patients were male, including 60.5% of patients with AS and 60.8% of matched controls. In both patients with AS and matched controls, the majority had preferred provider organization plans (60.2% and 58.2%, respectively), and one-third of patients were from the southern United States (33.3% and 34.8%, respectively).
The most common comorbidities at baseline were hypertension, dyslipidemia, and depression in patients with AS and hypertension, dyslipidemia, and diabetes in matched controls (Table S1 in the Electronic supplementary material, ESM). Patients with AS had higher incidence rates (P < 0.05) of most comorbidities, including cardiovascular disease, depression, malignancies, osteoporosis, sleep apnea, and spinal fracture, as well as inflammatory bowel disease, psoriasis, and uveitis. Patients with AS had a higher mean Deyo–Charlson Comorbidity Index score (P < 0.001) compared with matched controls (Table 1). 47.9% of the patients with AS had a history of prior biologic exposure, compared with 0.5% of the matched controls.
During the 12-month follow-up period, compared to matched controls, patients with AS had significantly higher rates of total all-cause inpatient admission (12% vs 6%), ED visits (23% vs 15%), nonhospital-based outpatient visits (100% vs 84%), hospital-based outpatient visits (68% vs 46%), and utilization of other outpatient services (97% vs 81%) (P < 0.001 for all; Table 2). Patients with AS had significantly higher rates of any medication use (97% vs 81%), antihypertensive use (43% vs 34%), and antidepressant use (38% vs 21%) than matched controls; however, patients with AS had significantly lower use of antidiabetics compared with matched controls (10% vs 9%) (P ≤ 0.005 for all; Fig. 2).
Medication use in patients with AS and matched controls (P ≤ 0.005 for all except lipid-lowering therapies: P = 0.939). acsDMARDs included auranofin, azathioprine, chloroquine, cyclophosphamide, cyclosporine, gold sodium thiomalate, hydroxychloroquine, leflunomide, methotrexate, minocycline hydrochloride, penicillamine, and sulfasalazine. bTNFis included adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab. cAS medications included both pharmacy claims and outpatient claims from National Drug Code claims and those billed by Healthcare Common Procedure Coding System codes. AS ankylosing spondylitis, csDMARDs conventional standard disease-modifying antirheumatic drugs, NSAIDs nonsteroidal anti-inflammatory drugs, TNFis tumor necrosis factor inhibitors
AS-specific healthcare utilizations and direct costs were those claims with a diagnosis code for AS (ICD-9-CM 720.0) and AS medications. A total of 94% of patients with AS had an AS-specific nonhospital-based outpatient visit, and 26% had an AS-specific hospital-based outpatient visit during the 12-month follow-up (Table 2). A small proportion of patients with AS had AS-specific inpatient admissions and AS-specific ED visits (1.1% and 2.3%, respectively). Patients with AS had significantly higher rates of use of NSAIDs (55% vs 20%), corticosteroids (47% vs 21%), TNFis (54% vs 0.5%), and csDMARDs (26% vs 2%) than matched controls (P < 0.001 for all; Fig. 2).
During the 12-month follow-up period, patients with AS had approximately threefold to fourfold higher mean and tenfold higher median total all-cause healthcare costs than matched controls [mean (SD): $33,285 ($46,363) vs $8310 ($32,260) per patient per year; median (IQR): $24,978 ($8205, $39,414) vs $2139 ($550, $6423) per patient per year], which was largely driven by increased use of medical outpatient services and outpatient pharmacy costs (Fig. 3a). The mean (SD) and median (IQR) outpatient pharmacy costs for patients with AS and matched controls were $14,074 ($16,381) and $6699 ($1124, $26,263), and $1737 ($6144) and $284 ($21, $1387), respectively. The mean (SD) and median (IQR) total AS-specific healthcare costs were $16,337 ($20,989) and $10,250 ($774, $28,824), respectively (Fig. 3b). The majority of the AS-specific total healthcare costs were related to AS medications; the mean (SD) and median AS-related drug costs were $14,595 ($19,119) and $7961, respectively.
