Abstract
Dual-phase steels provide an excellent combination of strength and ductility, as well as improved energy absorption and anti-corrosion protection properties. This research aims at evaluating the microstructure and corrosion behaviour of EN8 steel under different heat treatment temperatures in 0.5 M sulphuric acid solution (H2SO4) using the EIS, potentiodynamic polarization, and gravimetric method (weight-loss method). Austenitizing is performed at 973 K, 1023 K, 1063 K, and 1173 K for 2 h followed by quenching in water to form a ferrite–martensite (F–M) dual-phase structure. From the results, it is seen that the corrosion rate increased with different heat treatment conditions depending on the change in the phase when immersed in 0.5 M H2SO4 at the temperature of 303 K, 313 K, 323 K, and 333 K. This work investigates the energy of activation, enthalpy, and entropy of activation. For dual-phase steel containing ferrite and martensite, the corrosion behaviour depends on the amount of martensite and ferrite. As the austenitization temperature increases from 1023 to 1173 K, the amount of martensite increases. This is reflected in the increase of micro galvanic corrosion cells in the region between the ferrite and martensite phases, which acts as active corrosion centres. The normalized specimen showed greater corrosion resistance compared to the water-quenched specimen at 1173 K. This is due to the presence of lower carbon content for normalized dual-phase steel containing ferrite–pearlite phase than the ferrite–martensite phase present in specimen austenitized at 1173 K. Surface characterization and XRD confirmed the corrosion behaviour of the specimens under investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Steel is an important structural material that has applications in numerous fields, including automobiles, the marine industry, construction, etc. [1,2,3,4]. Corrosion of the materials can result in huge economic losses. Corrosion is involved mainly in destroying the metal through its reaction to the exposed medium. The reaction may be chemical or electrochemical that typically produces other metal oxides in the form of rust [5,6,7,8,9,10,11,12]. There is a steady rise in the requirement of low carbon steel in the aviation, defence, locomotive, and other manufacturing industries. This is due to their improved mechanical properties, excellent resistance to breakage, wear and fatigue [13,14,15]. Moreover, for practical application, the corrosion resistance of carbon steel is an important property. In a wide range of environments in industry, the low corrosion resistance of carbon steel leads to intense destruction. As a result, carbon steels have to be permanently replaced after a certain period, which entails significant maintenance costs [16,17,18]. Medium carbon steel is regarded as one of the most applicable forms of steel because of its ease of availability, its characteristic mechanical properties such as its high strength and hardness. A kind of non-alloy medium carbon steel is AISI 1040 steel or EN8 steel. EN 8 steel is an alloy-free medium carbon, with moderate strength steel and is a popular grade of bulk-hardening steel generally used in the normalized condition. Several automotive components such as axles, gears, bolts, shafts, and dowels are manufactured from this grade of steel [19]. Because of the good tensile strength, EN8 steel is used in chemical refineries and construction industries for the manufacture of gears, shafts, pressure vessels, bars, bolts, and studs. The process of corrosion is unavoidable that cannot be eliminated but can be reduced with the help of different control methods [20,21,22,23]. From a metallurgical perception, heat treatment is commonly used as a means of controlling corrosion [1, 24,25,26,27,28,29,30,31].
Some of the heat treatments may improve the corrosion resistance and alter the properties of steel [24, 32]. Heat treatment techniques such as normalizing, annealing, tempering, and hardening are used to modify the microstructure, corrosion, and mechanical properties of metals [18, 33,34,35]. Heat treatment consists of successive heating, followed by quenching and tempering to obtain the desired properties [36, 37]. During heat treatment, the material undergoes changes in phase transformation and crystal structure affecting corrosion and mechanical properties. Because of the decline of coarse grains during heating, there is a minor improvement in the hardening of steels [19, 38].
