Abstract
Purpose of Review
Wood-based adsorbents are increasingly used for environmental applications. They demonstrate considerable advantages, including renewable feedstock, relatively simple preparation processes, and advantageous structural and surface properties. In short, they provide environmentally friendly, effective, and economical sources for contaminant removal. This review summarizes recent advances in the preparation and use of selected modified wood-based residues (biochar, ash, and cellulose) as adsorbents for environmental applications (water, air, and soil remediation).
Recent Findings
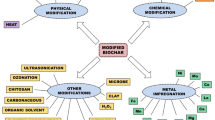
Although chemical modifications have produced better results for wood-based adsorbents, the inherent corrosion problems and safety issues have made physical modifications more feasible on an industrial scale. For environmental remediation, inorganic contaminants can be removed by raw and modified wood-based adsorbents, mainly via electrostatic interaction, surface complexation, pore filling, and ion exchange. Organic contaminants are removed via van der Waals forces between unsaturated polycyclic molecules, pore filling, and hydrogen bonding. Specific surface area and porosity are critical parameters for effective contaminant adsorption, mostly from water and air. A comparison of wood-based residues used for wastewater treatment ranked the efficiency as ash > cellulose > biochar versus cellulose > biochar > ash for air remediation. Adding modified wood residues to soil enhances the fertility and biological characteristics in addition to remediation. Moreover, spent wood-based adsorbents can be used in construction materials, soil fertilizers, and catalysts.
Summary
This review summarizes classical and new physical and chemical methods for modifying wood adsorbents and the impacts on physiochemical characteristics such as porosity, pore volume, surface area, and surface functional groups. Also addressed are the adsorption capacity and efficiency of raw and modified wood adsorbents for removing contaminants from synthetic effluents, mine water, air, and soil. Valorization methods for spent modified wood-based adsorbents are then outlined. Suggestions and prospects are given for future studies on environmental decontamination by wood residues.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zhang J, Koubaa A, Tao Y, Li P, Xing D. The emerging development of transparent wood: materials, characteristics, and applications. Curr For Rep. 2022;8(4):333–45. https://doi.org/10.1007/s40725-022-00172-z.
••Braghiroli FL, Passarini L. Valorizing biomass residues from forest operations and wood manufacturing presents many sustainable and innovative possibilities. Curr For Rep. 2020;6(2):172–83. https://doi.org/10.1007/s40725-020-00112-9. This review provides information on the valorization of forest biomass residues.
Durocher C, Thiffault E, Achim A, Auty D, Barrette J. Untapped volume of surplus forest growth as feedstock for bioenergy. Biomass Bioenergy. 2019;120:376–86. https://doi.org/10.1016/j.biombioe.2018.11.024.
Kizha AR, Han H-S. Forest residues recovered from whole-tree timber harvesting operations. Eur J For Eng. 2015;1(2):46–55. Retrieved from https://dergipark.org.tr/en/pub/ejfe/issue/17009/281374
Saravanakumar A, Vijayakumar P, Hoang AT, Kwon EE, Chen W-H. Thermochemical conversion of large-size woody biomass for carbon neutrality: principles, applications, and issues. Bioresour Technol. 2023;370:128562. https://doi.org/10.1016/j.biortech.2022.128562.
Spinelli R, Eliasson L, Han H-S. A critical review of comminution technology and operational logistics of wood chips. Curr For Rep. 2020;6(3):210–9. https://doi.org/10.1007/s40725-020-00120-9.
Shaheen SM, Niazi NK, Hassan NEE, Bibi I, Wang H, Tsang Daniel CW, et al. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: a critical review. Int Mater Rev. 2019;64(4):216–47. https://doi.org/10.1080/09506608.2018.1473096.
Oliveira FR, Patel AK, Jaisi DP, Adhikari S, Lu H, Khanal SK. Environmental application of biochar: current status and perspectives. Bioresour Technol. 2017;246:110–22. https://doi.org/10.1016/j.biortech.2017.08.122.
•Zhang L, Guo L, Wei G. Recent advances in the fabrication and environmental science applications of cellulose nanofibril-based functional materials. Materials. 2021;14(18). https://doi.org/10.3390/ma14185390. This paper reviews cellulose functionalization strategies for environmental science applications.
Braghiroli FL, Bouafif H, Neculita CM, Koubaa A. Activated biochar as an effective sorbent for organic and inorganic contaminants in water. Wat Air Soil Poll. 2018;229(7):230. https://doi.org/10.1007/s11270-018-3889-8.
Zhang X, Sun P, Wei K, Huang X, Zhang X. Enhanced H2O2 activation and sulfamethoxazole degradation by Fe-impregnated biochar. Chem Eng J. 2020;385:123921. https://doi.org/10.1016/j.cej.2019.123921.
