Abstract
Shoreline oiling poses a risk to coastal ecosystems and resources. Understanding the natural attenuation potential and impact of different sediment types is important for choosing appropriate intervention strategies and priority areas following a spill. Simulated IFO-40 oil spills on artificial beach mesocosms were carried out using different sediment types: sandy beach and sandy tidal flat, under low energy tidal cycles over a 5-day period. Chemical and biological analysis of leachate and sediment was conducted to understand the movement of oil through these mesocosms. Rapid oil movement from the oil slick to the surface sediment layer was observed in the sandy beach enclosures, while slower oil movement was observed in the sandy tidal flat enclosures. Increased hydrocarbon dissolution was observed in the sandy beach enclosures, marked by higher concentrations of low molecular weight n-Alkanes (C12 − 15) and naphthalenes (C0 − 3) in sandy beach leachate compared to sandy tidal flat samples. Despite the increase in hydrocarbons, there were no major shifts in microbial communities observed in the leachate and sediment compartments for either sediment type. Both prokaryote and microeukaryote communities differed between the two sediment types, with little overlap between dominant sequences. Our results indicate that limited oil penetration occurs within sandy tidal flat shorelines resulting in oil accumulation suggesting that sorbent or vacuuming could be used as emergency response to minimize the environmental and ecological impacts of spilled oil.
Highlights
Oil fate and behaviour differed between sandy beach and sandy tidal flat shorelines.
Oil movement and hydrocarbon dissolution was limited in sandy tidal flat beaches.
Oiling did not cause large changes in sediment microbial communities.
Bulk oil removal mitigation may be applicable to sandy tidal flat shorelines.
Biostimulation may be considered for oiled sandy beach shorelines.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
As of 2021, the seaborne crude oil trade had reached 1846 millions of tons, and the trade value was greater than 1.5 trillion USD for oil, gas and coal (United Nations Conference on Trade and Development 2023). High global trade and energy demands will lead to increases in marine vessels, off-shore oil extraction and transportation of oil through pipelines. These activities pose an increased risk of oil being accidently released from tankers, off-shore drilling platforms, or through routine operations and transportation (National Research Council 2003; ITOPF 2022). Depending on the location of the release and environmental conditions, currents and tides can rapidly transport spilled oil toward the shoreline. Spilled oil deposited on shorelines can have significant impacts on various ecological systems. Marine mammals, turtles and birds are sensitive when exposed to spilled oil as slicks can coat the fur or feathers of these animals resulting in the loss of insulation (Williams et al. 1988; Lipscomb et al. 1993; Albers 2003; Feng et al. 2021). In addition, animals may ingest the oil resulting in toxic effects (Williams et al. 1988; Lipscomb et al. 1993; Albers 2003; Feng et al. 2021). Sea turtles that were identified as moderately impacted by the 2010 BP Deepwater Horizon incident were estimated to have mortalities of 85% (Mitchelmore et al. 2017). Sea turtle hatchings are particularly at risk of severe impacts on sandy beaches affected by oil spills (Asif et al. 2022). Oil stranded on tidal flats can negatively impact diverse members of the ecosystem, including birds and macrobenthic communities (Chung et al. 2004; IPIECA 2016). Severe declines of ghost crabs and other burrowing crab populations were reported as a result of oiled tidal flats during the 1991 Gulf War spill incident (IPIECA 2016).
A timely and effective intervention and remediation strategy is important to minimize the potential impacts from stranded oil and to promote natural attenuation and ecosystem recovery (Owens and Sergy 2010; IPIECA 2016). Countermeasure deployment, including physical barriers, mechanical removal, pressure washing and bioremediation, may be considered during an oil spill response (Owens et al. 2003; Yang et al. 2021; Asif et al. 2022; Michel et al. 2022). Physical barriers, such as hard booms or filter fences, may be used to protect wetlands, marshes and mangroves, but these barriers may restrict wildlife from accessing the shoreline and require maintenance throughout the response (Asif et al. 2022; Michel et al. 2022). If oil reaches the shoreline, there are some clean-up options, with different levels of impacts and effectiveness. Mechanical removal of oiled shoreline material is possible, but is seldom used because of the large amount oiled waste generated and the greater risks for damaging sensitive areas (e.g. marshes) (Feng et al. 2021; Michel et al. 2022). Pressure washing is mainly recommended for the rocky and gravel shorelines as the high pressure water can cause sediment erosion (Asif et al. 2022) and relocates the oil, but does not remove it from the system. While a lower pressure flushing alternative can be used for sensitive areas like marshes, several challenges need to be overcome, including mobilizing people, equipment and recovery devices, acquiring a clean water source with equivalent parameters (salinity, tidal current and vegetation considerations), in order to effectively use the technique (Michel et al. 2022). Bioremediation is one of the least intrusive option, where the hydrocarbons are allowed to be degraded by natural microbial processes (Lee et al. 2018; Michel et al. 2022). In general, aggressive treatment methods may hinder recovery of shorelines. In the case of the Amoco Cadiz, a treated salt marsh took > 12 years to recover following excessive removal of oiled soil, while an untreated marsh recovered within 4–8 years (reviewed by Michel et al. 2022).
The selection of appropriate intervention and remediation strategies requires an understanding of the fate and transport of spilled oil (Wang et al. 2021). Depending on the shoreline type and environmental factors (wind, waves and tides), spilled oil may distribute on a shoreline as surface oil residues, subsurface oil residues, and/or submerged oil mats (Wang et al. 2021). Through advection, evaporation, dispersion, dissolution and sedimentation processes, spilled oil can be weathered, transported, remobilized and removed within the shoreline sediments and neighbouring water bodies (Wang et al. 2021; Asif et al. 2022). As spilled oil weathers, hydrocarbons with lower molecular weights tend to be depleted, thereby increasing the density and viscosity of the remaining material. Depending on oil type and the extent of weathering, spilled oil could be remobilized and redistributed, especially when the spilled oil density is lower than that of seawater (Taylor et al. 2021; Wang et al. 2021).
Shoreline sediment composition is an important factor affecting oil penetration and retention within the sediment (Wang et al. 2021; Asif et al. 2022). Dispersed spilled oil and dissolved hydrocarbons can be transported through sediment via advective processes, resulting in partitioning and sequestration within the sediments. Several studies have reported different oil penetration behavior between coarse sand and fine-grain sediments (Vandermeulen et al. 1988; Humphrey et al. 1993; Harper et al. 1995, Michel et al. 2002). A model constructed using data from the Exxon Valdez incident identified sediment grain size and spilled oil properties as important parameters in determining the fate of oil on beaches (Humphrey et al. 1993). A comprehensive column study was conducted by Harper et al. (1995), where the penetration and retention potential of various oil types and properties were examined using different grain sizes (from pebbles, granules to coarse sand). These authors observed that oil penetration was proportional to sediment grain size. Oil loading experiments using intact tidal flat sediment cores showed that the oil penetration and sediment hydrocarbon concentrations were inversely associated with the fine-grain sediment (silt and clay) content (Vandermeulen et al. 1988). These authors concluded that the presence of fine-grain sediment as low as 2% can greatly reduce oil penetration (Vandermeulen et al. 1988). This could reduce the oil persistence on the shoreline as exposed oil would be subjected to natural weathering processes (reviewed by Wang et al. 2021). However, it is still possible for some amount of oil to penetration into the subsurface of fine grain sediment due to animal perturbation (reviewed by Wang et al. 2021).
The hydrocarbon distribution within the sediment matrix is another the important parameter in understanding the fate of subsurface oil. The presence of natural organic matter in the sediment is another important factor affecting the partitioning of dispersed spilled oil and dissolved hydrocarbons. The fraction of natural organic matter (foc) contributes to the partitioning coefficient (KD), with higher foc increasing KD (King et al. 2014; Maletic et al. 2019). Reid et al. (2000) summarized that PAHs can become entrapped within humic acid complexes or absorb into the surface of organic matter in sediments, decreasing mobility of the PAHs.
Biodegradation is a cost effective remediation option, where the native bacterial communities are allowed to adapt to degrade hydrocarbons from the spilled oil. Biodegradation potential within coastal sediments is well documented in field and laboratory studies (Chaerun et al. 2004; Kostka et al. 2011; Pontes et al. 2013; Lee et al. 2018, 2020). However, salinity, nutrient status, organic matter concentrations, hydrocarbon partitioning and bioavailability have a strong influence on microbial communities, thereby affecting hydrocarbon biodegradation efficiency (Powell et al. 2003; Ikenaga et al. 2010).
To make informed decisions regarding response activities during a spill, it is important to understand how different types of shoreline affect the fate and biodegradation potential behaviour of stranded oil. The objective of this study was to integrate analysis of hydrocarbon sediment infiltration with observations of microbial dynamics using artificial beach experiments to better understand hydrocarbon degradation potential within different sediment types. Sandy beach and sandy tidal flat sediments represent contrasting sediment compositions, sediment nutrient status and hydrogeological characteristics. These two sediments were studied under low-energy tidal cycles following the addition of a weathered fuel oil (IFO-40). The use of IFO-40, a highly viscous marine intermediate fuel oil, provided an opportunity to understand the movement of various hydrocarbons through the sandy beach and sandy tidal flat sediment matrices as this fuel is comprised of a wide range of n-Alkanes and PAHs.
This study has taken a holistic approach to investigate how sediment type and hydrocarbon partitioning impacts the microbial community structure, both in the sediment and the adjacent water. Data generated from this study would provide emergency responders better support in decision making for protection and respond strategies in shoreline oiling scenario.
2 Materials and Methods
2.1 Sediment Collection
Sediment was collected from two shorelines of eastern Nova Scotia, Canada, which differ in sediment grain size distribution, inorganic nutrient concentrations and organic matter content. Sediment for the first experiment was collected on July 6, 2018 from Conrad’s Beach (44°38.67’ N 63° 22.74’W), an exposed fine sandy beach. Sediment for the second experiment was collected on July 20, 2018 from Cow Bay (44°39.31’ N 63°25.38’W), a sandy tidal flat. Sediment was collected from both locations at low tide at the mid-level water mark of the beach by scraping the top (∼ 10 cm) layer with shovels into clean 20 L Ropak plastic buckets. Sediment was immediately transported to the Bedford Institute of Oceanography (BIO) to be added to mesocosm enclosures within 2 h of collection. After collecting sediment, the temperature and salinity of the water at each site (∼ 30 cm deep) was measured with a YSI hand-held meter.