Recently, due to its combination of strength and formability, emphasis has been placed on improving the properties of dual-phase (DP) steel [39]. Unlike most types of steel that have a single microstructural phase, dual-phase steel usually has a multiphase phase, composed of bainite, ferrite, and martensite phases. The high ductility and resistance are due to the unique microstructure of the dual-phase steel. As a result, dual-phase steel has acquired scientific and technical importance over the past decade [35, 40]. The microstructure of DP steel consists of a ferrite matrix, in which a second phase composed of martensite or perlite is observed as small patches. DP steel with ferrite–martensite phases has received considerable attention due to its unique properties such as strength, formability, and ductility. A variety of studies on the properties of these DP steels have revealed the unique possession of characteristic properties such as high elongation, and high tensile strength, which are utilized for the production of sheet materials in auto sectors [18, 24, 41].
Several mineral acids are used for various treatments such as descaling, pickling, acidification of oil wells, etc. Degradation of parts, particularly in acidic environments, has continued to become a high priority for researchers to find a lasting solution to the problem. The present study aims to analyse the true potential of heat-treated EN8 steel as a replacement material for various components in industries, improved mechanical properties, and corrosion resistance behaviour. Since limited literature is available regarding the effect of the morphology of phase constituents of EN8 steel on the corrosion behaviour, this study focuses on evaluating the corrosion behaviour of EN8 steel under different heat treatment temperatures in 0.5 M sulphuric acid solution (H2SO4) using the electrochemical and gravimetric methods.
2 Methodology
2.1 Material and Specimen Preparation
Table 1 shows the chemical composition of EN8 steel used in the study.
A medium carbon, moderate strength, non-alloy EN 8 steel was manufactured by Vizag Steel Plant, Visakhapatnam, and supplied by Hi-Tech Sales Corporation, Mangalore, India. It is used in the present investigation. Heat-treated test samples were prepared as a cylindrical rod 19 mm in diameter and 8 mm high. The samples were abraded using different grades of emery paper (80–800 µm) and finally on the disc-polishing wheel with different grades (3 to 0.25 µm) of diamond paste [42]. The abraded specimens were then washed with distilled water, cleaned with acetone, and eventually dried before immersing in the selected acid medium.
2.2 Heat Treatment Process of EN8 Steel
Initially, the samples were heated in a muffle furnace at the predetermined austenitization temperature (1173 K) [28, 42]. Figure 1 represents the process of austenitization. The samples were held at the austenitization temperature for 2 h. After an isothermal retention period of 2 h, the samples are immediately removed from the furnace for air cooling to obtain the ambient temperature structure. Austenitization is conducted at 973 K, 1023 K, 1063 K, and 1173 K for 2 h, followed by quenching in water to form dual-phase ferrite–martensite (F–M) structure.
2.3 Corrosive Medium Preparation
The environment chosen for the study is standardized sulphuric acid. A large quantity of sulphuric acid solution was prepared by diluting a reactive analytical grade sulphuric acid to a suitable volume. The concentration solution (0.5 M) was prepared by appropriately diluting the stock solution. The experiments were conducted at temperatures ranging from 303, 313, 323, and 333 K [42].
2.4 EIS and PDP Method
A moulded sample of EN8 steel with an exposed surface of 0.7 cm2 was used as a working electrode for measuring EIS and PDP. The platinum electrode and the calomel electrode were used as the counter electrode and the reference electrode, respectively. All three electrodes were immersed in the corrosive medium (0.5 M H2SO4) are connected to potentiostat CH600E. All electrochemical measurements were performed by maintaining a temperature of 303 K under aerated unstirred conditions. The sample reached steady-state open-circuit (OCP) potential after 30 min.
For EIS measurements, a frequency range of 100 Hz to 10 Hz and an amplitude of 10 mV of sinusoidal AC voltage have been selected. All the experiments were carried out three times and the average is reported. From the Nyquist curve obtained, the polarization resistance (Rp) is computed by the addition of the charge transfer resistance (Rct) and the solution resistance (Rs).
For the PDP studies, the working electrode was polarized with a potential of − 250 mV cathodic and + 250 mV anodically at an acquisition frequency of 1.0 mV−1. Extrapolation of the curves of the resulting tafel trace will yield a corrosion potential (Ecorr) and a corrosion current (Icorr).
