Abstract
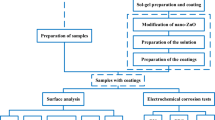
Application of biopolymers as biomaterials has greatly impacted the development of modern medicine. Specifically, biopolymers have a lot of applications in biomedical field because of its capability to tie through bone and soft tissues. Present study shows Zn alloy improves biocompatibility and the corrosion resistance ability when it is coated with Chitin-Ni-doped ZnO biocomposite material. Here, the electrochemical corrosion behavior of coated Zn alloy is examined in Simulated body fluid (SBF) solution and the concentration of Ni doped ZnO is investigated in Chitin-Ni doped ZnO biocomposites. To study the structural properties and to the presence of different Functional groups in coated materials X-ray diffraction (XRD) and Fourier transform infrared spectroscopy with KBr pellets (KBr-FTIR) techniques were used. Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDAX) techniques were used to understand the surface morphology with elemental analysis of Chitin-Ni doped ZnO biocomposite material. The chemical composition of Chitin and Ni/ZnO in Chitin-Ni doped ZnO biocomposites were examined by XPS studies. AFM and the contact angle measurement shows that Chitin-Ni doped ZnO biocomposites coating improves anticorrosion performance by enhancing the surface roughness and hydrophilic nature of Zn alloy. The Electrochemical analysis of Zn alloy was performed to evaluate corrosion resistance in SBF solution for 24 h. It shows a decreasing trend in the current density with increase in corrosion resistance and the corrosion potential which shifts towards the positive direction from potentiodynamic polarization studies. Electrochemical impedance spectroscopy (EIS) study reveals a good capacitor behavior and high resistance value for Chitin-Ni doped ZnO-coated Zn alloy substrate.










Similar content being viewed by others
References
Bowen PK, Drelich J, Goldman J (2013) Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv Mater 25:2577–2582. https://doi.org/10.1002/adma.201300226
Murni NS, Dambatta MS, Yeap SK et al (2015) Cytotoxicity evaluation of biodegradable Zn-3Mg alloy toward normal human osteoblast cells. Mater Sci Eng C 49:560–566. https://doi.org/10.1016/j.msec.2015.01.056
Li H, Zheng Y, Qin L (2014) Progress of biodegradable metals. Prog Nat Sci Mater Int 24:414–422. https://doi.org/10.1016/j.pnsc.2014.08.014
Purnama A, Hermawan H, Mantovani D (2014) Biodegradable metal stents: a focused review on materials and clinical studies. J Biomater Tissue Eng 4:1–6. https://doi.org/10.1166/jbt.2014.1263
Yang H, Wang C, Liu C et al (2017) Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 145:92–105. https://doi.org/10.1016/j.biomaterials.2017.08.022
Törne K, Larsson M, Norlin A, Weissenrieder J (2016) Degradation of zinc in saline solutions, plasma, and whole blood. J Biomed Mater Res Part B 104:1141–1151. https://doi.org/10.1002/jbm.b.33458
Zhang S, Zhang X, Zhao C et al (2010) Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater 6:626–640. https://doi.org/10.1016/j.actbio.2009.06.028
Li HF, Xie XH, Zheng YF et al (2015) Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci Rep 5:1–14. https://doi.org/10.1038/srep10719
Chandra RK (1984) Excessive intake of zinc impairs immune responses. JAMA J Am Med Assoc 252:1443–1446. https://doi.org/10.1001/jama.1984.03350110043027
Martins R, Nathan A, Barros R et al (2011) Complementary metal oxide semiconductor technology with and on paper. Adv Mater 23:4491–4496. https://doi.org/10.1002/adma.201102232
MacDonald RS (2000) The role of zinc in growth and cell proliferation. J Nutr 130(5):1500S–1508S. https://doi.org/10.1093/jn/130.5.1500s