2.2 Beach Mesocosm Construction
Artificial beach mesocosms (6 per experiment) were constructed from polypropylene trays with dimensions of 40 cm L x 30 cm W x 20 cm D (Dynalon Labware, Rochester, NY, USA) (Fig. SM1). Sediment was mixed and added to the trays forming artificial beaches 8–10 cm deep (approximately 14.4 kg of sediment). The beach mesocosms were securely mounted outdoors over a flume tank platform located adjacent to the Bedford Basin. The ‘landward’ end of the beach rested on a 1 inch board to provide a gentle slope to the beach. To simulate daily tidal flushing, ∼ 2.5 L fresh, unfiltered seawater, drawn daily from the Bedford Basin (∼ 2 m below the surface), was pumped through a 30 cm long platinum cured Masterflex® silicone tubing (Cole-Parmer Co., Chicago, IL), which was perforated with holes every 2 cm and secured on top of the mesocosm, using a peristaltic pump (Fig. SM1). Each beach mesocosm had a drain port mounted to the bottom at the lowest point of the beach covered with a stainless steel screen to prevent clogging (Fig. SM1). Platinum cured silicone tubing was attached and regulated via a clamp to allow leachate sample collection. The mesocosms were uncovered and exposed to outdoor conditions. The sediments in the mesocosms were allowed to equilibrate with Bedford Basin seawater for 72 h prior to the start of the experiment. Subsamples of sediment from each location were retained for sediment characterization.
2.3 Weathering of IFO-40 oil
Intermediate fuel oil (IFO-40) was weathered for 3 d at in situ temperature (16.9 °C and 18.5 °C for each experiment, respectively) measured during sediment collection with Bedford Basin seawater. Temperature and salinity of the water was recorded. For the oiled treatments, 40 mL of IFO-40 was measured in a glass beaker and poured into an open 500-mL baffled flask containing 200 mL seawater. The oil-seawater mixture was weathered using an orbital shaker at 150 rpm for 72 h, which likely resulted in some emulsification, as the oil layer increased in thickness, but was not investigated further. Two additional oil-seawater flasks were prepared for physical and chemical characterization, and these flasks were weathered in the same manner as above and stored at -20 °C prior to analysis. For the control treatment, three additional flasks with 200 mL of seawater only were prepared in the same manner without the addition of IFO-40.
2.4 Experimental Conditions
After 72 h, the equilibrated mesocosms were drained and replenished with 2.5 L of freshly collected seawater prior to receiving treatment. For the oil treatment, flasks with oil and seawater were weighed and weathered IFO-40-seawater mixtures were poured directly onto three randomly selected mesocosms (T = 0). Empty flasks were weighed to determine residual oil remaining on the sides of the flask. For control treatments, the shaken seawater was poured directly onto the remaining three mesocosms (T = 0). Leachate and sediment core samples were collected immediately after the addition of the treatment and then daily for a total of 5 d.
2.5 Sampling
2.5.1 Leachate Samples
Leachate samples were collected by opening the clamp on the tube and allowing the water to drain into a sterile 2 L media bottle (Fig. SM2). The leachate was immediately subsampled and processed. For hydrocarbon analysis (total petroleum hydrocarbons (TPH) and concentrations of n-Alkanes, polycyclic aromatic hydrocarbons (PAHs) and alkylated PAHs), ∼ 200 mL of unfiltered leachate samples were dispensed into 500 mL amber, pre-cleaned, EPA-certified, wide-mouth bottles with PTFE lined caps. Bottles with water were weighed and 20 mL of dichloromethane (DCM) was added then shaken by hand to mix the combined contents. Samples were stored at 4 °C until analysis. Additional 5 mL of leachate was subsampled and immediately processed to estimate potential toxicity using the Microtox® assay (Modern Water Inc., New Castle, DE, USA). Subsamples for quantification of prokaryotes (980 µL) were pipetted into 2 mL cryovials, fixed with EM-grade glutaraldehyde (0.5% final concentration), placed in the dark on ice for 10 min, then flash frozen in liquid nitrogen before storage at -86 °C (Ortmann et al. 2019). For 16 S and 18 S rRNA gene amplicon analysis, 100 mL of sample were filtered through a 47 mm Millipore Durapore® PVDF 0.22 μm pore size filter (Millipore, USA). The filter was immediately placed in a sterile 50 mL centrifuge tube and flash frozen with liquid nitrogen before storage at -86 °C. Duplicate 15 mL acid cleaned tubes were rinsed twice with the filtrate, filled to ∼ 10 mL and frozen (-20 °C) for analysis of inorganic nutrients. A final subsample for carbon analysis was collected in an acid-clean polycarbonate bottle and frozen at -20 ℃.
2.5.2 Sediment Samples
To minimize perturbation during sampling, sediment cores were collected from the mesocosms using a soil recovery auger plastic liner (5.08 cm Diameter x 20.32 cm Length) and secured with plastic end caps (AMS, American Falls, ID, USA). The liner and caps were cleaned and rinsed with hexane and distilled water before and after each use. Six sample locations were identified in each beach (2 rows of 3 sites near the lowest end). At each time point, a core was collected from one of the six locations that were randomly assigned to a control/oil beach pair (Fig. SM2). The cores from the control beaches were immediately processed in the lab and subdivided into three ∼ 3 cm sections (surface, middle and bottom). For the oil-treated cores, an additional layer (top), representing the oil slick, was collected for hydrocarbon analysis by removing the layer of oil from the surface of the sediment core with a clean sterile spatula. In the case where oil was not observed, a thin layer of sediment (∼ 0.5 cm) was collected for analysis. Subsamples (∼ 5 g, mass recorded) from each layer (surface, middle and bottom) were collected for hydrocarbon analysis and stored at -20 °C until extraction and analysis. A 7 g subsample was immediately analyzed using the Microtox® assay. For prokaryote abundances, 2 g of sediment was dispensed into a pre-weighed 50 mL centrifuge tube and preserved with 0.5% glutaraldehyde in 0.22 μm filtered seawater. The remaining sediment was placed in a 50 mL centrifuge tube for DNA analysis. The tubes for DNA analysis and prokaryote abundances were flash frozen in liquid nitrogen and stored at -86 °C.
2.6 Sample Analysis
2.6.1 Sediment Characterization
Sediment samples were analyzed to determine bulk density, moisture content and grain size distribution. Dry bulk density was measured from a 5 cm3 sediment sample, which was dried for 24 h at 60 °C (Brady and Weil 2010; Brannock et al. 2016). The grain size distribution for each field sample was determined using a Beckman Coulter Laser Diffraction Particle Size Analyser (LS 13 320; Beckman Coulter, Pasadena, CA, USA) for particles between 0.375 and 2000 μm and sieves for particles > 2000 μm. Approximately 3 g of sediment was digested with ∼ 10 mL of 70% hydrogen peroxide (H2O2) at 60 °C to remove all organic material. Prior to analysis, sediment was suspended in ultra-pure water and sonicated for 1 min. Sediments were then rinsed with ultra-pure water into the sample chamber through a 2000 μm sieve to remove large particles and sieved material was processed by the particle size analyzer. Sieve sizes of 2360 μm, 2800 μm, 3350 μm and 4000 μm were used to complete analysis of particles greater than 2000 μm. Datasets were then merged and normalized to 100% for the complete grain size distribution.
2.6.2 Leachate Carbon and Inorganic Nutrient Analysis
Inorganic nutrients (NO3−, NH4+, PO43− and dissolved silicate (DSi) were measured using a SEAL Analytical AA3 continuous segmented flow autoanalyzer (SEAL Analytical, Inc.) following standard methods Brady and Weil (2010). The total organic (TOC) and inorganic (TIC) carbon concentrations were measured using a VARIO® Select TOC analyzer (Elementar Americas Inc.) at 650 °C for the combustion column temperature with ultrapure air as the carrier gas (Becker et al. 2020).
2.6.3 Hydrocarbon Analysis
The weathering flasks were thawed and allowed to settle and separate, with the oil fraction collected for analysis. The weathered and fresh non-weathered oil samples were subjected to chemical characterization using gas chromatography linked to mass spectroscopy (GC-MS) (an Agilent 6890GC coupled to an Agilent 5975B MS) under selected ion monitoring (SIM) mode.
For the leachate samples, detailed methods for extracting and measuring TPH, n-Alkanes, PAHs, and alkylated PAHs were previously described (Ortmann et al. 2020). In brief, TPH concentrations from sample extracts were quantified using an Agilent 7890B GC connected to an FID. The abundance of specific Alkanes, PAHs and alkylated PAHs were measured using GC-MS method as above.
Frozen sediment samples were allowed to thaw and transferred into a 20 mL glass beaker where they were mixed with 10 g of granular anhydrous sodium sulphate and 2 g of granular copper (20–30 mesh). The mixture was transferred to a cellulose thimble which was then placed in a SOXHLET extraction manifold and extracted for 18 h with DCM. Following extraction, the solvent was concentrated using a TurboVap II (Biotage, Sweden) to a final volume of 2 mL, which was split 1:1 for TPH and GC-MS analysis as above. The extracts of the samples were subjected to TPH analysis without further processing. Prior to GC-MS analysis, the extracted samples were spiked with certified deuterate-labelled surrogates and solvent exchanged into hexane and purified using a silica gel column (US EPA 1996). The purified extracts were solvent exchanged to iso-octane using an N-evap (Organomation, USA) and concentrated to a final volume of 1 mL.
2.6.4 Biological Analysis
Microtox® assays were conducted to determine the biological availability of hydrocarbons as a proxy for potential toxicity. Leachate samples were analyzed using the 100% Acute Toxicity Test following the manufacturer’s 15 min protocol. For sediment samples, the solid-phase test (SPT) protocol was followed (Environment Canada 2002). All analyses used 0.1 μm filtered seawater as the diluent and photoluminescence was measured on a M500 analyzer. EC50 values could not be determined for most samples, so the % light inhibition was calculated (Ortmann et al. 2020).
Prokaryotes from the leachate samples were enumerated with a BD FACSLyric™ (BD Biosciences, USA) flow cytometer following previously described protocols (Cobanli et al. 2022). Prokaryote cells were extracted from sediment samples using a modification of the protocol described by Danovaro et al. (2002). The preserved sediment samples were thawed in a 37 °C water bath and resuspended with 30 mL of 0.1 M sodium pyrophosphate and 0.1% Tween 80. Samples were incubated at 4 °C for 30 min prior to centrifugation at 1000 x g for 1 min. The extraction was carried out twice and the two supernatants were analyzed following the protocol for the leachate samples. Data from each supernatant were pooled to determine the cell abundance.