2.5 Gravimetric Method
The gravimetric method of corrosion measurements is very simple, mainly because they do not require specialized tools apart from a precise balance. Experiments are typically performed according to a standard method (e.g. ASTM G1-90, 1996). The gravimetric method is mainly slower than other techniques, but many samples can be carried out simultaneously. This method provides the average corrosion rate (CR) over extended periods based on Eq. 1 [42]:
where, K = a constant (3.45 × 106), W = difference between the initial and final weight of the sample in gms, D = density of the sample in g cm−3, A = area of the sample exposed to corrosive medium in cm2, and T = immersion time in hours.
The gravimetric method is one of the simplest methods for calculating the inhibition performance of inhibitors and has high reliability.
2.6 Effect of Temperature and Kinetic Factors
Various kinetic parameters such as activation energy (Ea), activation enthalpy (ΔH#), and activation entropy (ΔS#) were calculated from the CRs obtained at different temperatures (303–333 K) using Eqs. 2 and 3:
where R = 8.314 J mol−1 K−1, T = absolute temperature (K), and B = a constant, which varies for different metals.
where h = Plank’s constant (6.626 × 10−34 J s), N = Avogadro’s number (6.022 × 1023 mol−1), and T = absolute temperature (K).
2.7 Scanning Electron Microscopy (SEM) and X-ray Diffraction Analysis (XRD)
The heat-treated EN 8 steel test samples were abraded using different grades of emery paper (100–2000 µm) and finally on the disc-polishing wheel with different grades (3 to 0.25 µm) of diamond paste. The abraded specimens were then washed with distilled water, cleaned with acetone, and dried. Then the polished samples are immersed in the Nital solution (etchant) to reveal the microstructural details. The surface morphology of the heat-treated EN8 steel under different conditions (Normalized, quenched at 973 K, 1023 K, 1063 K, and 1173 K) was determined using SEM. EVO MA18 at 5000 X magnification has been used to obtain the SEM images with an accelerated voltage of 15 kV. The XRD images of EN8 steel in different conditions (Normalized, quenched at 973 K, 1023 K, 1063 K, and 1173 K) were performed using Minifex 600 model instruments.
3 Results
3.1 Heat Treatment of EN8 Steel
Figure 2a represents the SEM image of the normalized condition—the microstructure of the steel. The microstructure has been characterized by fine and lamellar perlite and proeutectoid ferrite. Fine pearlite enhances the mechanical properties of the steel. Figure 2b–e shows the SEM images of the microstructure having ferrite and martensite under different heat treatment conditions. When the steel is maintained in the intercritical temperatures and is soaked in a liquid at room temperature, the austenite transforms into martensite and the proeutectoid ferrite remains unchanged. As the cooling takes place, the carbon atoms do not have sufficient time to diffuse from the austenite which results in the formation of a deformed martensite structure. Figure 2c–e indicates an increase in the amount of martensite in line with the increase in intercritical temperatures. As a result, Fig. 2e shows a greater area of martensite than Fig. 2c and d.
3.2 EIS Method
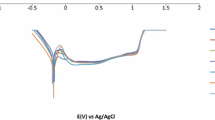
The Nyquist plot obtained for the dual-phase ferrite–martensite EN8 steel in 0.5 M H2SO4 under various heat treatment conditions is given in Fig. 3. The Nyquist diagram shows semicircles with a depressed capacitive-like loop at high frequency (HF) under different heat treatment conditions. The curves obtained are fitted using an equivalent circuit as shown in Fig. 3, which consists of Rs, Rct, and a constant phase element (CPE) using ZSimpWin version 3.1 software, and the values are represented in Table 2. The ideal double-layer capacitance has been replaced by CPE in the circuit due to the inhomogeneity displayed at the surface and this accounts for the depressive capacitive loop observed in the HF region. For this circuit, Cdl is calculated using Eq. (4):
where \(f_{\max }\) is the frequency maximum. The polarization resistance (Rp) is calculated using Eq. (5):
When comparing Rp values for different heat treatment conditions, EN8 DP steel quenched at 973 K exhibits the highest Rp value indicating the highest corrosion resistance. As the austenitization temperature increases from 1023 to 1173 K, there is a decrease in the Rp values which indicates a decrease in the corrosion resistance.
