Abstract
River ecosystem metabolism (REM) is a measure of ecological function which integrates gross primary production (GPP) and ecosystem respiration (ER). Urban rivers often receive effluents from wastewater treatment plants (WWTP) which frequently alter nutrient concentrations and modify temperature regimes of receiving water bodies. To investigate how variations in nutrients and water temperature affect REM, we applied the night-time slope modelling to estimate diurnal REM at sites above and below a wastewater outfall on the River Wandle, UK. Overall, estimated GPP (0–21.2 mgO2·L− 1·d− 1) and ER (5.5–10.1 mgO2·L− 1·d− 1) from our study sites were similar to those of urban impacted rivers in other countries. GPP values were similar between sites, but downstream ER values were significantly higher affected by the WWTP effluent. GPP/ER ratios were < 1 indicating heterotrophic conditions and the river as a carbon source during the study. We found that sites had similar activation energy associated with ER suggesting our work provides a useful reference for estimating temperature corrected metabolic processes for other urban rivers in the region. Furthermore, structural equation modelling revealed that nutrient supply, water temperature and light availability were the main factors driving REM. This research highlights the major environmental factors affecting REM, which helps to understand the response of river metabolism and river regulation of regional carbon cycle to future climate change and provide evidence to inform river restoration and future in-stream management.
Article highlights
Sewage effluent resulted in elevated metabolic rates.
Nutrient supply and water temperature jointly promoted metabolic rates.
Inexpensive loggers to estimate river metabolism are needed for river restoration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
River ecosystem metabolism (REM) is a measure of ecosystem function to regulate dissolved oxygen (DO) dynamic balance and regional carbon cycles, which integrates gross primary production (GPP) and ecosystem respiration (ER) (Fellows et al. 2006; Rodríguez-Castillo et al. 2019). On a global scale, a major contributor to atmospheric CO2 is from the respiration of riverine organic matter (Beaulieu et al. 2013; Raymond et al. 2013; Jauhiainen et al. 2014), while GPP is a process of CO2 fixation and important support for aquatic biodiversity (Strayer and Dudgeon 2010; Ferreira et al. 2020). A wide range of environmental factors affects both GPP and ER (Mulholland et al. 2008; Gernot et al. 2010; Jankowski et al. 2021; Fig. 1a), notably stressors associated with the urban stream syndrome (Meyer et al. 2005; Walsh et al. 2005). For example, increased nutrients from anthropogenic activities, urban impervious surfaces and wastewater treatment plant (WWTP) effluents entering rivers can significantly increase REM in urban rivers by promoting biological activity (Meyer et al. 2005; Dodds 2007; Beaulieu et al. 2013; Fig. 1b). The difference between GPP and ER indicates the carbon budget in the river ecosystem; it is therefore referred to as net ecosystem production (NEP) (Bernhardt et al. 2018). Some studies also use the ratio of GPP to ER to determine whether a river is autotrophic or heterotrophic (Grace and Imberger 2006; Tank et al. 2010; Escoffier et al. 2018). Considering complex interactions among hydrology, climate, chemistry and biology, REM has direct implications for water quality and food webs and is an excellent functional indicator of ecological response for environmental change (Tank et al. 2010; Rodríguez-Castillo et al. 2019; Jankowski et al. 2021), which can be used as a holistic tool for the classification of river ecosystem health (Young et al. 2008; Table 1).
It is widely accepted that polluted rivers with high nutrient and organic matter loads have higher ER which often results in anoxia and hypoxia, a serious water quality problem (Bernhardt et al. 2018). Additionally, WWTP effluents are frequently warmer than ambient stream temperatures which elevate respiratory enzyme activity, then increasing ER rate in urban rivers (Beaulieu et al. 2013; Jauhiainen et al. 2014). For GPP, photosynthesis is strongly influenced by nutrient supply, light availability, and water temperatures (Roberts et al. 2007; Heffernan and Cohen 2010; Finlay 2011). For example, Bernhardt et al. (2018) noted that GPP often peaks in high nutrient concentrations, warming temperatures and lengthening days; Bernot et al. (2010) noted that reduced riparian vegetation due to urban land use allows more intensive light to enter rivers and promotes GPP.