Lansdown ABG, Mirastschijski U, Stubbs N et al (2007) Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair Regen 15:2–16
Jacob J, Haponiuk JT, Thomas S, Gopi S (2018) Biopolymer based nanomaterials in drug delivery systems: a review. Mater Today Chem 9:43–55. https://doi.org/10.1016/j.mtchem.2018.05.002
Oh JK, Lee DI, Park JM (2009) Biopolymer-based microgels/nanogels for drug delivery applications. Prog Polym Sci 34:1261–1282. https://doi.org/10.1016/j.progpolymsci.2009.08.001
Suner SS, Sahiner M, Sengel SB et al (2018) Responsive biopolymer-based microgels/nanogels for drug delivery applications. Elsevier Ltd, Amsterdam
Rebelo R, Fernandes M, Fangueiro R (2017) Biopolymers in medical implants: a brief review. Proc Eng 200:236–243. https://doi.org/10.1016/j.proeng.2017.07.034
Degeratu C (2014) Biopolymer based structures for biological tissue reconstruction Cristinel-Nicolae DEGERATU Structures de biopolymères pour la reconstruction de tissus biologiques Biopolymer based structures for biological tissue reconstruction
Bressan E, Favero V, Gardin C et al (2011) Biopolymers for hard and soft engineered tissues: application in odontoiatric and plastic surgery field. Polymers (Basel) 3:509–526. https://doi.org/10.3390/polym3010509
Jaganathan SK, Supriyanto E, Murugesan S et al (2014) Biomaterials in cardiovascular research: applications and clinical implications. Biomed Res Int. https://doi.org/10.1155/2014/459465
Ramesan MT, Siji C, Kalaprasad G et al (2018) Effect of silver doped zinc oxide as nanofiller for the development of biopolymer nanocomposites from chitin and cashew gum. J Polym Environ 26:2983–2991. https://doi.org/10.1007/s10924-018-1187-6
Abolghassem S, Molaei S, Javanshir S (2019) Preparation of α-chitin-based nanocomposite as an effective biocatalyst for microwave aided domino reaction. Heliyon 5:e02036. https://doi.org/10.1016/j.heliyon.2019.e02036
Feng M, Lu X, Jiang K et al (2018) One-step preparation of an antibacterial chitin/Zn composite from shrimp shells using urea-Zn(OAc)2·2H2O aqueous solution. Green Chem 20:2212–2217. https://doi.org/10.1039/c8gc00767e
Cader Mhd Haniffa MA, Ching YC, Abdullah LC et al (2016) Review of bionanocomposite coating films and their applications. Polymers (Basel) 8:1–33. https://doi.org/10.3390/polym8070246
Göpferich A, Langer R (1993) Modeling of polymer erosion. Macromolecules 26:4105–4112. https://doi.org/10.1021/ma00068a006
Zhang Z, Kuijer R, Bulstra SK et al (2006) The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials 27:1741–1748. https://doi.org/10.1016/j.biomaterials.2005.09.017
Abe K, Ifuku S, Kawata M (2014) Preparation of tough hydrogels based on b -chitin nanofibers via NaOH treatment. Cellulose 21:535–540. https://doi.org/10.1007/s10570-013-0095-0
Chem JM, Chang C, Chen S, Zhang L (2011) Novel hydrogels prepared via direct dissolution of chitin at low temperature: structure and biocompatibility. J Mater Chem 21:3865–3871. https://doi.org/10.1039/c0jm03075a
Jayakumar R, Prabaharan M, Nair SV, Tamura H (2010) Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv 28:142–150. https://doi.org/10.1016/j.biotechadv.2009.11.001
Cooper A, Zhong C, Kinoshita Y et al (2012) Self-assembled chitin nanofiber templates for artificial neural networks. J Mater Chem 22:3105–3109. https://doi.org/10.1039/c2jm15487k
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
Hassanzadeh P, Kharaziha M, Nikkhah M et al (2013) Chitin nanofiber micropatterned flexible substrates for tissue engineering. J Mater Chem B 1:4217–4224. https://doi.org/10.1039/c3tb20782j
Fan Y, Saito T, Isogai A (2010) Individual chitin nano-whiskers prepared from partially deacetylated a -chitin by fibril surface cationization. Carbohydr Polym 79:1046–1051. https://doi.org/10.1016/j.carbpol.2009.10.044