Liu C, Zhang H-X. Modified-biochar adsorbents (MBAs) for heavy-metal ions adsorption: a critical review. J Environ Chem Eng. 2022;10(2):107393. https://doi.org/10.1016/j.jece.2022.107393.
Kumar M, Dutta S, You S, Luo G, Zhang S, Show PL, et al. A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. J Clean Prod. 2021;305:127143. https://doi.org/10.1016/j.jclepro.2021.127143.
Li Q, Yu W, Guo L, Wang Y, Zhao S, Zhou L, et al. Sorption of sulfamethoxazole on inorganic acid solution-etched biochar derived from alfalfa. Materials. 2021;14(4). https://doi.org/10.3390/ma14041033.
Cheng N, Wang B, Wu P, Lee X, Xing Y, Chen M, et al. Adsorption of emerging contaminants from water and wastewater by modified biochar: a review. Environ Pollut. 2021;273:116448. https://doi.org/10.1016/j.envpol.2021.116448.
Zhu S, Zhao J, Zhao N, Yang X, Chen C, Shang J. Goethite modified biochar as a multifunctional amendment for cationic Cd(II), anionic As(III), roxarsone, and phosphorus in soil and water. J Clean Prod. 2020;247:119579. https://doi.org/10.1016/j.jclepro.2019.119579.
Huang K, Hu C, Tan Q, Yu M, Shabala S, Yang L, et al. Highly efficient removal of cadmium from aqueous solution by ammonium polyphosphate-modified biochar. Chemosphere. 2022;305:135471. https://doi.org/10.1016/j.chemosphere.2022.135471.
Zhang P, Tian X, Fu D. CO2 removal in tray tower by using AAILs activated MDEA aqueous solution. Energy. 2018;161:1122–32. https://doi.org/10.1016/j.energy.2018.07.162.
Calugaru IL, Neculita CM, Genty T, Zagury GJ. Metals and metalloids treatment in contaminated neutral effluents using modified materials. J Environ Manage. 2018;212:142–59. https://doi.org/10.1016/j.jenvman.2018.02.002.
Hussain Z, Chang N, Sun J, Xiang S, Ayaz T, Zhang H, et al. Modification of coal fly ash and its use as a low-cost adsorbent for the removal of directive, acid, and reactive dyes. J Hazard Mater. 2022;422:126778. https://doi.org/10.1016/j.jhazmat.2021.126778.
Qiu R, Cheng F, Huang H. Removal of Cd2+ from aqueous solution using hydrothermally modified circulating fluidized bed fly ash resulting from coal gangue power plant. J Clean Prod. 2018;172:1918–27. https://doi.org/10.1016/j.jclepro.2017.11.236.
Gao M, Ma Q, Lin Q, Chang J, Bao W, Ma H. Combined modification of fly ash with Ca(OH)2/Na2FeO4 and its adsorption of methyl orange. Appl Surf Sci. 2015;359:323–30. https://doi.org/10.1016/j.apsusc.2015.10.135.
Attari M, Bukhari SS, Kazemian H, Rohani S. A low-cost adsorbent from coal fly ash for mercury removal from industrial wastewater. J Environ Chem Eng. 2017;5(1):391–9. https://doi.org/10.1016/j.jece.2016.12.014.
Ranasinghe RAJC, Hansima MACK, Nanayakkara KGN. Adsorptive removal of fluoride from water by chemically modified coal fly ash: synthesis, characterization, kinetics, and mechanisms. Ground Sustain Dev. 2022;16:100699. https://doi.org/10.1016/j.gsd.2021.100699.
Chai F, Wang R, Yan L, Li G, Cai Y, Xi C. Facile fabrication of pH-sensitive nanoparticles based on nanocellulose for fast and efficient As(V) removal. Carbohydr Polym. 2020;245:116511. https://doi.org/10.1016/j.carbpol.2020.116511.
Zhao F, Repo E, Song Y, Yin D, Hammouda SB, Chen L, et al. Polyethylenimine-cross-linked cellulose nanocrystals for highly efficient recovery of rare earth elements from water and a mechanism study. Green Chem. 2017;19(20):4816–28. https://doi.org/10.1039/C7GC01770G.
Dufresne A. Nanocellulose processing properties and potential applications. Curr For Rep. 2019;5(2):76–89. https://doi.org/10.1007/s40725-019-00088-1.
Georgouvelas D, Jalvo B, Valencia L, Papawassiliou W, Pell AJ, Edlund U, et al. Residual lignin and zwitterionic polymer grafts on cellulose nanocrystals for antifouling and antibacterial applications. ACS Appl Polym Mater. 2020;2(8):3060–71. https://doi.org/10.1021/acsapm.0c00212.
Lei C, Gao J, Ren W, Xie Y, Abdalkarim SYH, Wang S, et al. Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr Polym. 2019;205:35–41. https://doi.org/10.1016/j.carbpol.2018.10.029.