Filters and sediment samples for 16 S and 18 S rRNA gene amplicon analysis were shipped overnight on dry ice to the National Research Council lab in Montreal, QC. Filters were extracted using a modified hexadecyltrimethylammonium bromide (CTAB) method (Tremblay et al. 2017) and sediment samples were extracted (0.25 g) using DNeasy Power soil kit. The V4-V5 region of the 16 S rRNA gene was amplified with the 515 F-Y (5-GTGYCAGCMGCCGCGGTAA3) and 926R (5-CCGYCAATTYMTTTRAGTTT-3) primer pair, which was used to target both Archaea and Bacteria (Schreiber et al. 2019). The V4 region of the 18 S rRNA gene was amplified with the 565-F (5-CCAGCASCYGCGGTAATTCC-3) and 948-R (5-ACTTTCGTTCTTGATYRA-3) primer pair to amplify microeukaryote diversity. Sequencing was carried out on an Illumina MiSeq platform (Illumina Inc., San Diego, CA) and reads from all experiments were pooled and processed following the Amplicon Tagger pipeline to generate amplicon sequence variant (ASV) tables (Tremblay and Yergeau 2019). Sequences were deposited to NCBI (Bioproject ID: PRJNA973985).
2.6.5 Data Analysis
R program (version 4.2.2) (R Core Team 2020) was used to perform statistical analysis and generate graphical representations. To determine effects of sediment type and treatment on hydrocarbon concentrations, prokaryotic cell density and light inhibition data, linear mixed models were constructed using lme4 and lmerTest packages (Bates et al. 2015; Kuznetsova et al. 2017). The sediment type, treatment (control and IFO-40 treatment) and day were all included as fixed factors, with enclosure being a random factor. The post-hoc pairwise comparison of sediment depth was performed using emmeans package (Russell 2023). Comparison between the chemical and biological parameters were carried out using Spearman correlation calculated using R commander and all pairwise combinations (Fox et al. 2019). P-values were adjusted using the Holms correction for multiple comparisons. Data was visualized using the ggplot2 package (Wickham 2009).
The GC-MS individual hydrocarbon data was processed to produce the following summations: ∑Alkanes is the sum of each individually measured n-Alkane and ∑PAHs is the sum of parent and alkylated PAHs concentrations. To monitor the extraction efficiency for hydrocarbon (GC-MS) analysis, deuterium labeled surrogates were added to the sample during the extraction process. The percent recovery for these labeled surrogates were below 70% in many leachate samples from the tidal flat enclosures. To evaluate the impact of lower extraction efficiency on the overall hydrocarbon patterns on tidal flat leachate samples, the concentration of each Alkane and PAH compounds were extrapolated based on the extraction efficiencies. To monitor partitioning of n-Alkanes, the mean background concentration from each n-Alkane (C10 − 35) measured from control samples were subtracted from the mean concentration of the oil-treated samples. Changes in these values over time were identified for the leachate and sediment.
Amplicon tables were filtered to remove ASVs affiliated with chloroplasts and mitochondria from the 16 S rRNA gene table and metazoans and terrestrial plants from the 18 S rRNA gene table (Cobanli et al. 2022). The tables were rarefied to 9369 reads and 4189 reads for the 16 S rRNA gene and 18 S rRNA gene, respectively, using the ampvis2 package (Andersen et al. 2018). These tables were used to calculate the Shannon diversity and Chao1 richness of each sample. Community structure was analyzed using the vegan package (Oksanen et al. 2022) for each experiment and substrate separately due to the large differences in the initial communities (e.g. between leachate and sediment and between sediment type). Using the avgdist function, 100 iterations were used to generate the mean Bray Curtis dissimilarity of rarefied ASV tables. Permutational analysis of variance (PERMANOVA) was carried out using these average distance matrices and the adonis2 function. The initial analysis included the time and treatment (control vs. IFO-40) interaction, but final models only included those terms that were significant. Permutations were restricted by beach using the permute package. The ten most abundant prokaryotic and eukaryotic ASVs were identified for the beach and tidal flat in the sediment and leachate compartments. The average relative abundances of ASVs were calculated for each time point by treatment.
3 Results
3.1 Initial Conditions
Both sediment types were comprised of sand, silt and clay. However, samples from the tidal flat at Cow Bay contained ∼ 2.2-fold and ∼ 1.7-fold more silt and clay, respectively, compared to Conrad’s Beach samples (Table SM1). Approximately 10% of sand from the beach sediment was greater than 0.5 mm in grain size (coarse and very coarse sand), while sand with the grain size greater than 0.34 mm was not detected from the tidal flat samples (Fig. SM3). Sediments from both locations shared similar physical properties including bulk density, porosity and moisture content (Table SM1). When the sediment was collected, water temperature was 16.9 ℃ and 18.5 ℃ at the beach and tidal flat, respectively. Salinity was 27.0 and 27.9. At the beginning of each experiment, water collected from Bedford Basin was also measured. The temperature was 18.2 ℃ and 16.9 ℃, with salinity 26.7 and 27.5.
The hydrocarbon composition for fresh and weathered IFO-40 used in the experiment was characterized. The distribution of hydrocarbon compounds in the weathered IFO-40 were similar between the two experiments, with weathering occurring at similar temperatures (Table 1). A slight reduction of total Alkanes was observed in the weathered IFO-40-water mixture samples compared to the fresh IFO-40. The weathering process resulted in increased concentrations of ∑alkylated PAHs (Table 1). Different patterns were observed depending on the type of PAH, with phenanthrenes (PHEN) and pyrenes (PYR) increasing, but decreasing amounts of naphthalenes (NAP) and benzonaphthothiophenes (BNT) (Fig. 1). Weathering of IFO-40 resulted in an increase in viscosity and a slight increase in density compared to the fresh IFO-40 (Table 1). Of note, higher viscosity was measured from the weathered IFO-40 used in the tidal flat experiment compared to the beach experiment (Table 1).
Initial PAHs distribution for fresh IFO-40 and weathered IFO-40 water mixture. Parent PAHs are grouped with their alkylated homologs; naphthalene (NAP), phenanthrene (PHEN), dibenzothiophene (DBT), fluorene (FLU), pyrene (PYR), naphthobenzothiophene (BNT), chrysene (CHR), and with other group includes Biphenyl, Acenaphthylene, Acenaphthene, Anthracene, Benz(a)anthracene, Benzo(b)fluoranthene, Benzo(k)fluoranthene, Benzo(e)pyrene, Benzo(a)pyrene, Ideno(1,2,3-cd)pyrene, Dibenzo(a, h)anthracene and Benzo(g, h,i)perylene. Error bars are standard deviation of n = 2
The sediment from the tidal flat appeared black or dark brown in color suggesting it was rich in organic matter (Dragun 1988). Higher TOC and TIC concentrations were measured in the tidal flat leachate compared to the beach samples at day 0 (Table. SM2). With respect to inorganic nutrients, PO43−, DSi and NH4+ concentrations were one to three orders of magnitude higher in the tidal flat leachate compared to the beach samples at day 0 (Table. SM2).
A storm with average winds of 31.5 km/h and with maximum 2.2 mm of rain occurred during the tidal flat experiment on day 3. Otherwise the ambient conditions were similar between the two experiments, with temperatures ranging from 15.6 to 23.5 ℃, no other precipitation was reported and average wind speeds between 10 and 19.8 km/h were observed.
3.2 Hydrocarbon Distribution
3.2.1 Sediment
During the experiments, it was observed that the oil slick re-floated during the “high tide period”, but varied in thickness, surface area and location within the artificial beach. This patchy motif was reflected on the sediment surface during the “low tide period” (after the seawater was drained). These observations, combined with the random sediment sampling scheme (Fig. SM1), may help explain the high variability in the sediment data as the oil slick and partitioning of hydrocarbon compounds in the sediment layers was not captured in all sediment cores. The location of the core was predetermined, which sometimes resulted in sampling through the oil slick, and other times sampled sediment had no visible slick.
The oil slick on the sediment surface was examined. The ∑Alkanes and ∑PAHs appeared to mimic the TPH pattern (Fig. 2). A significant Sediment type-Day interaction was detected with ∑Alkanes and ∑PAHs data (p < 0.015). In general, hydrocarbon concentrations in slicks were higher in tidal flat enclosures than the beach enclosures. Only 3 cores from the beach enclosures recorded high hydrocarbons concentrations and these were from days 3 and 5.
Linear mixed modelling identified a significant 2-way (Treatment-Depth; p < 0.05) interaction for TPH, ∑Alkanes and ∑PAHs when analyzing the surface, middle and bottom layers. Post hoc analysis indicated that hydrocarbon concentrations in oiled surface samples were significantly higher than all other samples. Concentrations in middle and bottom layers were several orders of magnitude lower than observed in the slick and surface layers with no significant difference between oil and control samples (Fig. SM4).
During sampling, a thin dark band was observed directly below the surface of the cores, which was more evident in the oiled beach samples. This was likely due to IFO-40 residues. The patchy distribution of the oil slick, reflected in the high variability of hydrocarbons in the slick, was also observed in the surface sediments, with spikes in concentration detected in some, but not all, cores at a given time point (Fig. 3a). The ∑Alkanes and ∑PAHs concentrations in the surface sediment mimicked the TPH pattern (Fig. 3b & c).
3.2.2 Leachate
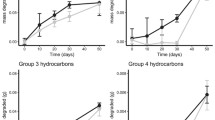
Oil addition resulted in elevated TPH and ∑PAHs concentrations in the leachate samples over a 5-day period (Fig. 4). Using linear mixed modelling, significant two-way interactions (Treatment-Day and Sediment type-Day) were identified (p < 0.0001). With the exception of day 0, the overall TPH concentration pattern for oil-tidal flat samples was similar to that observed in the oil-beach experiment (Fig. 4a). The mean TPH concentrations peaked at day 3 and then plateaued (Fig. 4a).
The ∑Alkanes pattern showed a significant two-way (Sediment type-Day) interaction (p = 0.015) for the leachate. The apparent lack of a treatment effect is likely due to the high background concentrations measured in the control samples (Fig. 4b). In general, Alkanes with C11-C23 were detected in the beach samples from control mesocosms, while longer chain n-Alkanes, C11-C30, were detected in the tidal flat samples (data not shown). The concentrations for C19-C23 in the beach samples were almost 3 times higher compared to the tidal flat samples (data not shown). These n-Alkanes were likely derived from different biological sources present in the sediment prior to collection. Relative to the control mesocosms, lighter n-Alkanes (C12-C15) increased in concentration over time in the beach leachate samples (Fig. SM5a), which suggests the background biogenic n-Alkanes may mask the treatment effect in beach leachate. In tidal flat leachate, lighter n-Alkanes did not increase relative to the control enclosures over time.