Mean direct healthcare costs per patient per year over a 12-month follow-up period. a All-cause healthcare costs for patients with AS and matched controlsa, b. b AS-related healthcare costs for patients with ASc, d. aP < 0.001 for all. bAll costs were inflated to 2015 dollars, using the medical component of the Consumer Price Index. cIncludes medical and pharmacy costs. dAS-specific healthcare resource costs were defined as inpatient or outpatient claims with the diagnosis code for AS (ICD-9-CM 720.0) and AS medication. The outpatient diagnosis could be in any position. The inpatient diagnosis was required to be primary discharge diagnosis, and the whole hospitalization stay was considered AS specific. eAS medications included both pharmacy claims and outpatient claims from National Drug Code claims and those billed by the Healthcare Common Procedure Coding System codes. AS ankylosing spondylitis, ICD-9-CM International Classification of Diseases, Ninth Revision, Clinical Modification, USD United States dollars
The mean (SD) durations of follow-up for patients with AS and the matched controls were 739 (139) and 740 (138) days, respectively. The trends in total all-cause healthcare utilization and direct costs for patients with AS compared with matched controls observed in the 12-month fixed follow-up period were similar when examined during the full, variable-length follow-up time (data not shown).
Discussion
In this descriptive study using a large, US-based administrative claims database, patients with AS had substantially higher healthcare utilizations and direct costs than matched controls. Whereas prior studies have examined healthcare utilizations and costs of AS [16, 18, 26, 27], this study is among the first to do so in comparison with a matched cohort of patients who do not have AS or another rheumatic disease. In the present study, the tenfold-higher median total all-cause healthcare costs in patients with AS than in matched controls was largely driven by increased outpatient pharmacy costs, including the use of TNFis, other AS-related medications, antihypertensives, and antidepressants. These results highlight the substantial burden of disease in patients with AS.
One possible contributor to these increased utilizations and costs was the higher number of comorbidities in patients with AS than in the matched controls. Patients with AS had higher incidence rates (P < 0.05) of most comorbidities, including cardiovascular disease, depression, malignancies, osteoporosis, sleep apnea, and spinal fracture, as well as inflammatory bowel disease, psoriasis, and uveitis. Patients with AS also had a higher mean Deyo–Charlson Comorbidity Index score (P < 0.001) compared with matched controls. These results are consistent with previous studies, mostly conducted outside the United States, that have shown that patients with AS are at a higher risk for comorbidities [19, 21,22,23, 28]. Comorbidities may make the management of each disease more difficult, resulting in increased utilizations and costs.
A prospective, longitudinal study conducted in 1999, before the approval of TNFis for the treatment of AS, found that indirect costs ($4945 per year) related to AS were higher than direct costs ($1755 per year) [16]. After the introduction of TNFis, costs related to AS increased, as evidenced by a 2013 US study of healthcare claims data that showed that the total annual cost for all patients with AS treated with adalimumab, etanercept, or infliximab ranged from $16,375 to $27,084 [27]; however, as the 2013 study included only patients who received a TNFi, it cannot be directly compared with the present study, in which some study patients did not receive treatment with a TNFi [27]. Further, a 2016 study using US claims data showed that the mean (SD) annual direct medical costs in patients with AS was $6514 ($32,982), but the study included only the costs of TNFis and csDMARDs when calculating medication costs [18]. Healthcare utilizations in the present study, including the percentage of patients with AS who had inpatient admissions, ED visits, and outpatient visits, were similar to the most recent study of healthcare utilizations in patients with AS [18]. Therefore, the findings of this current study using real-world data and a comparison of utilization and costs between patients with AS and matched controls add important information to the existing knowledge of healthcare utilization and costs in US patients with AS.
The mean age of patients included in the study was older than reported in previous studies of patients with AS; however, the patient population in this study included both patients with incident and prevalent AS. The older age may also be partially explained by the inclusion criteria, which required continuous enrollment for 12 months before and after the index date. Younger patients may be more likely to switch jobs [29] and thus switch insurance carriers. Furthermore, younger people may have fewer visits with healthcare providers and thus fewer opportunities for an AS diagnosis.
The high proportion of women included in this study was also unexpected. One possible explanation is that some women may have had nonradiographic or peripheral spondyloarthritis (SpA) without AS (there are no specific diagnostic codes for these subtypes). Because women more frequently have nonradiographic SpA and peripheral SpA than men [30], women may have been misclassified as having AS more frequently than men.