3.3 PDP Method
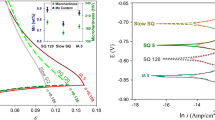
The corrosion of DP EN8 steel under different heat-treated conditions (normalized, quenched at 973 K, 1023 K, 1063 K, and 1173 K) was carried out in 0.5 M H2SO4 solution at 303 K and the Tafel plot obtained is depicted in Fig. 4. The sequence of result obtained from the PDP studies was in agreement with the result obtained from EIS measurement.
The icorr values obtained are reported in Table 3. The icorr value of DP EN8 steel quenched at 973 K is the lowest, which indicates a greater corrosion resistance. As the temperature at which quenching takes place increases (973 K to 1173 K), the value of icorr also increases. This indicates an increase in the corrosion rate with an increase in the temperature of heat treatment. The icorr value of the normalized DP EN8 steel was found to be lesser than the quenched specimen at 1173 K, which indicates a greater corrosion resistance for the normalized specimen than the other. The sequence of result obtained from the PDP studies was in agreement with the result obtained from EIS measurement.
3.4 Gravimetric Method
The effect of the weight loss of DP EN8 steel under different heat treatment conditions in 0.5 M H2SO4 at different temperatures is represented in Fig. 5. For all DP EN8 steel specimens, the weight loss was found to increase with the increase in the temperature of the immersed acidic medium which suggests an increase in the corrosion of the DP EN8 steel in relation to the temperature of the environment. The increase in weight loss with the increase in temperature as far as heat treatment is concerned indicates a higher corrosion rate for DP EN8 steel quenched at 1173 K. The result obtained from weight loss happens to be in line with that of the electrochemical study of corrosion.
3.5 Effect of Temperature and Kinetic Factors
The Arrhenius plots and the plot of ln (CR/T) vs 1/T of DP EN8 steel under different heat treatment conditions and temperature of immersion are represented in Fig. 6. The different activation parameters for DP EN8 steel in 0.5 M H2SO4 are illustrated in Table 4. At all austenitization temperatures, the activation energy (Ea) is found to be greater than 20 kJ mol−1. The value of ΔH# obtained is in line with the Ea values. The entropy of activation (ΔS#) for all the DP EN8 steel specimens is negative and has a large value. The ΔS# value becomes more negative as the austenitization temperature increases.
3.6 SEM and XRD Analysis
The XRD profiles of the EN8 steel under different heat treatment conditions are shown in Fig. 7. It is observed that the peak ferrite phase (110) is predominant for all samples irrespective of the type of heat treatment. Since the ferrite and martensite peaks overlap, it is very difficult to differentiate these two phases using the XRD [43, 44]. A further peak (200) can be seen for the metal sample, which also indicates the ferrite phase of the DP EN8 steel. As the austenitization temperature increases, the intensity of the two (110) and (200) decreases, and at 1173 K, this peak almost disappears.
The SEM image of the DP EN8 Steel under varying heat treatment conditions immersed in a 0.5 M H2SO4 medium is presented in Fig. 8. It is observed that the degradation of the surface caused by the corrosion process increases with the increase of the austenitization temperature from 973 to 1173 K of the quenched sample. When the normalized DP EN8 steel specimen is compared with the specimen quenched at 1173 K, the surface irregularity increases, which increases the corrosion for the later.
4 Discussions
In Sect. 3.1 (Fig. 2), the heat-treated samples showed a martensite phase which has a strongly distorted structure this is due to the steady diffusion of carbon particles from austenite [27]. According to the literature, the ferrite stabilizer (Cr) increases the quantity and morphology of the martensite in a dual-phase structure. The higher number of martensite leads to better ultimate tensile strength and hardness, but it has an adverse impact on the resistance and ductility. Hence, microstructure influences the mechanical characteristics of the steels [19].