The ∑PAHs pattern was significantly different between oil-treated samples from the two experiments (Fig. 4c). Linear mixed modeling indicated there was a significant three-way interaction (Day-Treatment-Sediment type) for the ∑PAHs (p < 0.001). Concentrations increased over time in oil treated mesocosms, but there were clear differences between sediment types. Overall lower ∑PAHs concentrations were measured in the oil-tidal flat samples throughout the experiment compared to the oil-beach samples, with differences increasing over time (Fig. 4c). The ∑PAHs was 5.07 ± 2.83-fold higher in leachate from oil-beach enclosures compared to tidal flat enclosures at day 5.
In addition to oil emulsion formation during the hydrocarbon extraction process, low extraction efficiencies were observed for most of the tidal flat samples compared to beach samples. However, even with the concentrations adjusted to 100% recovery efficiency, the interactions observed with the corrected data remained significant for ∑PAHs (Sediment type-Treatment-Day; p < 0.013) the ∑Alkanes (Sediment type-Day; p < 0.0001) (Fig. SM6). Corrected concentrations of all PAHs in the tidal flat leachate were still significantly lower than concentrations in the leachate from the beach indicating this pattern is not solely due to the low extraction efficiencies (Fig. SM5b).
Within the leachate, parent (C0) naphthalene accounted for > 80% of the mean parent PAHs (data not shown). A significant 3-way (Sediment type-Treatment-Day) interaction was observed for parent (C0) and alkylated (C1 − 3) naphthalenes (p < 0.001) (Fig. SM5b). Oil treatment resulted in an initial increase of the mean concentration of parent (C0) naphthalene in both beach and tidal flat samples, although concentrations were higher in beach mesocosms. Methylnaphthalene (C1) appeared to follow the parent naphthalene pattern in both beach and tidal flat samples, while increases in dimethylnaphthalene (C2) was only observed in oil-beach samples (Fig. SM4b). Trimethylnaphthalene (C3) was also detected in the oil-beach enclosure, but overall concentrations were < 500 ng/L throughout the experiment. With respect to other PAH groups, an increase in concentrations for PHEN, FLU and BNT groups were also observed in oil treated leachate samples, although the maximum mean concentrations for these groups were < 1000 ng/L (data not shown).
3.2.3 Potential Toxicity
Light inhibition in sediment samples differed from the leachate. Percent light inhibition remained low for the beach control samples; however, for all surface sediment samples, the oil treatment resulted in an increase in light inhibition (Fig. SM7a). For the tidal flat mesocosms, high percent light inhibition was observed in all samples, regardless of treatment (data not shown). In addition to the black coloration observed in the initial tidal flat sediments, a strong odour was also noted from these samples, which could be attributed to hydrogen sulfide and/or methane generated under anaerobic conditions within the tidal flat. The presence of hydrogen sulfide could result in higher light inhibition in the absence of oil. Oil treatment resulted in an increase in light inhibition in the surface sediment layer for some time points for the tidal flat samples. Overall light inhibition results showed a significant correlation with TPH and ∑PAHs concentrations within the surface sediment (Padjusted <0.0001, ρ = 0.79 and 0.72 respectively).
For the leachate samples, the light inhibition patterns followed that of the hydrocarbon concentration patterns with similar light inhibition in leachate from both sediment types after 2 d (Fig. SM7b). Collectively, light inhibition from the leachate samples correlated strongly with TPH and ∑PAHs (Padjusted <0.0001, ρ = 0.79 and 0.76).
3.3 Biological Analyses
3.3.1 Cell Density
In general, prokaryotic cell density was one order of magnitude higher in the tidal flat sediment samples compared to beach samples. For most time points, there was high variability between estimates from the three cores. There was no significant effect of treatment or time on the cell abundances for either the beach or the tidal flat (Fig. 5a).
As was observed for sediment samples, higher overall prokaryotic cell abundances were measured in the tidal flat leachate compared to the beach, and a significant 2-way (Day-Sediment type) interaction was identified (p < 0.0001) (Fig. 5b). Cell abundances for the control treatment was similar to that of oil treatment, and hydrocarbon concentrations were poorly correlated with the prokaryotes cell density regardless of treatment (ρ < 0.3 for TPH, ∑Alkanes and ∑PAHs).
Amplicon sequencing data was used to examine prokaryotic and eukaryotic communities in both leachate and sediment samples (Fig. SM8 and SM9). With respect to the surface sediment samples, a two-way (Day-Sediment type; p < 0.05) interaction was observed in prokaryotic species richness while neither sediment type nor treatment were identified as factors in eukaryotic species richness. The prokaryotic Chao1 estimates decreased over time in the beach sediment samples, while a slight increase in prokaryotic Chao1 estimates over time in the tidal flat sediment samples was observed (Fig. SM8a). In general, the eukaryotic Chao1 estimates decreased over time in both beach and tidal flat sediment samples. The prokaryotic Shannon diversity decreased over time in the beach samples, while a slight increase was observed in the tidal flat samples (Fig. SM9a). While no change was observed in the beach samples, the eukaryotic Shannon diversity decreased with time in the tidal flat surface samples.
With respect to leachate samples, a significant Day-Sediment type interaction was observed in prokaryotic species richness, and significant Day-Treatment-Sediment type interaction was observed in the eukaryotic species richness (p < 0.05) (Fig. SM8b). Indeed, higher prokaryotic Chao1 estimates were observed in the tidal flat samples compared to the beach samples, regardless of treatment. The mean eukaryotic Chao1 estimates increased over time in the oil-beach samples, resulting in higher values compared to the control samples, with the opposite pattern observed for the tidal flat samples (Fig. SM9b). Shannon diversity patterns mimicked the prokaryotic and eukaryotic Chao1 pattern (Fig. SM9b).
PERMANOVA of the prokaryotic communities detected significant effects of Day and Treatment for both tidal flat and beach surface sediment samples (Table SM3). Similarly, Day and Treatment were significant for the eukaryote communities in both tidal flat and beach experiment. Though significant, the treatment term in these analyses was only able to account for < 10% of the variation observed in these communities (p = 0.002). A significant Day-Treatment interaction was observed for the tidal flat eukaryotic communities, but accounted for only 4% of the community variation observed (p = 0.012) (Table SM3).
With respect to the leachate, PERMANOVA detected Day and Treatment effects for both tidal flat and beach samples as well as for the eukaryote community from the tidal flat leachate (Table SM4). Though significant, the Treatment term only accounted for < 10% of the variation observed (p = 0.002). The Day-Treatment interaction term was significant for the eukaryotic community in the beach leachate, but also accounted for < 10% of the variation observed (Table SM4).
PERMANOVA indicated that the effect of IFO-40 treatment on microbial communities was minor, but significant in both sediment and leachate compartments. To identify differences due to oil, the 10 most abundant prokaryotic and eukaryotic ASVs in the surface sediment for each sediment type were identified. Few ASVs were identified from both sediment types. Both the prokaryote and eukaryote communities show that abundant ASVs in beach sediments contributed a larger fraction of the whole community compared to the tidal flat sediment. In general, minor changes in relative abundance was observed in oil treatment compared to the control enclosures (Fig. 6). Many of the abundant prokaryotic ASVs were identified as genera with known hydrocarbon degraders. This includes Pseudoalteromonas, Oceanospirillum, Ulvibacter, Alteromonas and Desulfosarcina (Achberger et al. 2021; Voordouw et al. 1996; Watanabe et al. 2017; Pequin et al. 2022). The eukaryotic community was dominated by dinoflagellates and diatoms.
The average relative abundance of 10 most abundant prokaryote (top) and eukaryote (bottom) ASVs from each sediment type over time in the surface sediment compartment. Average abundances in the control beaches are outlined in white, while oil treatments are outlined in black. ASVs are identified to genus where possible. Asterisks (*) indicate where the lowest taxonomic level was family or higher. Values are averages of 3 replicates per treatment and site
Similarly, abundant ASVs from both the prokaryotic and eukaryotic communities differed between the beach and tidal flat leachate (Fig. 7). Some of the same ASVs were identified as abundant in both the leachate and the surface sediment with five shared prokaryotic and six shared eukaryotic ASVs. As with sediment, the leachate had several ASVs that may represent organisms capable of hydrocarbon degradation. This includes Pseudoalteromonas, Oceanospirillum, Vibrio and Alteromonas (Achberger et al. 2021; Pequin et al. 2022; Kampouris et al. 2023). While dinoflagellates were also abundant in the leachate, the abundant eukaryotic ASVs also included green algae and fewer diatoms. Overall, minor changes in relative abundance of these ASVs was observed between the two treatments (Fig. 7). In general, there were higher numbers of prokaryotic and eukaryotic ASVs identified in the tidal flat that are typical of sulfur rich and potentially anaerobic environments (Desulfosarcina, Sva0081 sediment group, Desulfofrigus, Bicosoecida and Marichromatium) (Ferrera et al. 2007; Wylezich and Jurgens 2011; Watanabe et al. 2017; Ramirez et al. 2020; Song et al. 2021).
The average relative abundance of 10 most abundant prokaryote (top) and eukaryote (bottom) ASVs from each sediment type over time in the leachate compartment. Average abundances in the control beaches are outlined in white, while oil treatments are outlined in black. ASVs are identified to genus where possible. Asterisks (*) indicate where the lowest taxonomic level was family or higher. Values are averages of 3 replicates per treatment and site
4 Discussion
The mesocosm experiments described here aimed to understand how weathered medium viscosity oil, under low energy tidal cycles, would move through sediment from different shoreline types. The experiments investigated how the microbial community would respond to differences in hydrocarbon patterns. The mesocosms represented sandy beach and sandy tidal flat shoreline types, with distinct sediment composition (grain size and composition), nutrient regimes and microbial community structure, all of which influenced the oil movement within the sediment compartment, the hydrocarbon dissolution and the microbial responses to treatment in different compartments.
High variability in hydrocarbon partitioning was observed among replicates for both sediment type (tidal flat and beach), which was likely introduced through movement of the oil slick during the artificial tidal cycles. The density of the weathered IFO-40 was < 0.97 g/cm3 (at 15 °C), lower than that of the Bedford Basin seawater (1.019 g/cm3) used in this experiment, and thus the slick refloated and remobilized during the experiment (Owens et al. 2021). In addition to the daily tidal cycles, high winds (max 48 km/hr) occurred on day 4 of the tidal flat experiment and contributed to the variable distribution of the oil slick. Remobilization of oil slicks and variability in the distribution of oil can happen on larger scales. For example, during the Baffin Island Oil Spill experiment in 1981, a rapid refloating of spilled medium crude oil was documented during the first 24–48 h (Owens et al. 2008). During an early visual survey conducted after the initial Arrow incident, patchy oil was observed along the south shore (e.g. Black Duck cove and Half Island cove) (Drapeau 1970). Highly variable TPH concentrations were reported from different sites along the same shoreline following a second oil release in 2015 (Yang et al. 2018).