Wang Y, Gong Y, Lin N, Jiang H, Wei X, Liu N, et al. Cellulose hydrogel coated nanometer zero-valent iron intercalated montmorillonite (CH-MMT-nFe0) for enhanced reductive removal of Cr(VI): characterization, performance, and mechanisms. J Mol Liq. 2022;347:118355. https://doi.org/10.1016/j.molliq.2021.118355.
Ambika S, Kumar M, Pisharody L, Malhotra M, Kumar G, Sreedharan V, et al. Modified biochar as a green adsorbent for removal of hexavalent chromium from various environmental matrices: mechanisms, methods, and prospects. Chem Eng J. 2022;439:135716. https://doi.org/10.1016/j.cej.2022.135716.
Lyu H, Gao B, He F, Zimmerman AR, Ding C, Tang J, et al. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem Eng J. 2018;335:110–9. https://doi.org/10.1016/j.cej.2017.10.130.
••Sajjadi B, Chen W-Y, Egiebor NO. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev Chem Eng. 2019;35(6):735–76. https://doi.org/10.1515/revce-2017-0113. Excellent review paper on physical modification methods and their impact on physiochemical properties of biochar.
Oyehan TA, Salami BA, Badmus SO, Saleh TA. Technological trends in activation and modification of palm oil fuel ash for advanced water and wastewater treatment – a review. Sustain Chem Pharm. 2022;29:100754. https://doi.org/10.1016/j.scp.2022.100754.
Tan S, Zhou G, Yang Q, Ge S, Liu J, Cheng YW, et al. Utilization of current pyrolysis technology to convert biomass and manure waste into biochar for soil remediation: a review. Sci Total Environ. 2023;864:160990. https://doi.org/10.1016/j.scitotenv.2022.160990.
Kumar A, Singh E, Mishra R, Kumar S. Biochar as environmental armor and its diverse role towards protecting soil, water, and air. Sci Total Environ. 2022;806:150444. https://doi.org/10.1016/j.scitotenv.2021.150444.
Guo Y, Zhao C, Chen X, Li C. CO2 capture and sorbent regeneration performances of some wood ash materials. Appl Energy. 2015;137:26–36. https://doi.org/10.1016/j.apenergy.2014.09.086.
Braghiroli FL, Bouafif H, Neculita CM, Koubaa A. Influence of pyro-gasification and activation conditions on the porosity of activated biochars: a literature review. Waste Biomass Valorization. 2020;11(9):5079–98. https://doi.org/10.1007/s12649-019-00797-5.
Braghiroli FL, Bouafif H, Koubaa A. Enhanced SO2 adsorption and desorption on chemically and physically activated biochar made from wood residues. Ind Crops Prod. 2019;138:111456. https://doi.org/10.1016/j.indcrop.2019.06.019.
••Gargiulo V, Gomis-Berenguer A, Giudicianni P, Ania CO, Ragucci R, Alfè M. Assessing the potential of biochars prepared by steam-assisted slow pyrolysis for CO2 adsorption and separation. Energy Fuels. 2018;32(10):10218–27. https://doi.org/10.1021/acs.energyfuels.8b01058. This study investigated how the surface area and porosity of modified wood-based biochar affected SO2 adsorption.
•Braghiroli FL, Bouafif H, Hamza N, Neculita CM, Koubaa A. Production, characterization, and potential of activated biochar as adsorbent for phenolic compounds from leachates in a lumber industry site. Environ Sci Pollut Res. 2018;25(26):26562–75. https://doi.org/10.1007/s11356-018-2712-9. This paper analyzes the effect of steam-assisted pyrolysis method on biochar for CO2 adsorption.
Wang B, Gao B, Fang J. Recent advances in engineered biochar productions and applications. Crit Rev Environ Sci Technol. 2017;47(22):2158–207. https://doi.org/10.1080/10643389.2017.1418580.
Xiang W, Zhang X, Chen K, Fang J, He F, Hu X, et al. Enhanced adsorption performance and governing mechanisms of ball-milled biochar for the removal of volatile organic compounds (VOCs). Chem Eng J. 2020;385:123842. https://doi.org/10.1016/j.cej.2019.123842.
Huang J, Zimmerman AR, Chen H, Gao B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ Pollut. 2020;258:113809. https://doi.org/10.1016/j.envpol.2019.113809.
Zhuang J, Rong N, Wang X, Chen C, Xu Z. Adsorption of small size microplastics based on cellulose nanofiber aerogel modified by quaternary ammonium salt in water. Sep Purif Technol. 2022;293:121133. https://doi.org/10.1016/j.seppur.2022.121133.