Jiang Y, Song Y, Miao M et al (2015) Transparent nanocellulose hybrid films functionalized with ZnO nanostructures for UV-blocking. J Mater Chem C 3:6717–6724. https://doi.org/10.1039/c5tc00812c
Marx KA (2003) Quartz crystal microbalance: a useful tool for studying thin polymer films and complex biomolecular systems at the solution—surface interface. Biomacromol 4:1099–1120
Mohanty S, Nayak SK (2016) Polyvinyl alcohol-modified pithecellobium clypearia benth herbal residue fiberpolypropylene composites. Polym Compos 37:915–924
Ramesan MT, Nidhisha V, Jayakrishnan P (2017) Synthesis, characterization and conducting properties of novel poly (vinyl cinnamate)/zinc oxide nanocomposites via in situ polymerization. Mater Sci Semicond Process 63:253–260. https://doi.org/10.1016/j.mssp.2017.02.027
Yadollahi M, Farhoudian S, Barkhordari S et al (2016) Facile synthesis of chitosan/ZnO bio-nanocomposite hydrogel beads as drug delivery systems. Int J Biol Macromol 82:273–278. https://doi.org/10.1016/j.ijbiomac.2015.09.064
Lv S, Zhang K, Zhu L, Tang D (2019) ZIF-8-Assisted NaYF4:Yb, Tm@ZnO converter with exonuclease III-powered DNA walker for near-infrared light responsive biosensor. Anal Chem. https://doi.org/10.1021/acs.analchem.9b04710
Wang S, Zhu B, Liu M et al (2019) Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl Catal B 243:19–26. https://doi.org/10.1016/j.apcatb.2018.10.019
Rodlamul P, Tamura S, Imanaka N (2019) Effect of p- or n-type semiconductor on CO sensing performance of catalytic combustion-type CO gas sensor with CeO2-ZrO2-ZnO based catalyst. Bull Chem Soc Jpn 92:585–591. https://doi.org/10.1246/bcsj.20180284
Kallappa D, Venkatarangaiah VT (2018) Synthesis of CeO2 doped ZnO nanoparticles and their application in Zn-composite coating on mild steel. Arab J Chem 13:2309–2317. https://doi.org/10.1016/j.arabjc.2018.04.014
Risbud MV, Bhonde RR (2000) Polyacrylamide-chitosan hydrogels: in vitro biocompatibility and sustained antibiotic release studies. Drug Deliv J Deliv Target Ther Agents 7:69–75. https://doi.org/10.1080/107175400266623
Lin ZF, Wang Y, Zhang D, Li XB (2016) Corrosion resistance research of ZnO/polyelectrolyte composite film. Int J Electrochem Sci 11:8512–8519. https://doi.org/10.20964/2016.10.37
Deyá MC, Romagnoli R, Romagnoli R, Del Amo B (2004) The influence of zinc oxide on the anticorrosive behaviour of eco—friendly paints. Corros Rev 22:1–18. https://doi.org/10.1515/CORRREV.2004.22.1.1
Chandrappa KG, Venkatesha TV, Nayana KO, Punithkumar MK (2012) Generation of nanocrystalline NiO particles by solution combustion method and its Zn-NiO composite coating for corrosion protection. Mater Corros 63:445–455. https://doi.org/10.1002/maco.201005966
Müller L, Müller FA (2006) Preparation of SBF with different HCO3- content and its influence on the composition of biomimetic apatites. Acta Biomater 2:181–189. https://doi.org/10.1016/j.actbio.2005.11.001
Arthanari S, KwangSeon S, Rajendran N (2016) Influence of bicarbonate concentration on the conversion layer formation onto AZ31 Magnesium alloy and its electrochemical corrosion behaviour in simulated body fluid. RSC Adv 6:49910–49922. https://doi.org/10.1039/C6RA08478H
GEARY SAAL, Metals (1957) Electrochemical polarization. J Electrochem Soc 104:56–63. https://doi.org/10.1149/1.2428496
Huang VM-W, Vivier V, Orazem ME et al (2007) The apparent constant-phase-element behavior of an ideally polarized blocking electrode. J Electrochem Soc 154:C81. https://doi.org/10.1149/1.2398882
Hsu CH, Mansfeld F (2001) Concernng the conversion of the constant phase element parameter Y0into a capacitance. Corrosion 57:747–748. https://doi.org/10.5006/1.3280607