The respiration process of aquatic organisms is associated with activation energy, which is related to temperature (Yvon-Durocher et al. 2010; Hill et al. 2018). Within a certain temperature range, higher water temperature can promote the activity of enzymes related to respiration, thus improving ER rates and higher activation energy is required, especially for urban rivers receiving WWTP effluents with warmer water temperature (Beaulieu et al. 2013; Jauhiainen et al. 2014). The sensitivity of activation energy to temperature provides a new insight into river water quality management. For example, elevated riverine ER and associated activation energy in summer indicate higher DO consumption, and DO supplementary measures need to be implemented (Perkins et al. 2012).
There are two important sources of river DO supply, one is the GPP process, the other is the reaeration process, which refers to the transfer of oxygen in the atmosphere into the water across the air-water interface (Hill et al. 2018). The reaeration rate is related to oxygen deficit and reaeration coefficient k, where k measures the efficiency of the reaeration process (Jin et al. 2012; Omole et al. 2013; Soares et al. 2013) noted that k is proportional to the flow velocity and river channel slope, suggesting that disturbances that speed up water flow can accelerate reaeration (e.g., flooding; Raymond et al. 2012).
Urban rivers often receive WWTP effluents (Preisner 2020; Zhang et al. 2021), but there are few studies on the mechanism of REM responding to effluents. Furthermore, considering that elevated nutrients and water temperature in effluents can be used as indicators for REM prediction under environmental changes, we measured REM of the River Wandle, an urban tributary to the River Thames, which is affected by WWTP effluent. Using the WWTP effluent as a natural experiment, we evaluated how both variations in nutrients and water temperatures affect REM. Many studies have used a variety of methods to measure GPP (e.g., 14 C tracer was used by Son et al. (2005) and Wen et al. (2012)), ER (e.g., incubation in light/dark bottles were used by Wang et al. (2014) and Pei et al. (2018)), and reaeration (e.g., tracer gas was used by Nifong et al. (2019)), but these methods are complex or expensive. In this study, we applied low-cost DO loggers and night-time slope modelling to simultaneously obtain rich river metabolic information from DO concentration changes (see Sect. 2.3). Additionally, we employed structural equation modelling (SEM), which has been widely used in ecology, to provide regression models to investigate the causality of environmental factors affecting REM (Fan et al. 2016). With this knowledge, we then sought to use our River Wandle case study to inform further river restoration and future in-stream management options.
2 Materials and Methods
2.1 Study Sites
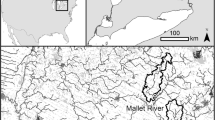
The River Wandle, a tributary of the River Thames in southwest London, has a catchment area of 11 km2 and urban coverage of ~ 50% (Smith and Chadwick 2014; Lavelle et al. 2019). The river is 14 km in length with an average discharge of 1.70 m3/s (River Wandle Catchment Plan 2014). Within the catchment, Beddington WWTP serves 360,000 people and discharges into the Wandle (Schnell et al. 2015). The upstream site is located 0.2 km above WWTP effluent outfall (henceforth U0.2 in the paper); the downstream site, located 3 km downstream of the effluent outfall, is referred to as D3.0 (Fig. 2). U0.2 has abundant riparian vegetation and submerged macrophytes; D3.0 flows through a residential area.
2.2 Water Quality and Light Data
50 mL water samples were collected and water temperature was measured at each study site (Hanna HI98194 multiparameter meter). Water samples were stored in coolbox separately during transport to the laboratory. In the laboratory, water samples were passed through 0.22 μm membrane filters before nutrient measurements. NO3− and PO43− concentrations were measured by ion chromatography (Dionex Aquion). NH4+ concentration was measured using Turner Designs AquaFluor handheld fluorometer (Holmes et al. 1999). Dissolved organic carbon (DOC) concentration was measured by total organic carbon (TOC) analyzer (SHIMADZU TOC-L). The website http://environment.data.gov.uk provides data of photosynthetic active radiation (PAR) for the study reaches, which are used as a measure of light availability.