Xiao W-D, Xiao L-P, Xiao W-Z, Liu K, Zhang Y, Zhang H-Y, et al. Cellulose-based bio-adsorbent from TEMPO-oxidized natural loofah for effective removal of Pb(II) and methylene blue. Int J Biol Macromol. 2022;218:285–94. https://doi.org/10.1016/j.ijbiomac.2022.07.130.
Qin C-C, Abdalkarim SYH, Zhou Y, Yu H-Y, He X. Ultrahigh water-retention cellulose hydrogels as soil amendments for early seed germination under harsh conditions. J Clean Prod. 2022;370:133602. https://doi.org/10.1016/j.jclepro.2022.133602.
Shafawi AN, Mohamed AR, Lahijani P, Mohammadi M. Recent advances in developing engineered biochar for CO2 capture: an insight into the biochar modification approaches. J Environ Chem Eng. 2021;9(6):106869. https://doi.org/10.1016/j.jece.2021.106869.
Guo T, Ma N, Pan Y, Bedane AH, Xiao H, Eić M, et al. Characteristics of CO2 adsorption on biochar derived from biomass pyrolysis in molten salt. Can J Chem Eng. 2018;96(11):2352–60. https://doi.org/10.1002/cjce.23153.
Ma Q, Chen W, Jin Z, Chen L, Zhou Q, Jiang X. One-step synthesis of microporous nitrogen-doped biochar for efficient removal of CO2 and H2S. Fuel. 2021;289:119932. https://doi.org/10.1016/j.fuel.2020.119932.
Chatterjee R, Sajjadi B, Mattern DL, Chen W-Y, Zubatiuk T, Leszczynska D, et al. Ultrasound cavitation intensified amine functionalization: a feasible strategy for enhancing CO2 capture capacity of biochar. Fuel. 2018;225:287–98. https://doi.org/10.1016/j.fuel.2018.03.145.
Truong HB, Ike IA, Ok YS, Hur J. Polyethyleneimine modification of activated fly ash and biochar for enhanced removal of natural organic matter from water via adsorption. Chemosphere. 2020;243:125454. https://doi.org/10.1016/j.chemosphere.2019.125454.
Jawad AH, Malek NNA, Abdulhameed AS, Razuan R. Synthesis of magnetic chitosan-fly ash/Fe3O4 composite for adsorption of reactive orange 16 dye: optimization by Box-Behnken design. J Polym Environ. 2020;28(3):1068–82. https://doi.org/10.1007/s10924-020-01669-z.
Cimirro NFGM, Lima EC, Cunha MR, Thue PS, Grimm A, dos Reis GS, et al. Removal of diphenols using pine biochar. Kinetics, equilibrium, thermodynamics, and mechanism of uptake. J Mol Liq. 2022;364:119979. https://doi.org/10.1016/j.molliq.2022.119979.
Zhou Z, Liu P, Wang S, Finfrock YZ, Ye Z, Feng Y, et al. Iron-modified biochar-based bilayer permeable reactive barrier for Cr(VI) removal. J Hazard Mater. 2022;439:129636. https://doi.org/10.1016/j.jhazmat.2022.129636.
Liao W, Zhang X, Shao J, Yang H, Zhang S, Chen H. Simultaneous removal of cadmium and lead by biochar modified with layered double hydroxide. Fuel Process Technol. 2022;235:107389. https://doi.org/10.1016/j.fuproc.2022.107389.
Xu B, Shi W-Y, Sun W, Pan L-M, Dong Y-Q. Investigation on synergistic effect of CuCl2 and FeCl3 impregnated into fly ash on mercury removal by experiment and density functional theory. Appl Surf Sci. 2021;565:150484. https://doi.org/10.1016/j.apsusc.2021.150484.
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, et al. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014;99:19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071.
Aigbe UO, Ukhurebor KE, Onyancha RB, Osibote OA, Darmokoesoemo H, Kusuma HS. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review. J Mater Res Technol. 2021;14:2751–74. https://doi.org/10.1016/j.jmrt.2021.07.140.
Huang J, Zimmerman AR, Chen H, Wan Y, Zheng Y, Yang Y, et al. Fixed bed column performance of Al-modified biochar for the removal of sulfamethoxazole and sulfapyridine antibiotics from wastewater. Chemosphere. 2022;305:135475. https://doi.org/10.1016/j.chemosphere.2022.135475.
Zheng Y, Yang Y, Zhang Y, Zou W, Luo Y, Dong L, et al. Facile one-step synthesis of graphitic carbon nitride-modified biochar for the removal of reactive red 120 through adsorption and photocatalytic degradation. Biochar. 2019;1(1):89–96. https://doi.org/10.1007/s42773-019-00007-4.
Filipinas JQ, Rivera KKP, Ong DC, Pingul-Ong SMB, Abarca RRM, de Luna MDG. Removal of sodium diclofenac from aqueous solutions by rice hull biochar. Biochar. 2021;3(2):189–200. https://doi.org/10.1007/s42773-020-00079-7.