Marchessault RH, Pearson FG, Liang CY (1960) Infrared spectra of crystalline polysaccharides. Biochim Biophys Acta 45:499–507. https://doi.org/10.1016/0006-3002(60)91486-4
Kumirska J, Czerwicka M, Kaczyński Z et al (2010) Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar Drugs 8:1567–1636. https://doi.org/10.3390/md8051567
Ashwin Kumar N, Sanoj Rejinold N, Anjali P et al (2013) Preparation of chitin nanogels containing nickel nanoparticles. Carbohydr Polym 97:469–474. https://doi.org/10.1016/j.carbpol.2013.05.009
Sohi MH, Jalali M (2003) Study of the corrosion properties of zinc-nickel alloy electrodeposits before and after chromating. J Mater Process Technol 138:63–66. https://doi.org/10.1016/S0924-0136(03)00050-5
Blawert C, Bala Srinivasan P (2010) Plasma electrolytic oxidation treatment of magnesium alloys. Surf Eng Light Alloy Alum Magnes Titan Alloy. https://doi.org/10.1533/9781845699451.2.155
Nuss J, Wedig U, Kirfel A, Jansen M (2010) The structural anomaly of zinc: Evolution of lattice constants and parameters of thermal motion in the temperature range of 40 to 500 K. Z Anorg und Allg Chem 636:309–313. https://doi.org/10.1002/zaac.200900460
McMurdie HF, Evans EH, Morris MC et al (1986) Standard X-ray diffraction powder patterns from the JCPDS research associateship. Powder Diffr. https://doi.org/10.1017/S0885715600011593
Vogler EA (2013) Surface modification for biocompatibility. engineered biomimicry. Elsevier Inc, Amsterdam, pp 189–220
Drelich J, Chibowski E, Meng DD, Terpilowski K (2011) Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 7:9804–9828. https://doi.org/10.1039/c1sm05849e
Rudolph A, Teske M, Illner S et al (2015) Surface modification of biodegradable polymers towards better biocompatibility and lower thrombogenicity. PLoS ONE 10:1–17. https://doi.org/10.1371/journal.pone.0142075
Lusty JR, Chan HSO, Khor E, Peeling J (1985) The synthesis and characterisation of some xanthine complexes: evidence for the existence of oxygen involvement using O(6) and O(2). Inorg Chim Acta 106:209–218. https://doi.org/10.1016/S0020-1693(00)82271-9
Wayne J (1977) X-ray photoelectron spectra and electronic structure of some diamine compounds. J Electron Spectrosc Relat Phenom 11:123–127
Arfelli M, Cossu G, Mattogno G et al (1990) X-ray spectroscopic characterization of Cu2+-phenanthroline complexes intercalated in α-zirconium phosphate. J Incl Phenom Mol Recognit Chem 9:161–170. https://doi.org/10.1007/BF01041260
Barbaux Y, Dekiouk M, Le Maguer D et al (1992) Bulk and surface analysis of a Fe-P-O oxydehydrogenation catalyst. Appl Catal A 90:51–60. https://doi.org/10.1016/0926-860X(92)80247-A
Landis WJ, Martin JR (1984) X-ray photoelectron spectroscopy applied to gold-decorated mineral standards of biological interest. J Vac Sci Technol A 2:1108–1111. https://doi.org/10.1116/1.572680
Stipp SLS, Hochella MF (1991) Structure and bonding at the calcite surface as observed with X-ray photoelectron spectroscopy (XPS) and (LEED). Geochim Cosmochim Acta 55:1723–1736
Acknowledgements
Deepti Jain greatfully acknowledge financial support from the Dr. Ramdas Pai Scholarship, Manipal University Jaipur, India (Ref: DOR/RPSA/2018-19/08-06) and Shubhra Pareek from the UGC-DAE CSR, Kalpakkam, India (Grant Ref. No: CSR-KN/CRS-96) for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jain, D., Pareek, S., Chattopadhyay, S. et al. Ni-Doped ZnO-Chitin Composites for Anti-Corrosive Coating on Zn Alloy in Simulated Body Fluid Solution. J Bio Tribo Corros 6, 113 (2020). https://doi.org/10.1007/s40735-020-00411-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00411-5