2.3 Measurement of River Metabolism
During the study period (19 to 22 September 2019), water levels at U0.2 and D3.0 remained at 0.20–0.25 m, 0.35–0.40 m, respectively (https://flood-warning-information.service.gov.uk). The stable flow conditions were suitable for using the open-water, single station method (Grace and Imberger 2006; Demars et al. 2015). We measured DO and water temperature at both sites simultaneously (Supplementary Material (SM); Table SM1). Specifically, the night-time slope modelling (NSM) was applied for GPP, ER and reaeration estimates in this study (Hill et al. 2018; Tables SM2 to SM4), which is based on the principle of no GPP during night-time to simplify the oxygen mass balance equation (Eqs. 1 and 2) to establish the linear regression between DO change rate and ER:
where CDO is the DO concentration (mg/L); CDOs is the DO saturated concentration (mg/L); k is the reaeration coefficient (d− 1); k(CDOs-CDO) is the reaeration rate (mg/(L d)). Due to the limited number of loggers, these measurements were made using two lab calibrated data loggers (MiniDOT logger and Arduino logger (Chan et al. 2020); collection frequency was once per minute for the MiniDOT logger and 9 times per minute for the Arduino logger.
In our study, given the uniform nature of the river channel and river velocities along the study reaches the night-time slope modelling approach was appropriate for estimating k (Grace and Imberger 2006; Hill et al. 2018).
CDOs was derived from Eq. (3):
where T is mean water temperature (K).
The night-time period was selected from the time of sunset to the time when the difference between DO saturation concentration and DO concentration (DO deficit) reached the maximum (Hill et al. 2018). The ER obtained by Eq. (2) requires temperature correction, which is related to Arrhenius activation energy Ea, (i.e., the energy required for respiration of aquatic organisms; Gillooly et al. 2001). Higher Ea indicates that ER is more sensitive to temperature changes (Hill et al. 2018), which depends on resident biodiversity, especially heterotrophic organisms that do not change significantly on seasonal scales (Perkins et al. 2012; González-Pinzón et al. 2016). Therefore, the calculated ER is used to obtain GPP values in Eq. (1).
ER requires temperature correction, and the coefficient \({{\theta }}_{\text{R}}\)was derived from Eq. (4):
where Ea is Arrhenius activation energy (eV) that was derived from Eq. (5):
where kB is Boltzmann constant (8.62 × 10− 5 eV/K); T is mean water temperature (K); T0 is reference water temperature (288.15 K); ER0 is respiration rate at T0.
The temperature corrected ER was derived from Eq. (6):
GPP required temperature correction:
where θP = 1.066, the coefficient.
The temperature-corrected reaeration rate was derived from Eq. (8):
where θa = 1.024, the coefficient.
t in Eqs. (6)-(8) is the water temperature (°C).
GPP, ER and reaeration rate were temperature corrected to obtain the diurnal variation data during the experimental period. Differences in REM between sites were evaluated using Paired t-tests. Correlations between environmental variables were analyzed using the Pearson correlation coefficients. All data analysis was performed using Excel and SPSS 26.0.
2.4 Structural Equation Modelling (SEM)
By evaluating potential interactions among hydrology, climate, chemistry and biology (Fig. 1), we established pathway analysis (Fig. 3) to investigate the influence of the WWTP effluent (i.e., 4 nutrient concentrations in the effluent and elevated water temperatures) on downstream water quality and REM. The arrow points at the affected factor; among them, the nutrient concentrations in the WWTP effluent were derived from Zhang et al. (2021), the PAR data was from http://environment.data.gov.uk, other data, except bioactivity, were derived from our measurements and estimates. Bioactivity (e.g., phytoplankton and aquatic animals) is a variable we did not measure and cannot obtain data directly from the literature or websites (referred to as central latent variable, in the oval) but aquatic organisms are the main body of river metabolic processes. Structural equation modelling (SEM) allows us to include bioactivity in pathways and estimate its correlations with other measured variables (in the box) (Fan et al. 2016). The SEM was performed in SPSS 21.0 AMOS.
3 Results
3.1 Light and Water Flow
The data of light availability were measured by PAR for the River Wandle (Table 2). The water flow data from the South Wimbledon flow monitoring station near to D3.0 (http://nrfa.ceh.ac.uk/data/station/info/39003) provides an opportunity to further study the effect of water flow Q on DO dynamics (e.g., reaeration coefficient k, Table 3).
3.2 DO Profiles
During the experimental period, the sunrise and sunset were around 6:00 am and 6:40 pm, respectively. At both sites, there were strong diurnal patterns in DO with the maximum DO deficit occurring at around 3:40 am at U0.2 and around 6:30 am at D3.0 (Fig. 4). The mean saturated DO concentrations at U0.2 and D3.0 were 10.29 ± 0.16 mg/L and 9.50 ± 0.13 mg/L, respectively.