Li Y, Shang H, Cao Y, Yang C, Feng Y, Yu Y. High performance removal of sulfamethoxazole using large specific area of biochar derived from corncob xylose residue. Biochar. 2022;4(1):11. https://doi.org/10.1007/s42773-021-00128-9.
Güleç F, Williams O, Kostas ET, Samson A, Stevens LA, Lester E. A comprehensive comparative study on methylene blue removal from aqueous solution using biochars produced from rapeseed, whitewood, and seaweed via different thermal conversion technologies. Fuel. 2022;330:125428. https://doi.org/10.1016/j.fuel.2022.125428.
Pinto AH, Taylor JK, Chandradat R, Lam E, Liu Y, Leung ACW, et al. Wood-based cellulose nanocrystals as adsorbent of cationic toxic dye, Auramine O, for water treatment. J Environ Chem Eng. 2020;8(5):104187. https://doi.org/10.1016/j.jece.2020.104187.
Olabemiwo FA, Tawabini BS, Patel F, Oyehan TA, Khaled M, Laoui T. Cadmium removal from contaminated water using polyelectrolyte-coated industrial waste fly ash. Bioinorg Chem Appl. 2017;2017:7298351. https://doi.org/10.1155/2017/7298351.
Ihsanullah AA, Al-Amer AM, Laoui T, Al-Marri MJ, Nasser MS, et al. Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol. 2016;157:141–61. https://doi.org/10.1016/j.seppur.2015.11.039.
Keshvardoostchokami M, Majidi M, Zamani A, Liu B. A review on the use of chitosan and chitosan derivatives as the bio-adsorbents for the water treatment: removal of nitrogen-containing pollutants. Carbohydr Polym. 2021;273:118625. https://doi.org/10.1016/j.carbpol.2021.118625.
Braghiroli FL, Bouafif H, Neculita CM, Koubaa A. Performance of physically and chemically activated biochars in copper removal from contaminated mine effluents. Wat Air Soil Poll. 2019;230(8):178. https://doi.org/10.1007/s11270-019-4233-7.
Braghiroli FL, Calugaru IL, Gonzalez-Merchan C, Neculita CM, Bouafif H, Koubaa A. Efficiency of eight modified materials for As(V) removal from synthetic and real mine effluents. Miner Eng. 2020;151:106310. https://doi.org/10.1016/j.mineng.2020.106310.
Mehmood T, Khan AU, Raj Dandamudi KP, Deng S, Helal MH, Ali HM, et al. Oil tea shell synthesized biochar adsorptive utilization for the nitrate removal from aqueous media. Chemosphere. 2022;307:136045. https://doi.org/10.1016/j.chemosphere.2022.136045.
Cruz H, Luckman P, Seviour T, Verstraete W, Laycock B, Pikaar I. Rapid removal of ammonium from domestic wastewater using polymer hydrogels. Sci Rep. 2018;8(1):2912. https://doi.org/10.1038/s41598-018-21204-4.
Keshvardoostchokami M, Babaei S, Piri F, Zamani A. Nitrate removal from aqueous solutions by ZnO nanoparticles and chitosan-polystyrene–Zn nanocomposite: kinetic, isotherm, batch and fixed-bed studies. Int J Biol Macromol. 2017;101:922–30. https://doi.org/10.1016/j.ijbiomac.2017.03.162.
Zhu S, Qu T, Irshad MK, Shang J. Simultaneous removal of Cd(II) and As(III) from co-contaminated aqueous solution by α-FeOOH modified biochar. Biochar. 2020;2(1):81–92. https://doi.org/10.1007/s42773-020-00040-8.
Li Y, Shaheen SM, Azeem M, Zhang L, Feng C, Peng J, et al. Removal of lead (Pb+2) from contaminated water using a novel MoO3-biochar composite: performance and mechanism. Environ Pollut. 2022;308:119693. https://doi.org/10.1016/j.envpol.2022.119693.
Calugaru IL, Neculita CM, Genty T, Zagury GJ. Removal efficiency of As(V) and Sb(III) in contaminated neutral drainage by Fe-loaded biochar. Environ Sci Pollut Res. 2019;26(9):9322–32. https://doi.org/10.1007/s11356-019-04381-1.
Braghiroli FL, Calugaru IL, Gonzalez-Merchan C, Neculita CM, Bouafif H, Koubaa A. Efficiency of eight modified materials for As(V) removal from synthetic and real mine effluents. Mine Eng. 2020;151:106310. https://doi.org/10.1016/j.mineng.2020.106310.