The depressed capacitive-like loop at high frequency (HF) obtained from the Nyquist plots (Sect. 3.2, Fig. 3) under different heat treatment conditions is due to the frequency dispersion effect [45]. For DP EN8 steels containing ferrite and martensite, the corrosion behaviour depends on the amount of martensite and ferrite they contain. As the austenitization temperature increases from 1023 to 1173 K, the amount of martensite increases. This results in the increase in the micro galvanic corrosion cells in the region between the ferrite and martensite phases (lamellas), which acts as active corrosion centres [46, 47]. The normalized specimen showed greater corrosion resistance when compared to the water-quenched specimen at 1173 K, since the Rp value of the normalized specimen is greater than the other. This is due to the presence of lesser carbon content for normalized DP EN8 steel containing ferrite–pearlite phase than ferrite–martensite phase present in quenched specimen austenitized at 1173 K [48].
The Tafel plots (Fig. 4) and icorr values (Table 3) obtained indicate active dissolution of the metal. As the temperature at which quenching takes place increases (973 K to 1173 K), suggesting the reduction in corrosion resistance of DP steels. This indicates a change in the microstructure of DP steels with the increasing temperature, which can be attributed to an increase in the amount of martensite. Since the martensite phase is regarded as a supersaturated solid solution of carbon in the ferrite phase, there is a difference in the electrode potential between the martensite and ferrite phases, which leads to a formation of a galvanic cell between them, in which ferrite phase acted as anode and martensite phase as a cathode [49]. The leaching of the oxide layer from the electrode–electrolyte interface will lead to the further dissolution of the metal, which controls the cathodic reduction reaction. Therefore, there is a certain relationship between the dissolution rate of ferrite and the area ratio of ferrite to martensite in the form of galvanic couples [49]. Therefore, as the area of martensite increases in the material with an increase in the temperature of quenching from 973 to 1173 K, there is an increase in corrosion rate. The icorr value of the normalized DP EN8 steel was found to be lesser than the quenched specimen at 1173 K, which indicates a greater corrosion resistance for the normalized specimen as indicated in the discussion of EIS studies.
The effect of the weight loss of DP EN8 steel under different heat treatment conditions in 0.5 M H2SO4 (Fig. 5) is in line with EIS and Tafel studies. The effect of temperature on the corrosion behaviour of each heat-treated material was performed and the kinetic and thermodynamic parameters are obtained. The value of activation energy (Ea) is greater than 20 kJ mol−1 indicates that the process of oxidation is controlled by the surface reactions which is due to activation polarization [50]. The large and negative value of entropy of activation (ΔS#) indicates that the activation complex formed during the rate determination stage represents disassociation [51]. The increase in the negative value of ΔS# value with austenitization temperature is due to an increase in the kinetic energy of the surface molecules, which will increase the disorder.
The decrease in the intensity of the ferrite peak has been obtained from the XRD profiles (Fig. 7) of the EN8 steel with an increase in the temperature of heat treatment is due to the reduction in the volume fraction of the ferrite phase with an increase in the austenitization temperature [32]. The increased degradation of the surface with austenitization temperature (973 K to 1173 K) that is evident from the SEM images (Fig. 8) of the DP EN8 Steel under varying heat treatment conditions immersed in a 0.5 M H2SO4 medium is due to the increase in the amount of pits, through which the penetration of the corrosive medium occurs.
5 Conclusions
This research was conducted to understand the corrosion behaviour of different heat treatment conditions on EN8 dual-phase steel in 0.5 M sulphuric acid solution (H2SO4). Heat treatment was carried out on EN8 steel at 973 K, 1023 K, 1063 K, and 1173 K for 2 h followed by quenching in water to form a ferrite–martensite (F–M) dual-phase structure. The corrosion rate was characterized by EIS, PDP, and Gravimetric methods. Based on the findings, the following conclusions are drawn:
-
The corrosion rate increased with various heat treatment conditions as a function of the phase change when it was immersed in 0.5 M H2SO4 at the temperature of 303 K, 313 K, 323 K, and 333 K.