3.3 Reaeration and Activation Energy Estimates
For both sites, the daily temperature-corrected reaeration rate in the night-time was significantly higher than in the daytime (p = 0.009; Fig. 5a). The highest reaeration rate was found at D3.0 (p = 0.238), where the reaeration coefficient k was significantly lower than at U0.2 (p = 0.012; Table 4; Fig. 6) and presented as a function of discharge with a regression coefficient of 0.897 (Fig. 5b). The diurnal reaeration rate was not correlated with ER (r2 = 0.775, see below for ER estimates), while the night-time reaeration rate was positively correlated with ER (r2 = 0.013). A similar activation energy required for ER was observed between the two sites (U0.2 = 0.97 eV, D3.0 = 0.95 eV).
(a) Diurnal variation of reaeration rate during the study period for the two study sites. Reaeration was estimated using the night-time slope modelling; (b) Reaeration coefficient calculated from the NSM method for D3.0 normalized at 20 °C with θa during the study period. The flow data was derived from the South Wimbledon flow monitoring station near to D3.0 site (http://nrfa.ceh.ac.uk/data/station/info/39003)
3.4 GPP, ER and NEP Estimates
For both sites, GPP and NEP peaked around 1:00–3:00 pm (Fig. 7); GPP ranged from 0 to 23.4 mgO2·L− 1·d− 1 and NEP ranged from − 13.7 to 13.2 mgO2·L− 1·d− 1 (Table 4). No significant difference in GPP was observed between U0.2 and D3.0 (p = 0.196). GPP was positively correlated with ER, water temperature and regional photosynthetic radiation (Table 5). ER ranged from 5.4 to 16.3 mgO2·L− 1·d− 1 (Table 4) and was significantly higher at D3.0 (11.9 ± 2.0 mgO2·L− 1·d− 1) compared with U0.2 (7.1 ± 1.2 mgO2·L− 1·d− 1; p = 0.020). ER was positively correlated with water temperature (r2 = 0.889; p < 0.001). At both sites, negative NEP indicated carbon depletion in the river. For D3.0, the higher ER supported a significant higher rate of carbon depletion (p = 0.024). Further, ratios of daily GPP to ER were < 1 (Table 6), indicating that the river was in a heterotrophic state.
Diurnal variations of GPP, ER and NEP during the study period. Daytime hours were 7:00 am − 6:40 pm, night-time hours were 6:40 pm − 7:00 am. Data measurement for U0.2 and D3.0 started at 1:00 pm and 3:00 pm on September 19 and ended at 12:00 pm and 1:00 pm on September 22, respectively. GPP = gross primary production; ER = ecosystem respiration; NEP = net ecosystem production; U0.2 = upstream of WWTP; D3.0 = downstream of WWTP
Nitrate and phosphate concentrations of the Wandle were positively correlated with ER, and a negative correlation between phosphate and GPP was observed (Table 5), suggesting significant impacts of nutrient supply on aquatic biological activities involved in river metabolism. Further, significant positive correlations between GPP and PAR were recorded at both sites (r2 = 0.921, p < 0.001 for U0.2; r2 = 0.829, p < 0.001 for D3.0; Fig. 8), suggesting that the GPP determined by the NSM method was reasonable.
3.5 SEM Regression Analysis
To investigate the effects of downstream nutrients (at site D3.0) affected by the WWTP effluent and light availability and water temperature on river metabolism, we obtained correlation coefficients from running SEM (Fig. 9; Tables SM5, SM6). The SEM allows to include bioactivity without data into the pathways and estimate the correlations between bioactivity and metabolic processes (i.e., GPP and ER), water quality (i.e., nutrients and water temperature) and light availability through regression calculations.
The four nutrients in the WWTP effluent all contributed significantly to the elevated nutrient concentrations in the WWTP-affected reach (Fig. 8), resulting in differences in the rates of REM-related processes (Table 6). Although no significant effect of DOC on bioactivity was observed, we still noted that other nutrients and water temperature contributed significantly to bioactivity, thus affecting ER, which played a decisive role in reaeration rates, suggesting that the DO consumed by ER will be significantly supplemented by reaeration. For GPP, it was directly controlled by light availability (PAR).
4 Discussion
4.1 Comparison with Other Studies
We compared our results with other rivers also receiving WWTP effluents or in similar conditions (e.g., elevated temperature and nutrients) in different regions. Overall, reported GPP and ER values for these studies were within the range of our results (our results were multiplied by the water depth to make the units consistent, U0.2 = 0.25 m, D3.0 = 0.40 m; Table 8), supporting the role of WWTP effluents and associated conditions in regulating REM, despite different channel characteristics, nutrient loads, aquatic organism composition, and local climate might contribute to the differences in these results.