Sediment composition appeared to have the greatest effect on oil movement and hydrocarbon dissolution processes in this study. Overall, higher amounts of dissolved hydrocarbons were measured in the beach leachate indicating that dissolved hydrocarbons were more readily partitioned into the leachate compared to in the tidal flat enclosures. However, only a small fraction (< 0.01% C12 − 15n-Alkanes and < 0.15% C0 − 3 naphthalenes) of the total lower molecular weight hydrocarbons (Fig. SM5) were removed from the beach enclosures during the experiment, leaving most of hydrocarbons in the oil slick. Additionally, some lower molecular weight hydrocarbons were likely lost through volatilization. With coarse and very coarse sands (> 5 mm in grain size) present in the beach mesocosm sediment, oil should have percolated through the beach mesocosms readily. Although some oil movement between the slick to surface sediment was observed, low concentrations of TPH detected in the middle and bottom sediment in the beach enclosure were in contrast to a previous study (Harper et al. 1995). Harper et al. (1995) reported that at 15 °C the oil penetration depth for weathered IFO-180 was 9 cm and oil retention capacity was 128 L/m3 for very coarse sand sediment using a column experiment. Results from the current study suggest an oil penetration depth of < 3 cm. The apparent difference may be attributed to experimental design as the surface area was 70.9 cm2 for the column study and 1200 cm2 for the beach enclosure experiment. The larger surface area and lower oil loading (0.26 L/m2) in this study would allow for more horizontal movement of the oil in the mesocosms compared to the column study. Some limited oil movement was observed in the tidal flat enclosures as demonstrated by lower hydrocarbon concentrations in some slick samples after day 2 (Fig. 2) and a three-fold increase in TPH concentration in the surface layer by day 4 (Fig. 3). These observations are likely attributed to the slightly higher fine sediment content combined with slightly higher oil viscosity in the tidal flat enclosures compared to the sandy beach enclosures. Additionally, a gradual increase in hydrocarbons in the leachate suggests limited hydrocarbon dissolution in the tidal flat enclosures. This is in contrast to no oil penetration into the water-saturated sand flat sediment being observed following the M/T Westchester oil spill in the Mississippi River (Michel et al. 2002). Despite the oil viscosity for the M/T Westchester (8.3 cSt at 20 °C, non-weathered) being approximately 20-times lower than the weathered IFO-40 oil used in this study (167.9 cSt at 25 °C), the oil slick was characterized as a band of emulsified oil with 1–3 cm thickness on the sand flat following the M/T Westchester. The emulsification process may have increased the viscosity of the Westchester oil limiting oil penetration into the sand flat.
The response of V. fisheri in the Microtox® assay served as a proxy for hydrocarbon bioavailability and potential toxicity. A significant correlation was identified between light inhibition and TPH concentration in surface sediments for both sediment types. In addition to higher TPH concentrations compared to the beach samples, the apparent higher light inhibition observed in the tidal flat surface sediment layers in control enclosures, may be attributed to the presence of elemental and hydrogen sulfide in the sediment samples. Sediment samples (∼ 10 cm in depth), collected from the tidal flat, were likely stratified into oxygenic and anoxygenic layers, which were mixed together during the creation of the artificial beaches. The black appearance and strong sulfidic odor from the tidal flat sediments suggests high concentrations of sulfides (Brouwer and Murphy 1995; Pardos et al. 1999). A few sulfur-reducing bacteria ASVs (Desulfosarcina, Sva0081 sediment group and Desulfofrigus) as well as ASVs commonly detected in sulfur-rich environments were identified amongst the 10 most abundant genera in the tidal flat samples further indicative for the presence of reduced sulfur in these enclosures (Kuever et al., Ramirez et al. 2020; Hafez et al. 2023). Taken together, these results suggest that hydrocarbon bioavailability in the surface sediment layer for both beach and tidal flat enclosures increased light inhibition at the surface, but that anaerobic metabolic by-products contributed to the observed background toxicity in the tidal flat enclosures. The presences of sulfide in some shorelines may limit the usefulness of toxicity assays in detecting oil impacts as factors other than hydrocarbons may affect the results.
Low molecular weight n-Alkanes and naphthalenes, dominated the leachate compartment, which have high bioavailability. It is well documented that naphthalenes elicit toxicological effects due to their high solubility. This was further demonstrated by the strong correlation between light inhibition and ∑PAHs and TPH concentration in both oil-beach and oil-tidal flat leachate samples.
Shoreline type appears to have a great influence on oil removal. It has been reported that the decay rate of ∑PAHs for beaches (k = 0.016) was greater than that of muddy shorelines, including tidal flats (k = 0.001) following the Hebei Spirit oil spill incident (Kim et al. 2017). A similar pattern was observed in this study with higher overall ∑Alkanes and ∑PAHs concentrations measured in the beach leachate samples compared to tidal flat enclosure. It is well documented that hydrocarbons with lower molecular weight are more susceptible to dissolution due to their high solubility and lower hydrophobicity compared to higher molecular weight hydrocarbons (reviewed by Wang et al. 2021). In addition, PAHs with logKow values between 3.7 and 4.8 are more likely to partition into the aqueous phase compared to other higher molecular weight PAHs (Yang et al. 2020; Owens et al. 2021). The more water soluble PAHs include the NAP, PHEN, DBT and FLU groups, and amongst these groups, the NAP group has the lowest logKow and highest solubility values.
In this study, the differential mean concentrations of lower molecular weight n-Alkanes (C12 − 15) increased over time in oil-beach leachate samples, and the accumulation order followed closely with the solubility values; C13 > C12 > C14> C15. Substantial increases in ∑PAHs concentration were measured over time in oil-treated beach leachate samples, and a significant concentration increase for the NAP group was observed. The temporal pattern for parent and alkylated naphthalene compounds were consistent with logKow values (naphthalene > methylnaphthalene > dimethylnaphthalene; logKow=3.3, 3.87 and 4.3, respectively) (ChemSpider 2023). By contrast, the differential mean concentration of C12 decreased over time in the oil-tidal flat leachate sample. Only parent naphthalene and methylnaphthalene were detected in oil-tidal flat leachate samples with 2.5-20-fold and 8-114-fold lower mean concentrations compared to the oil-beach leachate. While many field studies have reported long-term oil burial and retention in shoreline sediment (Kim et al. 2017; Yang et al. 2018; Hunnie et al. 2023), this was not observed in this study, which was likely due to (1) the low energy experimental condition, (2) relatively short experimental period, and (3) the lack of burrowing animals in the enclosures. The low hydrocarbon concentrations in the middle and bottom layers of the cores in this study suggest very low hydrocarbon partitioning within these compartments. Though not examined in this study, oil evaporation and photo-oxidation processes could not be excluded as hydrocarbon loss mechanisms as these enclosures were exposed to the natural elements, such as wind and sunlight.
The physical partitioning of petroleum hydrocarbon can have a profound impact on indigenous microbial community in sediment (Chronopoulou et al. 2015; Sanni et al. 2015). The ability of the microbial community to respond and degrade the oil depends on the initial community composition (Schreiber et al. 2019; Cobanli et al. 2022) and the availability of nutrients (Personna et al. 2016; Williams et al. 2017; Ortmann et al. 2019). It is well documented that, in response to oil exposure, hydrocarbon degrading bacteria increase in relative abundance along with other microbial taxa that can tolerate the presence of hydrocarbons. This generally reduces the microbial diversity (Atlas et al. 2015; Valencia-Agami et al. 2019; Pequin et al. 2022).
In this study, the prokaryotic species richness and diversity increased over time for the tidal flat sediment under both treatment regimes, while decreasing in the beach sediment. Addition of oil produced significant, but marginal, effects on prokaryotic species richness and diversity in both beach and tidal flat enclosures. These opposing trends may be explained by differences in the carbon content (TOC) and nutrients status (NO3−, NH4+, PO43−) between the two experiments. Similar results were previously reported from examination of chronically PAHs-contaminated sediments along the Atlantic and the Mediterranean coasts (Jeanbille et al. 2016). In those samples, the prokaryotic alpha-diversity was strongly associated with salinity, temperature and organic carbon content (Jeanbille et al. 2016). Additionally, nutrient availability is a key factor for increasing microbial biomass and promoting hydrocarbon degradation (Ortmann et al. 2019; Bianco et al. 2020; Fragkou et al. 2021; Ellis et al. 2022; Kundu et al. 2022; Michel et al. 2022). Contrary to the results for the tidal flat in this study, several studies demonstrated nutrient addition significantly enriched hydrocarbon degraders and enhanced biodegradation rate (Chang et al. 2010; Kim et al. 2018; Bianco et al. 2020; Ellis et al. 2022). In the current study, addition of nutrients to the sandy beach enclosures could have increased the microbial response. Addition of nutrient to the already high nutrient tidal flat enclosures may not have had much of an impact. Very high nutrient concentrations could inhibit biodegradation, as observed in an intertidal zone field trial study conducted by Roberg et al. (2007). These authors reported the hydrocarbon degrader abundances for kerosene-fertilizer treated samples were similar to the fertilizer-alone control after > 78 days period, and concluded that fertilizer amendment may impact the hydrocarbon-degrading cell numbers.
The impacts of differences between the sediment types on the microbial communities were further exemplified by the overall higher prokaryote cell density in the tidal flat sediment and leachate in both treatments compared to the beach samples. The higher abundances are likely supported by the much higher organic carbon and inorganic nutrient concentrations measured in the tidal flat leachate at the beginning of the experiments compared to the beach. The presence of biogenic n-Alkanes (C24-C35) in the tidal flat leachate samples further supports the tidal flat sediment was rich in organic matter as these long chain n-Alkanes are likely derived from plants, bacteria and algae (Volkman et al. 2008; Yang et al. 2018). Slightly higher oil viscosity and oil emulsion interactions with fine sediment and organic matter likely contributed to the low hydrocarbon extraction efficiency observed for the tidal flat samples.
Several field and laboratory studies have shown that microbial hydrocarbon degraders become more abundant following oil (Roling et al. 2004; Atlas et al. 2015; Bargiela et al. 2015; Brakstad et al. 2015; Lee et al. 2018, 2019). In the current study, however, the most common prokaryotic ASVs were similar in both oil and control samples with minor difference in relative abundance. This pattern was observed for both the surface sediment and the leachate. The relative abundance of ASVs differed based on the sediment type. The lack of significant increase in the relative abundance of obligate hydrocarbon degraders in the oil treatment enclosures may be attributed to a priming effect of the vegetal organic matter and biogenic hydrocarbons on the microbial population in the sediment (Chaineau et al. 2003). Several of the ASVs that were identified as being abundant in both the sediment and the leachate are related to taxa known to carry out hydrocarbon degradation. This includes several taxa associated with n-Alkane degradation as well as PAH degradation (Voordouw et al. 1996; Watanabe et al. 2017; Achberger et al. 2021; Pequin et al. 2022; Kampouris et al. 2023). Because these taxa were abundant at the beginning of the experiment, they may have been able to shift to hydrocarbon degradation within the time frame of the experiment. To see a large community composition shift, a longer experiment may be needed. The five days used in this study may be too short of an incubation time given that seawater was replenished daily to mimic tidal cycle (Johnsen et al. 2007).