•Wang T, Zhang D, Fang K, Zhu W, Peng Q, Xie Z. Enhanced nitrate removal by physical activation and Mg/Al layered double hydroxide modified biochar derived from wood waste: adsorption characteristics and mechanisms. J Environ Chem Eng. 2021;9(4):105184. https://doi.org/10.1016/j.jece.2021.105184. This study showed that modification of wood biochar via metal-doping method significantly improved nitrate removal.
Wan Ngah WS, Endud CS, Mayanar R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React Funct Polym. 2002;50(2):181–90. https://doi.org/10.1016/S1381-5148(01)00113-4.
Park J-H, Eom J-H, Lee S-L, Hwang S-W, Kim S-H, Kang S-W, et al. Exploration of the potential capacity of fly ash and bottom ash derived from wood pellet-based thermal power plant for heavy metal removal. Sci Total Environ. 2020;740:140205. https://doi.org/10.1016/j.scitotenv.2020.140205.
Barbosa RFS, Souza AG, Maltez HF, Rosa DS. Chromium removal from contaminated wastewaters using biodegradable membranes containing cellulose nanostructures. Chem Eng J. 2020;395:125055. https://doi.org/10.1016/j.cej.2020.125055.
Wang Q, Zuo W, Tian Y, Kong L, Cai G, Zhang H, et al. Flexible brushite/nanofibrillated cellulose aerogels for efficient and selective removal of copper(II). Chem Eng J. 2022;450:138262. https://doi.org/10.1016/j.cej.2022.138262.
Oyewo OA, Mutesse B, Leswifi TY, Onyango MS. Highly efficient removal of nickel and cadmium from water using sawdust-derived cellulose nanocrystals. J Environ Chem Eng. 2019;7(4):103251. https://doi.org/10.1016/j.jece.2019.103251.
Georgouvelas D, Abdelhamid HN, Li J, Edlund U, Mathew AP. All-cellulose functional membranes for water treatment: adsorption of metal ions and catalytic decolorization of dyes. Carbohydr Polym. 2021;264:118044. https://doi.org/10.1016/j.carbpol.2021.118044.
Hokkanen S, Repo E, Bhatnagar A, Tang WZ, Sillanpää M. Adsorption of hydrogen sulphide from aqueous solutions using modified nano/micro fibrillated cellulose. Environ Technol. 2014;35(17–20):2334–46. https://doi.org/10.1080/09593330.2014.903300.
Calugaru IL, Neculita CM, Genty T, Zagury GJ. Removal and recovery of Ni and Zn from contaminated neutral drainage by thermally activated dolomite and hydrothermally activated wood ash. Wat Air and Soil Poll. 2020;231(5):226. https://doi.org/10.1007/s11270-020-04600-3.
Calugaru IL, Neculita CM, Genty T, Bussière B, Potvin R. Removal of Ni and Zn in contaminated neutral drainage by raw and modified wood ash. J Environ Sci Health A. 2017;52(2):117–26. https://doi.org/10.1080/10934529.2016.1237120.
Bandara T, Xu J, Potter ID, Franks A, Chathurika J, Tang C. Mechanisms for the removal of Cd(II) and Cu(II) from aqueous solution and mine water by biochars derived from agricultural wastes. Chemosphere. 2020;254:126745. https://doi.org/10.1016/j.chemosphere.2020.126745.
Veselská V, Šillerová H, Hudcová B, Ratié G, Lacina P, Lalinská-Voleková B, et al. Innovative in situ remediation of mine waters using a layered double hydroxide-biochar composite. J Hazard Mater. 2022;424:127136. https://doi.org/10.1016/j.jhazmat.2021.127136.
Genty T, Bussière B, Benzaazoua M, Zagury GJ. Capacity of wood ash filters to remove iron from acid mine drainage: assessment of retention mechanism. Mine Water Environ. 2012;31(4):273–86. https://doi.org/10.1007/s10230-012-0199-z.
Richard D, Neculita CM, Zagury GJ. Removal of nickel from neutral mine drainage using peat-calcite, compost, and wood ash in column reactors. Environ Sci Pollut Res. 2021;28(12):14854–66. https://doi.org/10.1007/s11356-020-11623-0.
Calugaru IL, Neculita CM, Genty T, Bussière B, Potvin R. Removal of Ni and Zn in contaminated neutral drainage by raw and modified wood ash. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2017;52(2):117–26. https://doi.org/10.1080/10934529.2016.1237120.
Hanyan Z. Evaluating the remediation potential of MgFe2O4-montmorillonite and its co-application with biochar on heavy metal-contaminated soils. Chemosphere. 2022;299:134217. https://doi.org/10.1016/j.chemosphere.2022.134217.
DaryabeigiZand A, Grathwohl P. Enhanced immobilization of polycyclic aromatic hydrocarbons in contaminated soil using forest wood-derived biochar and activated carbon under saturated conditions, and the importance of biochar particle size. Pol J Environ Stud. 2016;25(1):427–41. https://doi.org/10.15244/pjoes/60160.