The proportion of patients receiving corticosteroids in the present study was higher than anticipated; however, any filled claims for prescribed injectable or oral corticosteroids during the study period were counted regardless of whether patients took them (as claims data do have this information), which may have caused corticosteroid use to appear overinflated. Furthermore, patients in the present study may have had inflammatory conditions that are known to overlap with AS, such as ulcerative colitis, Crohn’s disease, and psoriasis, or the AS manifestations, including peripheral inflammatory arthritis or enthesitis, which may explain some of the corticosteroid use. The proportion of patients receiving corticosteroids in the present study (47%) was also comparable with that previously reported in another claims study of patients with AS (55%) [31].
Limitations
The limitations of this study are those inherent to any retrospective database analysis. Misclassification errors are possible when relying on diagnosis coding from administrative claims data, where the extent of under- or overcoding for AS is unknown. Because administrative claims data do not provide information on the severity of disease or other risk factors such as obesity or smoking, the effects of these factors could not be determined.
Patients with AS were required to have ≥ 2 outpatient diagnoses of AS, which required a second visit with a healthcare provider; matched controls were not required to have any visits with healthcare providers. Furthermore, because this study included only patients with commercial or private Medicare supplemental coverage, the results may not be generalizable to all patients with AS. This study was also limited to patients with AS who were clinically diagnosed; however, patients with undiagnosed AS may have incurred direct healthcare costs.
Another important limitation of our study is the lack of measurement of indirect costs; thus, the total financial burden of AS cannot be determined. In addition, due to the delay between the collection and the analyses of the data and the preparation of the manuscript, the financial data are not the most current; however, the data included in the present study still provide valuable information on the financial burden of AS on US patients and payers.
Despite these limitations, this study evaluated a large sample of patients from two sizeable insurance claims databases, and it provides a contemporary update on the healthcare utilizations and costs among patients with AS. Furthermore, the commercial and Medicare databases include adult patients with AS treated by clinicians across all US geographic regions and covered under various health plans.
Conclusions
In this retrospective, descriptive study of real-world health claims data in the United States, patients with AS had higher healthcare utilizations and direct costs, which were mostly driven by outpatient and pharmacy costs. These findings provide insights into the direct medical costs associated with healthcare utilization in patients with AS across the United States.
References
Reveille JD. The genetic basis of ankylosing spondylitis. Curr Opin Rheumatol. 2006;18:332–41.
Brionez TF, Reveille JD. The contribution of genes outside the major histocompatibility complex to susceptibility to ankylosing spondylitis. Curr Opin Rheumatol. 2008;20:384–91.
Brown MA. Breakthroughs in genetic studies of ankylosing spondylitis. Rheumatology (Oxford). 2008;47:132–7.
Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998;41:58–67.
Reveille JD, Weisman MH. The epidemiology of back pain, axial spondyloarthritis and HLA-B27 in the United States. Am J Med Sci. 2013;345:431–6.
Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374:2563–74.
Cawley MI, Chalmers TM, Ball J. Destructive lesions of vertebral bodies in ankylosing spondylitis. Ann Rheum Dis. 1971;30:539–40.
Hanson JA, Mirza S. Predisposition for spinal fracture in ankylosing spondylitis. AJR Am J Roentgenol. 2000;174:150.
Hunter T. The spinal complications of ankylosing spondylitis. Semin Arthritis Rheum. 1989;19:172–82.
Sutherland RI, Matheson D. Inflammatory involvement of vertebrae in ankylosing spondylitis. J Rheumatol. 1975;2:296–302.
Boonen A, Mau W. The economic burden of disease: comparison between rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol. 2009;27:S112–7.
Boonen A. A review of work-participation, cost-of-illness and cost-effectiveness studies in ankylosing spondylitis. Nat Clin Pract Rheumatol. 2006;2:546–53.
Boonen A, Brinkhuizen T, Landewe R, van der Heijde D, Severens JL. Impact of ankylosing spondylitis on sick leave, presenteeism and unpaid productivity, and estimation of the societal cost. Ann Rheum Dis. 2010;69:1123–8.
Ward MM, Reveille JD, Learch TJ, Davis JC Jr, Weisman MH. Impact of ankylosing spondylitis on work and family life: comparisons with the US population. Arthritis Rheum. 2008;59:497–503.
Reveille JD, Ximenes A, Ward MM. Economic considerations of the treatment of ankylosing spondylitis. Am J Med Sci. 2012;343:371–4.
Ward MM. Functional disability predicts total costs in patients with ankylosing spondylitis. Arthritis Rheum. 2002;46:223–31.