-
As the austenitization temperature increases from 1023 to 1173 K, the amount of martensite increases which acted as active corrosion centres.
-
The normalized specimen showed greater corrosion resistance when compared to the water-quenched specimen at 1173 K. This is due to the presence of lesser carbon content for normalized DP EN8 steel containing ferrite–pearlite phase than ferrite–martensite phase present in quenched specimen austenitizing at 1173 K.
-
The result obtained from weight loss happens to be in line with that of the electrochemical study of corrosion.
-
Surface characterization and XRD confirmed the corrosion behaviour of these heat-treated materials.
Data Availability
Inquiries about data availability should be directed to the corresponding author.
References
Abdo HS, Seikh AH, Mandal BB, Mohammed JA, Ragab SA, Abdo MS (2020) Microstructural characterization and corrosion-resistance behavior of dual-phase steels compared to conventional rebar. Crystals 10(11):1068
Sotelo-Mazon O et al (2020) Evaluation of corrosion inhibition of 1018 carbon steel using an avocado oil-based green corrosion inhibitor. Prot Met Phys Chem Surf 56(2):427–437
Martinez S, Metikoš-Huković M (2003) A nonlinear kinetic model introduced for the corrosion inhibitive properties of some organic inhibitors. J Appl Electrochem 33(12):1137–1142
Tansuǧ G, Tüken T, Kicir N, Erbil M (2014) Investigation of 2-aminoethanethiol as corrosion inhibitor for steel using response surface methodology (RSM). Ionics (Kiel) 20(2):287–294
Sharma L, Chhibber R (2019) Effect of heat treatment on mechanical properties and corrosion behaviour of API X70 linepipe steel in different environments. Trans Indian Inst Met 72(1):93–110
Berradja A (2019) Electrochemical techniques for corrosion and tribocorrosion monitoring: methods for the assessment of corrosion rates. IntechOpen, London
Olugbade T, Liu C, Lu J (2019) Enhanced passivation layer by Cr diffusion of 301 stainless steel facilitated by SMAT. Adv Eng Mater 21(8):1900125
Saxena A, Sharma V, Thakur KK, Bhardwaj N (2020) Electrochemical studies and the surface examination of low carbon steel by applying the extract of Citrus sinensis. J Bio-Tribo-Corrosion 6(2):41
Hanyang Zuo XL, Fan J, Liu F, Gong M, Zheng X, Meng J, Yang G (2021) Corrosion behavior of 3A21 aluminum alloy in water–ethylene glycol coolant under simulated engine working. Int J Electrochem Sci 16:17
Amin MA, Ahmed MA, Arida HA, Arslan T, Saracoglu M, Kandemirli F (2011) Monitoring corrosion and corrosion control of iron in HCl by non-ionic surfactants of the TRITON-X series—Part II. Temperature effect, activation energies and thermodynamics of adsorption. Corros Sci 53(2):540–548
Kayali Y, Anaturk B (2013) Investigation of electrochemical corrosion behavior in a 3.5 wt.% NaCl solution of boronized dual-phase steel. Mater Des 46:776–783
Hoai NT et al (2019) An improved corrosion resistance of steel in hydrochloric acid solution using Hibiscus sabdariffa leaf extract. Chem Pap 73(4):909–925
KhaniSanij MH, GhasemiBanadkouki SS, Mashreghi AR, Moshrefifar M (2012) The effect of single and double quenching and tempering heat treatments on the microstructure and mechanical properties of AISI 4140 steel. Mater Des 42:339–346
Bardelcik A, Worswick MJ, Wells MA (2014) The influence of martensite, bainite and ferrite on the as-quenched constitutive response of simultaneously quenched and deformed boron steel—experiments and model. Mater Des 55:509–525
Liu X, Xiao L, Wei C, Xu X, Luo M, Yan W (2018) Effect of multi-directional forging and annealing on abrasive wear behavior in a medium carbon low alloy steel. Tribol Int 119:608–613