Several studies have suggested that the prokaryotic communities are frequently subject to predation by microzooplankton, which could impact the oil degrading prokaryotic community abundance (Anderson et al. 2001; Gertler et al. 2010; Ortmann et al. 2012). However, as with the prokaryotic communities, there was little change due to treatment for eukaryotic communities, with the greatest differences between the sediment types. Dinoflagellates, some of which could be predatory, were the most abundant ASVs in the beach sediment, with many of the same ASVs abundant in the leachate. In contrast, the leachate from the tidal flat sediment was quite different from the sediment samples, potentially due to the higher sulfides selecting for different taxa in the tidal flat sediment. In contrast to this study, crude oil treatment resulted in a significant increase in marine ciliate and flagellate communities (Gertler et al. 2010). As with prokaryotic communities, a longer experimental period may be needed to see a larger eukaryotic community composition shift.
Taken together, results from this study suggest greater priority is needed to protect sandy tidal flat shorelines from oiling as slower natural attenuation was observed for these sediments compared to the sandy beach. In the event a shoreline is oiled, selecting an appropriate intervention strategy depends on the type of oil spilled, presence of vulnerable species and the degree of oiling. Low oil penetration in the oil-tidal flat enclosures in this study suggests that the oil mainly remains on the surface as a slick, only penetrating a small distance into the top 1–3 cm sediment layer. Additionally, the oil slick is easily refloated as the tide comes in. Large amounts of the oil slick could be recovered using sorbents (Michel et al. 2022). This method is effective on diverse oil types and produces the least impacts on habitat (Michel et al. 2022). For an oiled sandy beach, more cleanup techniques are available, which would address the deeper penetration of the oil into the beach. These may include mechanical removal of oiled sand, as was done during the Deepwater Horizon spill (Zengel et al. 2015; Owens et al. 2023) or biostimulation, similar to field study conducted by Jimenez et al. (2007) following the Prestige oil spill incident.
5 Conclusion
In the current study, our aim was to understand the effect of sediment type on the fate, transport and behaviour of stranded weathered-IFO-40 and the microbial community response, under a low energy tidal cycle regime. Overall our results showed weathered IFO-40 oil was more readily moved from the slick to the surface sediment layer in beach enclosures through low energy tidal flushing, with limited oil movement in tidal flat enclosures. Oil movement between slick and sediment was limited to the surface layer. With no oil burial and/or retention observed in this study, and with only limited amount of lower molecular weight hydrocarbon partitioned into the leachate compartment, physical weathering processes likely played an important role in the fate of the stranded oil, including photo-oxidation and evaporation. The addition of oil had minimal effects on the overall identity and abundances of the most abundant ASVs. Some of these abundant ASVs likely contributed to some hydrocarbon biodegradation in this study.
These results suggest that the slower natural attenuation could prolong the ecological and toxicological impacts on a sandy tidal flat compared to a beach shoreline. However, low penetration of the oil into the sediments may reduce the persistence of oil in these habitats, which also suggest bulk oil removal mitigation, such as sorbent application and oil vacuuming, may be deployed to minimize further environmental and ecological impacts. Mechanical removal and biostimulation could be deployed as a treatment method for oiled sand in sandy beach shorelines. High variability in the distribution and concentrations of hydrocarbons within any shoreline should be expected during an oil spill, which presents a challenge in estimating the fate and behaviour of oil deposited on a particular shoreline. This also limits predictions of potential ecological impacts, and in directing an effective emergency response to shoreline oiling.
Data Availability
The data supporting findings of this current study are available within the paper, its supplementary information file and upon reasonable request.
References
Achberger AM, Doyle SM, Mills MI, Holmes CP, Quigg A, Sylvan JB (2021) Bacteria-oil microaggregates are an important mechanism for Hydrocarbon Degradation in the Marine Water Column. Msystems 6(5). https://doi.org/10.1128/mSystems.01105-21
Albers PH, Loughlin TR (2003) Effects of PAHs on marine birds, mammals and reptiles. Chichester, England, Hoboken, NJ, USA, Wiley
Andersen KS, Kirkegaard RH, Karst SM, Albertsen M (2018) ampvis2: an R package to analyse and visualise 16S rRNA amplicon data. bioRxiv: 299537. https://doi.org/10.1101/299537
Anderson OR, Gorrell T, Bergen A, Kruzansky R, Levandowsky M (2001) Naked amoebas and bacteria in an oil-impacted salt marsh community. Microb Ecol 42(3):474–481. https://doi.org/10.1007/s00248-001-0008-x
Asif Z, Chen Z, An CJ, Dong JX (2022) Environmental Impacts and Challenges Associated with Oil spills on shorelines. J Mar Sci Eng 10(6). https://doi.org/10.3390/jmse10060762
Atlas RM, Stoeckel DM, Faith SA, Minard-Smith A, Thorn JR, Benotti MJ (2015) Oil biodegradation and oil-degrading microbial populations in Marsh Sediments impacted by oil from the Deepwater Horizon Well Blowout. Environ Sci Technol 49(14):8356–8366. https://doi.org/10.1021/acs.est.5b00413
Bargiela R, Mapelli F, Rojo D, Chouaia B, Tornes J, Borin S, Richter M, Del Pozo M, Cappello S, Gertler C, Genovese M, Denaro R, Martinez-Martinez M, Fodelianakis S, Amer R, Bigazzi D, Han X, Chen J, Chernikova T, Golyshina O, Mahjoubi M, Jaouanil A, Benzha F, Magagnini M, Hussein E, Al-Horani F, Cherif A, Blaghen M, Abdel-Fattah Y, Kalogerakis N, Barbas C, Malkawi H, Golyshin P, Yakimov M, Daffonchio D, Ferrer M (2015) Bacterial population and biodegradation potential in chronically crude oil-contaminated marine sediments are strongly linked to temperature. Sci Rep 5. https://doi.org/10.1038/srep11651
Bates D, Machler M, Bolker BM, Walker SC (2015) Fitting Linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Becker S, Aoyama M, Woodward EMS, Bakker K, Coverly S, Mahaffey C, Tanhua T (2020) GO-SHIP repeat Hydrography Nutrient Manual: the precise and accurate determination of dissolved inorganic nutrients in seawater, using continuous Flow Analysis methods. Front Mar Sci 7. https://doi.org/10.3389/fmars.2020.581790
Bianco F, Race M, Papirio S, Esposito G (2020) Removal of polycyclic aromatic hydrocarbons during anaerobic biostimulation of marine sediments. Sci Total Environ 709. https://doi.org/10.1016/j.scitotenv.2019.136141
Brady NC, Weil RR (2010) Elements of the nature and properties of soils. Pearson Educational International, Boston
Brakstad OG, Throne-Holst M, Netzer R, Stoeckel DM, Atlas RM (2015) Microbial communities related to biodegradation of dispersed Macondo oil at low seawater temperature with Norwegian coastal seawater. Microb Biotechnol 8(6):989–998. https://doi.org/10.1111/1751-7915.12303
Brannock PM, Wang L, Ortmann AC, Waits DS, Halanych KM (2016) Genetic assessment of meiobenthic community composition and spatial distribution in coastal sediments along northern Gulf of Mexico. Mar Environ Res 119. https://doi.org/10.1016/j.marenvres.2016.05.011
Brouwer H, Murphy T (1995) Volatile sulfides and their toxicity in fresh-water sediments. Environ Toxicol Chem 14(2):203–208. https://doi.org/10.1002/etc.5620140204
Chaerun SK, Tazaki K, Asada R, Kogure K (2004) Bioremediation of coastal areas 5 years after the Nakhodka oil spill in the sea of Japan: isolation and characterization of hydrocarbon-degrading bacteria. Environ Int 30(7):911–922. https://doi.org/10.1016/j.envint.2004.02.007
Chaineau CH, Yepremian C, Vidalie JF, Ducreux J, Ballerini D (2003) Bioremediation of a crude oil-polluted soil: Biodegradation, leaching and toxicity assessments. Water Air Soil Pollution 144(1):419–440. https://doi.org/10.1023/A:1022935600698
Chang WJ, Dyen M, Spagnuolo L, Simon P, Whyte L, Ghoshal S (2010) Biodegradation of semi- and non-volatile petroleum hydrocarbons in aged, contaminated soils from a sub-arctic site: Laboratory pilot-scale experiments. site Temp Chemosphere 80(3):319–326. https://doi.org/10.1016/j.chemosphere.2010.03.055
ChemSpider (2023) “ChemSpider” https://www.chemspider.com/, Accessed July 14, 2023
Chronopoulou PM, Sanni GO, Silas-Olu DI, van der Meer JR, Timmis KN, Brussaard CPD, McGenity TJ (2015) Generalist hydrocarbon-degrading bacterial communities in the oil-polluted water column of the North Sea. Microb Biotechnol 8(3):434–447. https://doi.org/10.1111/1751-7915.12176
Chung IY, Cho KJ, Hiraoka K, Mukai T, Nishijima W, Takimoto K, Okada M (2004) Effects of oil spill on seawater infiltration and macrobenthic community in tidal flats. Mar Pollut Bull 49(11–12):959–963. https://doi.org/10.1016/j.marpolbul.2004.06.021
Cobanli SE, Wohlgeschaffen G, Ryther C, MacDonald J, Gladwell A, Watts T, Greer CW, Elias M, Wasserscheid J, Robinson B, King TL, Ortmann AC (2022) Microbial community response to simulated diluted bitumen spills in coastal seawater and implications for oil spill response. FEMS Microbiol Ecol 98(5). https://doi.org/10.1093/femsec/fiac033
R Core Team (2020) R: A Language and Environment for Statistical Computing. from https://www.r-project.org/
Danovaro R, Manini E, Dell’Anno A (2002) Higher abundance of bacteria than of viruses in deep Mediterranean sediments. Appl Environ Microbiol 68(3):1468–1472. https://doi.org/10.1128/Aem.68.3.1468-1472.2002
Dragun J (1988) The soil chemistry of hazardous materials. Silver Spring, Maryland, Hazardous Materials Control Research Institute
Drapeau G (1970) Reconnaissance survey of oil pollution on south shore of Chedabucto Bay (March 24–25, 1970). Dartmouth, N.S, Atlantic Oceanographic Laboratory.