Palla I, Trieste L, Tani C, et al. A systematic literature review of the economic impact of ankylosing spondylitis. Clin Exp Rheumatol. 2012;30:S136–41.
Greenberg JD, Palmer JB, Li Y, Herrera V, Tsang Y, Liao M. Healthcare resource use and direct costs in patients with ankylosing spondylitis and psoriatic arthritis in a large US cohort. J Rheumatol. 2016;43:88–96.
Kang JH, Chen YH, Lin HC. Comorbidity profiles among patients with ankylosing spondylitis: a nationwide population-based study. Ann Rheum Dis. 2010;69:1165–8.
Meesters JJ, Bremander A, Bergman S, Petersson IF, Turkiewicz A, Englund M. The risk for depression in patients with ankylosing spondylitis: a population-based cohort study. Arthritis Res Ther. 2014;16:418.
Bremander A, Petersson IF, Bergman S, Englund M. Population-based estimates of common comorbidities and cardiovascular disease in ankylosing spondylitis. Arthritis Care Res (Hoboken). 2011;63:550–6.
Essers I, Stolwijk C, Boonen A, et al. Ankylosing spondylitis and risk of ischaemic heart disease: a population-based cohort study. Ann Rheum Dis. 2016;75:203–9.
Rosenbaum J, Chandran V. Management of comorbidities in ankylosing spondylitis. Am J Med Sci. 2012;343:364–6.
Ahmed N, Prior JA, Chen Y, Hayward R, Mallen CD, Hider SL. Prevalence of cardiovascular-related comorbidity in ankylosing spondylitis, psoriatic arthritis and psoriasis in primary care: a matched retrospective cohort study. Clin Rheumatol. 2016;35:3069–73.
Stolwijk C, Essers I, van Tubergen A, et al. The epidemiology of extra-articular manifestations in ankylosing spondylitis: a population-based matched cohort study. Ann Rheum Dis. 2015;74:1373–8.
Palmer JB, Li Y, Herrera V, Liao M, Ozturk Z. Treatment patterns and costs for ant-TNF alpha therapy in patients with ankylosing spondylitis. Rheumatology (Sunnyvale). https://doi.org/10.4172/2161-1149.S6-007.
Schabert VF, Watson C, Joseph GJ, Iversen P, Burudpakdee C, Harrison DJ. Costs of tumor necrosis factor blockers per treated patient using real-world drug data in a managed care population. J Manag Care Pharm. 2013;19:621–30.
Mercieca C, van der Horst-Bruinsma IE, Borg AA. Pulmonary, renal and neurological comorbidities in patients with ankylosing spondylitis; implications for clinical practice. Curr Rheumatol Rep. 2014;16:434.
US Department of Labor Bureau of Labor Statistics. Employee tenure in 2016. 2016;USDL-16-1867. https://www.bls.gov/news.release/archives/tenure_09222016.pdf
Tournadre A, Pereira B, Lhoste A, et al. Differences between women and men with recent-onset axial spondyloarthritis: results from a prospective multicenter French cohort. Arthritis Care Res (Hoboken). 2013;65:1482–9.
Walsh JA, Adejoro O, Chastek B, Park Y. Treatment patterns of biologics in US patients with ankylosing spondylitis: descriptive analyses from a claims database. J Comp Eff Res. 2018;7:369–80.
Acknowledgements
Funding
This study was sponsored by Novartis Pharmaceuticals Corporation, East Hanover, NJ. Article processing charges were funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and/or Editorial Assistance
Support for third-party writing assistance, furnished by Nicola Gillespie, DVM, of Health Interactions, Inc., was provided by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Disclosures
J. Walsh is a consultant for Novartis Pharmaceuticals Corporation. X. Song and G. Kim are employees of IBM Watson Health. Y. Park is an employee of Novartis Pharmaceuticals Corporation.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors. This study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data.
Data Availability
The MarketScan® databases used for the analyses in this paper are commercially available from Truven Health Analytics (IBM Watson Health) (http://truvenhealth.com). The license agreement to access these data does not give the authors permission to share this database.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6900545.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Walsh, J.A., Song, X., Kim, G. et al. Healthcare Utilization and Direct Costs in Patients with Ankylosing Spondylitis Using a Large US Administrative Claims Database. Rheumatol Ther 5, 463–474 (2018). https://doi.org/10.1007/s40744-018-0124-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-018-0124-4