Loto RT, Loto CA (2019) Data on the corrosion inhibition properties of centrimonium bromide with thiocarbanilide and with vanillin on low carbon steel in dilute acid media. Chem Data Collect 22:100250
Majd MT, Shahrabi T, Ramezanzadeh B (2019) Low carbon steel surface modification by an effective corrosion protective nanocomposite film based on neodymium-polyacrylic acid-benzimidazole. J Alloys Compd 783:952–968
Abedini O, Behroozi M, Marashi P, Ranjbarnodeh E, Pouranvari M (2019) Intercritical heat treatment temperature dependence of mechanical properties and corrosion resistance of dual phase steel. Mater Res. https://doi.org/10.1590/1980-5373-mr-2017-0969
Gurumurthy BM, Gowrishankar MC, Sharma S, Kini A, Shettar M, Hiremath P (2020) Microstructure authentication on mechanical property of medium carbon Low alloy duplex steels. J Mater Res Technol 9(3):5105–5111
Nathan CC (1973) Corrosion inhibitors. National Association of Corrosion Engineers, Houston
McCafferty E (2010) Introduction to corrosion science. Springer, New York
Fouda AS, El-shereafy EE, Hathoot AA, El-bahrawi NM (2020) Corrosion inhibition of aluminum by Cerumium rubrum extract in hydrochloric acid environment. J Bio-Tribo-Corrosion 6(2):37
Olugbade T, Lu J (2019) Enhanced corrosion properties of nanostructured 316 stainless steel in 0.6 M NaCl solution. J. Bio-Tribo-Corrosion 5(2):38
Ma J, Feng F, Yu B, Chen H, Fan L (2020) Effect of cooling temperature on the microstructure and corrosion behavior of X80 pipeline steel. Int J Miner Metall Mater 27(3):347–353
Katiyar PK, Misra S, Mondal K (2019) Comparative corrosion behavior of five microstructures (pearlite, bainite, spheroidized, martensite, and tempered martensite) made from a high carbon steel. Metall Mater Trans A 50(3):1489–1501
Song D et al (2019) Corrosion behavior and mechanism of Cr–Mo alloyed steel: role of ferrite/bainite duplex microstructure. J Alloys Compd 809:151787
Basantia SK, Bhattacharya A, Khutia N, Das D (2021) Plastic behavior of ferrite-pearlite, ferrite-bainite and ferrite–martensite steels: experiments and micromechanical modelling. Met Mater Int 27:1025
Xiong Z, Kostryzhev AG, Zhao Y, Pereloma EV (2019) Microstructure evolution during the production of dual phase and transformation induced plasticity steels using modified strip casting simulated in the laboratory. Metals 9(4):449
Keleştemur O, Aksoy M, Yildiz S (2009) Corrosion behavior of tempered dual-phase steel embedded in concrete. Int J Miner Metall Mater 16(1):43–50
Angkurarach L, Juijerm P (2020) Effects of high-temperature deep rolling on fatigue, work hardening, and residual stress relaxation of martensitic stainless steel AISI 420. J Mater Eng Perform 29(2):1416–1423
Fereiduni E, Ghasemi Banadkouki SS (2014) Improvement of mechanical properties in a dual-phase ferrite–martensite AISI4140 steel under tough-strong ferrite formation. Mater Des 56:232–240
Salamci FKE, Candan S (2017) Effect of microstructure on corrosion behavior of dual-phase steels. Kov Mater 5(2):133–139
Bignozzi MC et al (2020) Effect of heat treatment conditions on retained austenite and corrosion resistance of the X190CrVMo20-4-1 stainless steel. Met Mater Int 26(9):1318–1328
Zhang Q, Li Q, Chen X (2020) Effect of heat treatment on corrosion behavior of Mg–5Gd–3Y–0.5Zr alloy. RSC Adv 10(71):43371–43382
Prabhu D, Sharma S, Prabhu PR, Jomy J, Sadanand RV (2021) Analysis of the inhibiting action of pectin on corrosion of AISI1040 dual-phase steel with ferrite–martensite and ferrite–bainite structure: a comparison in 0.5 M sulphuric acid. J Iran Chem Soc 19:1109–1128