Srinivasan P, Sarmah AK. Characterization of agricultural waste-derived biochars and their sorption potential for sulfamethoxazole in pasture soil: a spectroscopic investigation. Sci Total Environ. 2015;502:471–80. https://doi.org/10.1016/j.scitotenv.2014.09.048.
Moon DH, Hwang I, Chang Y-Y, Koutsospyros A, Cheong KH, Ji WH, et al. Quality improvement of acidic soils by biochar derived from renewable materials. Environ Sci Pollut Res. 2017;24(4):4194–9. https://doi.org/10.1007/s11356-016-8142-7.
Liu Q, Liu B, Zhang Y, Lin Z, Zhu T, Sun R, et al. Can biochar alleviate soil compaction stress on wheat growth and mitigate soil N2O emissions? Soil Biol Biochem. 2017;104:8–17. https://doi.org/10.1016/j.soilbio.2016.10.006.
Chen C, Wang R, Shang J, Liu K, Irshad MK, Hu K, et al. Effect of biochar application on hydraulic properties of sandy soil under dry and wet conditions. Vadose Zone J. 2018;17(1):180101. https://doi.org/10.2136/vzj2018.05.0101.
McDonald MR, Bakker C, Motior MR. Evaluation of wood biochar and compost soil amendment on cabbage yield and quality. Can J Plant Sci. 2019;99(5):624–38. https://doi.org/10.1139/cjps-2018-0122.
Robert É, Braghiroli FL. Development of a biochar-based substrate added with nitrogen from a mining effluent for the production of picea mariana seedlings. Clean Technol. 2022. https://doi.org/10.3390/cleantechnol4030047.
Asadyar L, Xu C-Y, Wallace HM, Xu Z, Reverchon F, Bai SH. Soil-plant nitrogen isotope composition and nitrogen cycling after biochar applications. Environ Sci Pollut Res. 2021;28(6):6684–90. https://doi.org/10.1007/s11356-020-11016-3.
Bauli CR, Lima GF, de Souza AG, Ferreira RR, Rosa DS. Eco-friendly carboxymethyl cellulose hydrogels filled with nanocellulose or nanoclays for agriculture applications as soil conditioning and nutrient carrier and their impact on cucumber growing. Colloids Surf A: Physicochem Eng Asp. 2021;623:126771. https://doi.org/10.1016/j.colsurfa.2021.126771.
Pelletier C, Rogaume Y, Dieckhoff L, Bardeau G, Pons M-N, Dufour A. Effect of combustion technology and biogenic CO2 impact factor on global warming potential of wood-to-heat chains. Appl Energy. 2019;235:1381–8. https://doi.org/10.1016/j.apenergy.2018.11.060.
Nanda S, Reddy SN, Mitra SK, Kozinski JA. The progressive routes for carbon capture and sequestration. Energy Sci Eng. 2016;4(2):99–122. https://doi.org/10.1002/ese3.117.
D’Alessandro DM, Smit B, Long JR. Carbon dioxide capture: prospects for new materials. Angew Chem Int Ed. 2010;49(35):6058–82. https://doi.org/10.1002/anie.201000431.
Igalavithana AD, Choi SW, Shang J, Hanif A, Dissanayake PD, Tsang DCW, et al. Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: effect of porous structure and surface chemistry. Sci Total Environ. 2020;739:139845. https://doi.org/10.1016/j.scitotenv.2020.139845.
Li K, Zhang D, Niu X, Guo H, Yu Y, Tang Z, et al. Insights into CO2 adsorption on KOH-activated biochars derived from the mixed sewage sludge and pine sawdust. Sci Total Environ. 2022;826:154133. https://doi.org/10.1016/j.scitotenv.2022.154133.
Ben-Mansour R, Habib MA, Bamidele OE, Basha M, Qasem NAA, Peedikakkal A, et al. Carbon capture by physical adsorption: materials, experimental investigations, and numerical modeling and simulations – a review. Appl Energy. 2016;161:225–55. https://doi.org/10.1016/j.apenergy.2015.10.011.
Mulu E, M’Arimi MM, Ramkat RC, Mecha AC. Potential of wood ash in the purification of biogas. Energy Sustain Dev. 2021;65:45–52. https://doi.org/10.1016/j.esd.2021.09.009.
Lombardi L, Costa G, Spagnuolo R. Accelerated carbonation of wood combustion ash for CO2 removal from gaseous streams and storage in solid form. Environ Sci Pollut Res. 2018;25(36):35855–65. https://doi.org/10.1007/s11356-018-2159-z.
Wang P, Guo Y, Zhao C, Yan J, Lu P. Biomass derived wood ash with amine modification for post-combustion CO2 capture. Appl Energy. 2017;201:34–44. https://doi.org/10.1016/j.apenergy.2017.05.096.