Ellis M, Altshuler I, Schreiber L, Chen YJ, Okshevsky M, Lee K, Greer CW, Whyte LG (2022) Hydrocarbon biodegradation potential of microbial communities from high Arctic beaches in Canada’s Northwest Passage. Mar Pollut Bull 174. https://doi.org/10.1016/j.marpolbul.2021.113288
Environment Canada (2002) Biological Test Method. Reference method for determining the toxicity of sediment using luminescent Bacteria in a solid-phase test. Environment Canada
Feng Q, An CJ, Cao Y, Chen Z, Owens E, Taylor E, Wang Z, Saad EA (2021) An analysis of selected oil spill Case studies on the shorelines of Canada. J Environ Inf Lett. https://doi.org/10.3808/jeil.202100052
Ferrera I, Sanchez O, Mas J (2007) Characterization of a sulfide-oxidizing biofilm developed in a packed-column reactor. Int Microbiol 10(1):29–37. https://doi.org/10.2436/20.1501.01.5
Fox J, Bouchet-Valat M, Andronic L, Ash M, Boye T, Calza S, Chang A, Gegzna V, Grosjean P, Heiberger R (2019) Rcmdr: R Commander. R package version 2.5-2, Recuperado de https://cran. r-project. or g
Fragkou E, Antoniou E, Daliakopoulos I, Manios T, Theodorakopoulou M, Kalogerakis N (2021) In situ aerobic bioremediation of sediments polluted with Petroleum hydrocarbons: a critical review. J Mar Sci Eng 9(9). https://doi.org/10.3390/jmse9091003
Gertler C, Nather DJ, Gerdts G, Malpass MC, Golyshin PN (2010) A Mesocosm Study of the changes in Marine Flagellate and Ciliate communities in a Crude Oil Bioremediation Trial. Microb Ecol 60(1):180–191. https://doi.org/10.1007/s00248-010-9660-3
Hafez T, Ortiz-Zarragoitia M, Cagnon C, Cravo-Laureau C, Duran R (2023) Cold sediment microbial community shifts in response to crude oil water-accommodated fraction with or without dispersant: a microcosm study. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-25264-6
Harper JR, Kory M, Canada S, Environmental Protection and C. Environmental Technology (1995). Stranded oil in coarse sediment experiments (SOCSEX II). Ottawa, Ont, Environment Canada, Environmental Protection Service, Environmental Technology Centre
Humphrey B, Owens E, Sergy G (1993) DEVELOPMENT OF A STRANDED OIL IN COARSE SEDIMENT (SOCS) MODEL. Int Oil Spill Conf Proc 19931:575–582. https://doi.org/10.7901/2169-3358-1993-1-575
Hunnie BE, Schreiber L, Greer CW, Stern GA (2023) The recalcitrance and potential toxicity of polycyclic aromatic hydrocarbons within crude oil residues in beach sediments at the BIOS site, nearly forty years later. Environ Res 222:115329. https://doi.org/10.1016/j.envres.2023.115329
Ikenaga M, Guevara R, Dean AL, Pisani C, Boyer JN (2010) Changes in Community structure of sediment Bacteria along the Florida Coastal everglades Marsh-Mangrove-Seagrass Salinity Gradient. Microb Ecol 59(2):284–295. https://doi.org/10.1007/s00248-009-9572-2
IPIECA (2016) Impacts of oil spills on shorelines
ITOPF I (2022) Tanker spill statistics 2021
Jeanbille M, Gury J, Duran R, Tronczynski J, Ghiglione JF, Agogue H, Ben Said O, Taib N, Debroas D, Garnier C, Auguet JC (2016) Chronic polyaromatic hydrocarbon (PAH) contamination is a marginal driver for Community Diversity and Prokaryotic Predicted Functioning in Coastal Sediments. Front Microbiol 7. https://doi.org/10.3389/fmicb.2016.01303
Jimenez N, Viñas M, Bayona JM, Albaiges J, Solanas AM (2007) The Prestige oil spill: bacterial community dynamics during a field biostimulation assay. Appl Microbiol Biotechnol 77(4):935–945. https://doi.org/10.1007/s00253-007-1229-9
Johnsen AR, Schmidt S, Hybholt TK, Henriksen S, Jacobsen CS, Andersen O (2007) Strong impact on the polycyclic aromatic hydrocarbon (PAH)-degrading community of a PAH-polluted soil but marginal effect on PAH degradation when priming with bioremediated soil dominated by mycobacteria. Appl Environ Microbiol 73(5):1474–1480. https://doi.org/10.1128/Aem.02236-06
Kampouris ID, Grundger GF, Christensen JH, Greer C, Kjeldsen KU, Boone W, Meire L, Rysgaard S, Vergeynst L (2023) Long-term patterns of hydrocarbon biodegradation and bacterial community composition in epipelagic and mesopelagic zones of an Arctic fjord. J Hazard Mater 446. https://doi.org/10.1016/j.jhazmat.2022.130656
Kim M, Jung JH, Ha SY, An JG, Shim WJ, Yim UH (2017) Long-term monitoring of PAH Contamination in Sediment and Recovery after the Hebei Spirit Oil Spill. Arch Environ Contam Toxicol 73(1):93–102. https://doi.org/10.1007/s00244-017-0365-1
Kim J, Lee AH, Chang WJ (2018) Enhanced bioremediation of nutrient-amended, petroleum hydrocarbon-contaminated soils over a cold-climate winter: the rate and extent of hydrocarbon biodegradation and microbial response in a pilot-scale biopile subjected to natural seasonal freeze-thaw temperatures. Sci Total Environ 612:903–913. https://doi.org/10.1016/j.scitotenv.2017.08.227
King TL, Robinson B, Boufadel M, Lee K (2014) Flume tank studies to elucidate the fate and behavior of diluted bitumen spilled at sea. Mar Pollut Bull 83(1):32–37. https://doi.org/10.1016/j.marpolbul.2014.04.042
Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huettel M (2011) Hydrocarbon-degrading Bacteria and the Bacterial Community Response in Gulf of Mexico Beach sands impacted by the Deepwater Horizon Oil Spill. Appl Environ Microbiol 77(22):7962–7974. https://doi.org/10.1128/Aem.05402-11
Kuever J, Rainey FA and F. Widdel Desulfofrigus. Bergey’s Manual of Systematics of Archaea and Bacteria: 1–4
Kundu A, Harrisson O, Ghoshal S, (2022) ACS ES&T Eng 2(12): 2287–2300 DOI: https://doi.org/10.1021/acsestengg.2c00220
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest Package: tests in Linear mixed effects models. J Stat Softw 82(13):1–26. https://doi.org/10.18637/jss.v082.i13
Lee DW, Lee H, Lee AH, Kwon BO, Khim JS, Yim UH, Kim BS, Kim JJ (2018) Microbial community composition and PAHs removal potential of indigenous bacteria in oil contaminated sediment of Taean coast. Korea Environ Pollution 234:503–512. https://doi.org/10.1016/j.envpol.2017.11.097
Lee H, Lee DW, Kwon SL, Hen YM, Jang S, Kwon BO, Khim JS, Kim GH, Kim JJ (2019) Importance of functional diversity in assessing the recovery of the microbial community after the Hebei Spirit oil spill in Korea. Environ Int 128:89–94. https://doi.org/10.1016/j.envint.2019.04.039
Lee K, Wells P, Gordon D (2020) Reflecting on an anniversary. The 1970 SS Arrow oil spill in Chedabucto Bay, Nova Scotia. Can Mar Pollution Bull 157. https://doi.org/10.1016/j.marpolbul.2020.111332
Lipscomb TP, Harris RK, Moeller RB, Pletcher JM, Haebler RJ, Ballachey BE (1993) Histopathologic Lesions in Sea otters exposed to crude-oil. Vet Pathol 30(1):1–11
Maletic SP, Beljin JM, Roncevic SD, Grgic MG, Dalmacija BD (2019) State of the art and future challenges for polycyclic aromatic hydrocarbons is sediments: sources, fate, bioavailability and remediation techniques. J Hazard Mater 365:467–482. https://doi.org/10.1016/j.jhazmat.2018.11.020
Michel J, Henry CB, Thumm S (2002) Shoreline assessment and environmental impacts from the MIT Westchester oil spill in the Mississippi River. Spill Science & Technology Bulletin 7(3–4): 155–161 https://doi.org/10.1016/S1353-2561(02)00047-6
Michel JM, Rutherford NR, Zengel S (2022) Oil spills in marshes: planning & response considerations. Department of Commerce, National Oceanic and Atmospheric Administration, National Ocean Service, Office of Response and Restoration
Mitchelmore CL, Bishop CA, Collier TK (2017) Toxicological estimation of mortality of oceanic sea turtles oiled during the Deepwater Horizon oil spill. Endanger Species Res 33:39–50. https://doi.org/10.3354/esr00758
National Research Council (2003) Oil in the sea III: inputs, fates, and effects. National Academies, Washington, D.C.
Oksanen J, Simpson G, Blanchet FG, Kindt R, Legendre P, Minchin P, hara R, Solymos P, Stevens H, Szöcs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Cáceres M, Durand S, Weedon J (2022) vegan community ecology package version 2.6-2 April 2022.