Mohd Fauzi MA, Saud SN, Hamzah E, Mamat MF, Ming LJ (2019) In vitro microstructure, mechanical properties and corrosion behaviour of low, medium and high carbon steel under different heat treatments. J Bio-Tribo-Corrosion 5(2):1–11
Zhang D et al (2018) Effect of tempered martensite and ferrite/bainite on corrosion behavior of low alloy steel used for flexible pipe exposed to high-temperature brine environment. J Mater Eng Perform 27(9):4911–4920
Jacobo LR, García-Hernández R, López-Morelos VH, Contreras A (2020) Effect of acicular ferrite and bainite in API X70 steel obtained after applying a heat treatment on corrosion and cracking behaviour. Met Mater Int 27:3750–3764
Gerengi H, Sen N, Uygur I, Kaya E (2020) Corrosion behavior of dual phase 600 and 800 steels in 3.5 wt.% NaCl environment. J Adhes Sci Technol 34(8):903–915
Tasan CC et al (2015) An overview of dual-phase steels: advances in microstructure-oriented processing and micromechanically guided design. Annu Rev Mater Res 45(1):391–431
Ismail M, Muhammad B, Hamzah E, Keong T (2012) Corrosion behaviour of dual-phase and galvanized steels in concrete. Anti-Corrosion Methods Mater 59(3):132–138
Prabhu PR, Prabhu D, Chaturvedi A, KishoreDodhia P (2021) Corrosion inhibition of ferrite bainite AISI1040 steel in H2SO4 using biopolymer. Cogent Eng. 8(1):1950304
Li Z, Wei F, La P, Sheng J (2018) Enhancing strength of nanolaminate 1045 steel prepared by aluminothermic reaction through multiple warm rolling. Steel Res Int 89(2):1700304
García-Junceda A, Díaz-Rivera C, Gómez-Torralba V, Rincón M, Campos M, Torralba JM (2019) Analysis of the interface and mechanical properties of field-assisted sintered duplex stainless steels. Mater Sci Eng A 740–741(October):410–419
Rodríguez-Torres A, Olivares-Xometl O, Valladares-Cisneros MG, González-Rodríguez JG (2018) Effect of green corrosion inhibition by Prunus persica on AISI 1018 carbon steel in 0.5M H2SO4. Int J Electrochem Sci 13:3023–3049
Katiyar PK, Misra S, Mondal K (2018) Effect of different cooling rates on the corrosion behavior of high-carbon pearlitic steel. J Mater Eng Perform 27(4):1753–1762
Kumar S, Kumar A, Vinaya, Madhusudhan R, Sah R, Manjini S (2019) Mechanical and electrochemical behavior of dual-phase steels having varying ferrite–martensite volume fractions. J Mater Eng Perform 28(6):3600–3613
Osório WR, Peixoto LC, Garcia LR, Garcia A (2009) Electrochemical corrosion response of a low carbon heat treated steel in a NaCl solution. Mater Corros 60(10):804–812
Hao X et al (2020) Influence of intercritical quenching temperature on microstructure, mechanical properties and corrosion resistance of dual-phase steel. J Mater Eng Perform 29(7):4446–4456
Oguzie EE (2007) Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract. Corros Sci 49(3):1527–1539
Deepa P, Padmalatha R (2017) Corrosion behaviour of 6063 aluminium alloy in acidic and in alkaline media. Arab J Chem 10:S2234–S2244
Acknowledgements
The authors acknowledge the laboratory facility extended by the Department of Chemistry and Department of Mechanical & Manufacturing Engineering, Manipal Institute of Technology, Manipal, MAHE.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. The authors received no direct funding for this research article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author claims no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prabhu, D., Jomy, J. & Prabhu, P.R. Influence of Different Heat Treatment Temperatures on the Microstructure and Corrosion Behaviour of Dual-Phase EN8 Steel in 0.5 M Sulphuric Acid Solution. J Bio Tribo Corros 8, 88 (2022). https://doi.org/10.1007/s40735-022-00689-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-022-00689-7