Sepahvand S, Jonoobi M, Ashori A, Gauvin F, Brouwers HJH, Oksman K, et al. A promising process to modify cellulose nanofibers for carbon dioxide (CO2) adsorption. Carbohydr Polym. 2020;230:115571. https://doi.org/10.1016/j.carbpol.2019.115571.
Li Y, Lin Y, Xu Z, Wang B, Zhu T. Oxidation mechanisms of H2S by oxygen and oxygen-containing functional groups on activated carbon. Fuel Process Technol. 2019;189:110–9. https://doi.org/10.1016/j.fuproc.2019.03.006.
Liu HW, Feng S, Leung AK. Effects of nano-activated carbon on water and gas permeability and hydrogen sulfide removal in compacted kaolin. Appl Clay Sci. 2019;172:80–4. https://doi.org/10.1016/j.clay.2019.02.012.
Zhao Z, Wang B, Theng BKG, Lee X, Zhang X, Chen M, et al. Removal performance, mechanisms, and influencing factors of biochar for air pollutants: a critical review. Biochar. 2022;4(1):30. https://doi.org/10.1007/s42773-022-00156-z.
Shang G, Li Q, Liu L, Chen P, Huang X. Adsorption of hydrogen sulfide by biochars derived from pyrolysis of different agricultural/forestry wastes. J Air Waste Manag Assoc. 2016;66(1):8–16. https://doi.org/10.1080/10962247.2015.1094429.
Choudhury A, Lansing S. Adsorption of hydrogen sulfide in biogas using a novel iron-impregnated biochar scrubbing system. J Environ Chem Eng. 2021;9(1):104837. https://doi.org/10.1016/j.jece.2020.104837.
Zou W, Gao B, Ok YS, Dong L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: a critical review. Chemosphere. 2019;218:845–59. https://doi.org/10.1016/j.chemosphere.2018.11.175.
Shen Y, Zhang N. Facile synthesis of porous carbons from silica-rich rice husk char for volatile organic compounds (VOCs) sorption. Bioresour Technol. 2019;282:294–300. https://doi.org/10.1016/j.biortech.2019.03.025.
Kaikiti K, Stylianou M, Agapiou A. Development of food-origin biochars for the adsorption of selected volatile organic compounds (VOCs) for environmental matrices. Bioresour Technol. 2021;342:125881. https://doi.org/10.1016/j.biortech.2021.125881.
Zhang X, Gao B, Zheng Y, Hu X, Creamer AE, Annable MD, et al. Biochar for volatile organic compound (VOC) removal: sorption performance and governing mechanisms. Bioresour Technol. 2017;245:606–14. https://doi.org/10.1016/j.biortech.2017.09.025.
Collivignarelli MC, Canato M, Abbà A, CarnevaleMiino M. Biosolids: what are the different types of reuse? J Clean Prod. 2019;238:117844. https://doi.org/10.1016/j.jclepro.2019.117844.
Xu K, Lin F, Dou X, Zheng M, Tan W, Wang C. Recovery of ammonium and phosphate from urine as value-added fertilizer using wood waste biochar loaded with magnesium oxides. J Clean Prod. 2018;187:205–14. https://doi.org/10.1016/j.jclepro.2018.03.206.
Godiya CB, Cheng X, Li D, Chen Z, Lu X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J Hazard Mater. 2019;364:28–38. https://doi.org/10.1016/j.jhazmat.2018.09.076.
Karanac M, Đolić M, Veljović Đ, Rajaković-Ognjanović V, Veličković Z, Pavićević V, et al. The removal of Zn2+, Pb2+, and As(V) ions by lime-activated fly ash and valorization of the exhausted adsorbent. Waste Manage. 2018;78:366–78. https://doi.org/10.1016/j.wasman.2018.05.052.
Haddad K, Jellali S, Jeguirim M, Trabelsi ABH, Limousy L. Investigations on phosphorus recovery from aqueous solutions by biochars derived from magnesium-pretreated cypress sawdust. J Environ Manag. 2018;216:305–14. https://doi.org/10.1016/j.jenvman.2017.06.020.
Funding
The authors gratefully acknowledge the financial support of Mitacs Elevate Proposal (IT28891) and the Canada Research Chairs Program.
Author information
Authors and Affiliations
Contributions
M.K. wrote the main manuscript text and F.B., C.M.N. and A.K. reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keshvardoostchokami, M., Braghiroli, F.L., Neculita, C.M. et al. Advances in Modified Wood-Based Adsorbents for Contaminant Removal: Valorization Methods, Modification Mechanisms, and Environmental Applications. Curr. For. Rep. 9, 444–460 (2023). https://doi.org/10.1007/s40725-023-00200-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-023-00200-6