Ortmann AC, Anders J, Shelton N, Gong LM, Moss AG, Condon RH (2012) Dispersed oil disrupts Microbial pathways in Pelagic Food Webs. PLoS ONE 7(7). https://doi.org/10.1371/journal.pone.0042548
Ortmann AC, Cobanli SE, Wohlgeschaffen G, Thamer P, McIntyre C, Mason J, King TL (2019) Inorganic nutrients have a significant, but minimal, impact on a coastal microbial community’s response to fresh diluted bitumen. Mar Pollut Bull 139:381–389. https://doi.org/10.1016/j.marpolbul.2019.01.012
Ortmann AC, Cobanli SE, Wohlgeschaffen G, MacDonald J, Gladwell A, Davis A, Robinson B, Mason J, King TL (2020) Measuring the fate of different diluted bitumen products in coastal surface waters. Mar Pollut Bull 153. https://doi.org/10.1016/j.marpolbul.2020.111003
Owens EH, Sergy GA (2010) A field guide to oil spill response on Marine shorelines. Environment Canada
Owens EH, Sergy GA, Guénette CC, Prince RC, Lee K (2003) The reduction of stranded oil by In Situ shoreline treatment options. Spill Science & Technology Bulletin 8(3): 257–272 https://doi.org/10.1016/S1353-2561(03)00041-0
Owens EH, Taylor E, Humphrey B (2008) The persistence and character of stranded oil on coarse-sediment beaches. Mar Pollut Bull 56(1):14–26. https://doi.org/10.1016/j.marpolbul.2007.08.020
Owens E, Taylor E, Sergy G, Lee K, An C, Chen Z (2021) A practical model of the natural attenuation of oil on shorelines for decision support. J Environ Inf Lett 5:48–60
Owens EH, Taylor E, Graham A, Castle RW (2023) Sand Beach Treatment Studies and Field Trials Conducted During the Deepwater Horizon-Macondo Response Operation. International Oil Spill Conference Proceedings 2011(1): 265–265 https://doi.org/10.7901/2169-3358-2011.1.265
Pardos M, Benninghoff C, Thomas RL, Khim-Heang S (1999) Confirmation of elemental sulfur toxicity in the Microtox (R) assay during organic extracts assessment of freshwater sediments. Environ Toxicol Chem 18(2):188–193. https://doi.org/10.1002/etc.5620180213
Pequin B, Cai QH, Lee K, Greer CW (2022) Natural attenuation of oil in marine environments: a review. Mar Pollut Bull 176. https://doi.org/10.1016/j.marpolbul.2022.113464
Personna YR, Axe L, Boufadel MC, Zhang S, King T (2016) Dual effects of a dispersant and nutrient supplementation on weathered Endicott oil biodegradation in seawater. AIMS Environ Sci 3(4):739–751. https://doi.org/10.3934/environsci.2016.4.739
Pontes J, Mucha AP, Santos H, Reis I, Bordalo A, Basto MC, Bernabeu A, Almeida CMR (2013) Potential of bioremediation for buried oil removal in beaches after an oil spill. Mar Pollut Bull 76(1–2):258–265. https://doi.org/10.1016/j.marpolbul.2013.08.029
Powell SM, Bowman JP, Snape I, Stark JS (2003) Microbial community variation in pristine and polluted nearshore Antarctic sediments. FEMS Microbiol Ecol 45(2):135–145. https://doi.org/10.1016/S0168-6496(03)00135-1
Ramirez D, Vega-Alvarado L, Taboada B, Estradas-Romero A, Soto L, Juarez K (2020) Bacterial diversity in surface sediments from the continental shelf and slope of the North West Gulf of Mexico and the presence of hydrocarbon degrading bacteria. Mar Pollut Bull 150. https://doi.org/10.1016/j.marpolbul.2019.110590
Reid BJ, Jones KC, Semple KT (2000) Bioavailability of persistent organic pollutants in soils and sediments - a perspective on mechanisms. Consequences Assess Environ Pollution 108(1):103–112. https://doi.org/10.1016/S0269-7491(99)00206-7
Roberg S, Stormo SK, Landfald B (2007) Persistence and biodegradation of kerosene in high-arctic intertidal sediment. Mar Environ Res 64(4):417–428. https://doi.org/10.1016/j.marenvres.2007.03.003
Roling WFM, Milner MG, Jones DM, Fratepietro F, Swannell RPJ, Daniel F, Head IM (2004) Bacterial community dynamics and hydrocarbon degradation during a field-scale evaluation of bioremediation on a mudflat beach contaminated with buried oil. Appl Environ Microbiol 70(5):2603–2613. https://doi.org/10.1128/Aem.70.5.2603-2613.2004
Russell L (2023) Emmeans: estimated marginal means, aka least-squares means. 1
Sanni GO, Coulon F, McGenity TJ (2015) Dynamics and distribution of bacterial and archaeal communities in oil-contaminated temperate coastal mudflat mesocosms. Environ Sci Pollut Res 22(20):15230–15247. https://doi.org/10.1007/s11356-015-4313-1
Schreiber L, Fortin N, Tremblay J, Wassercheid J, Elias M, Mason J, Sanschagrin S, Cobanli S, King T, Lee K, Greer CW (2019) Potential for microbially mediated natural attenuation of diluted bitumen on the coast of British Columbia (Canada). Appl Environ Microbiol 85(10):e00086–e00019
Song J, Hwang J, Kang I, Cho JC (2021) A sulfate-reducing bacterial genus, Desulfosediminicola gen. nov., comprising two novel species cultivated from tidal-flat sediments. Sci Rep 11(1). https://doi.org/10.1038/s41598-021-99469-5
Taylor E, Owens E, Lee K, An C, Chen Z (2021) A review of Numerical models for Oil Penetration, Retention, and attenuation on shorelines. J Environ Inf Lett 5:27–38
Tremblay J, Yergeau E (2019) Systematic processing of ribosomal RNA gene amplicon sequencing data. Gigascience 8(12). https://doi.org/10.1093/gigascience/giz146
Tremblay J, Yergeau E, Fortin N, Cobanli S, Elias M, King TL, Lee K, Greer CW (2017) Chemical dispersants enhance the activity of oil- and gas condensate-degrading marine bacteria. ISME J 11(12):2793–2808. https://doi.org/10.1038/ismej.2017.129
United Nations Conference on Trade and Development (2023) Key Statistics and Trends in International Trade 2022. Geneva
US EPA (1996) SW-846 Test Method 3540 C: Soxhlet Extraction
Valencia-Agami SS, Cerqueda-Garcia D, Putzeys S, Uribe-Flores MM, Garcia-Cruz NU, Pech D, Herrera-Silveira J, Aguirre-Macedo ML, Garcia-Maldonado JQ (2019) Changes in the Bacterioplankton Community Structure from Southern Gulf of Mexico during a simulated crude oil spill at Mesocosm Scale. Microorganisms 7(10). https://doi.org/10.3390/microorganisms7100441
Vandermeulen JH, Harper J, Humphrey B (1988) Environmental factors influencing oil penetration and persistence in fine sediment tidal flats. Oil Chem Pollut 4(3):155–177. https://doi.org/10.1016/S0269-8579(88)80018-2
Volkman JK, Revill AT, Holdsworth DG, Fredericks D (2008) Organic matter sources in an enclosed coastal inlet assessed using lipid biomarkers and stable isotopes. Org Geochem 39(6):689–710. https://doi.org/10.1016/j.orggeochem.2008.02.014
Voordouw G, Armstrong SM, Reimer MF, Fouts B, Telang AJ, Shen Y, Gevertz D (1996) Characterization of 16S rRNA genes from oil field microbial communities indicates the presence of a variety of sulfate-reducing, fermentative, and sulfide-oxidizing bacteria. Appl Environ Microbiol 62(5):1623–1629. https://doi.org/10.1128/Aem.62.5.1623-1629.1996
Wang Z, An CJ, Lee K, Owens E, Chen Z, Boufadel M, Taylor E, Feng Q (2021) Factors influencing the fate of oil spilled on shorelines: a review. Environ Chem Lett 19(2):1611–1628. https://doi.org/10.1007/s10311-020-01097-4
Watanabe M, Higashioka Y, Kojima H, Fukui M (2017) Desulfosarcina Widdelii Sp Nov and Desulfosarcina alkanivorans Sp nov., hydrocarbon-degrading sulfate-reducing bacteria isolated from marine sediment and emended description of the genus Desulfosarcina. Int J Syst Evol MicroBiol 67(8):2994–2997. https://doi.org/10.1099/ijsem.0.002062
Wickham H (2009) Ggplot2: elegant graphics for data analysis. Dordrecht; New York, Springer
Williams TM, Kastelein RA, Davis RW, Thomas JA (1988) The effects of Oil Contamination and cleaning on Sea otters (Enhydra-Lutris).1. Thermoregulatory implications based on Pelt Studies. Can J Zool 66(12):2776–2781. https://doi.org/10.1139/z88-405
Williams AK, Bacosa HP, Quigg A (2017) The impact of dissolved inorganic nitrogen and phosphorous on responses of microbial plankton to the Texas City Y oil spill in Galveston Bay, Texas (USA). Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2017.05.033
Wylezich C, Jurgens K (2011) Protist diversity in suboxic and sulfidic waters of the Black Sea. Environ Microbiol 13(11):2939–2956. https://doi.org/10.1111/j.1462-2920.2011.02569.x
Yang Z, Shah K, Laforest S, Hollebone BP, Lambert P, Brown CE, Yang C, Goldthorp M (2018) A study of the 46-year-old Arrow oil spill: persistence of oil residues and variability in oil contamination along Chedabucto Bay, Nova Scotia, Canada. J Clean Prod 198:1459–1473. https://doi.org/10.1016/j.jclepro.2018.07.112
Yang ZY, Shah K, Laforest S, Hollebone BP, Situ J, Crevier C, Lambert P, Brown CE, Yang C (2020) Occurrence and weathering of petroleum hydrocarbons deposited on the shoreline of the North Saskatchewan River from the 2016 Husky oil spill. Environ Pollut 258. https://doi.org/10.1016/j.envpol.2019.113769
Yang ZY, Chen Z, Lee K, Owens E, Boufadel MC, An CJ, Taylor E (2021) Decision support tools for oil spill response (OSR-DSTs): approaches, challenges, and future research perspectives. Mar Pollut Bull 167. https://doi.org/10.1016/j.marpolbul.2021.112313
Zengel S, Bernik BM, Rutherford N, Nixon Z, Michel J (2015) Heavily oiled Salt Marsh following the Deepwater Horizon Oil spill, ecological comparisons of Shoreline cleanup treatments and recovery. PLoS ONE 10(7). https://doi.org/10.1371/journal.pone.0132324
Acknowledgements
We thank Linda Hutchison, Jennifer Mason, Melissa Faulkner and Claire McIntyre for their technical assistance in sample collection and processing. Sediment sampling permission obtained through Nova Scotia Natural Resources with Letter of Authority No. 55B18LA02.
Funding
Funding provided by the Government of Canada through Ocean Protection Plan.
Open access funding provided by Fisheries & Oceans Canada library.
Author information
Authors and Affiliations
Contributions
CRediT author statementHY.P.: Formal analysis, Data curation, Visualization, Writing- Original draft preparation. C.W. G.: Formal analysis, Methodology, Resources, Writing – review & editing. J.W.: Formal analysis, Investigation. M.E.: Formal analysis, Investigation. T. W.: Data curation, Investigation. G. W.: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. S.E.C.: Data curation, Investigation, Methodology, Writing – review & editing. B.R.: Formal analysis, Investigation, Methodology, Writing – review & editing. T.L. K.: Methodology, Resources, Writing – review & editing. A.C.O.: Conceptualization, Supervision, Investigation, Methodology, Formal Analysis, Resources, Writing – review & editing.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Poon, H.Y., Greer, C.W., Wasserscheid, J. et al. Measuring the Fate and Natural Attenuation Potential of a Viscous Marine Fuel on an Artificial Beach Mesocosm. Environ. Process. 11, 27 (2024). https://doi.org/10.1007/s40710-024-00711-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40710-024-00711-9