Levi and McIntyre (2020) estimated the mean ER in the effluent-impacted reaches was 6.9 times higher than in the reference reaches in six urban rivers in Wisconsin, USA, which was consistent with the significantly elevated ER we observed at D3.0. Fuß et al. (2017) reported that the ranges of GPP and ER in agricultural rivers with highly loaded dissolved organic matter (DOM) and nitrates (i.e., like the WWTP effluent) were higher than in forest rivers and covered the range at D3.0 in our study. Similarly, Rodríguez-Castillo et al. (2019) reported that the GPP and ER thresholds in the effluent-impacted reach of the River Cares, Spain, were within the range at D3.0. Arroita et al. (2018) showed that reduced nutrients significantly reduced GPP and ER after the operation of the WWTP on the River Oria, Spain. However, in many studies on river metabolism, including our study, GPP and ER estimates were based on river reaches and did not indicate which part of the river they came from (e.g., algae, aquatic macrophytes), which requires further research.
4.2 DO Profiles across Study Sites
The continuous monitoring of DO concentrations provides information about anoxia and hypoxia, which are vital for evaluating habitat suitability for aquatic organisms (Pollock et al. 2007; Rode et al. 2016; Elosegi et al. 2017). The magnitude of diurnal DO swings at D3.0 was higher than at U0.2 resulting from high rates of photosynthesis and organic matter respiration (Fig. 5), which can cause physiological stress in aquatic organisms (Pollock et al. 2007). Regarding spatial distributions of DO, affected by the WWTP effluent, the mean DO concentration at D3.0 was lower, but the DOC concentration was higher (Table 7), indicating that respiration supported by DOC contributed to DO consumption (Fig. 9). Additionally, high concentrations of DOC were partly attributed to the decomposition of organic matter, which also consumed DO and needs further investigation in these reaches (Ferreira et al. 2020).
The maximum DO deficit at the two study sites appeared before sunrise, around 3:40 am at U0.2 and around 6:30 am at D3.0. On the one hand, the lower night-time water temperature resulted in high concentrations of saturated DO. On the other hand, the DO concentration was lower in the night-time, leading to the maximum DO deficit at night (Hill et al. 2018). The SEM model revealed that the higher water temperature and nutrient supply of the WWTP effluent at D3.0 promoted the consumption of DO by ER (Fig. 9), which significantly increased the reaeration rate to supplement DO (Fig. 5a), resulting in the maximum DO deficit at D3.0 occurring later than at U0.2.
4.3 Factors Driving GPP and ER
WWTP effluent in this study was used as a natural experiment to investigate the effects of nutrient loads and elevated water temperatures on urban river ecosystem metabolism. We found that the WWTP effluent resulted in similar GPP (p = 0.196), but higher ER (p = 0.020) in the WWTP-affected reach (Fig. 7). These results are like other research which have evaluated REM in streams affected by similar stressors to our study (e.g., elevated temperatures and nutrients, Table 8). Further, based on the criteria reported in Table 1, our study sites would be classified as severely to mildly impaired. Although GPP and ER were measured at specific locations, they can represent the metabolic patterns of the studied reaches and be used to assess ecological functions, because of the natural movement and mixing of water in a river (Young et al. 2008). Overall, this suggests that both the WWTP effluent and the urbanized catchment have negative impacts on river ecological function and health.
The Beddington WWTP effluents had elevated nutrients, such as nitrates and phosphates, relative to the ambient River Wandle water quality during our study and this has been reported previously (Millier et al. 2010; South East Rivers Trust 2010). Available nitrogen and phosphorus often act as limiting nutrients for aquatic vegetation (Woodward et al. 2012; Bristow et al. 2017). The SEM model revealed that NO3−, NH4+ and PO43− were key nutrients affecting aquatic biological activity, and these increases can promote the growth and reproduction of aquatic organisms (e.g., phytoplankton), thus accelerating GPP and ER (Tank and Dodds 2003; Noormets 2009; Aristi et al. 2015) referred to the increase in GPP and ER in heterotrophic rivers as the “subsidy” effect of WWTP effluents. As a result of this, a positive correlation between GPP and ER was found (r2 = 0.900, p < 0.001), which has also been widely reported by many studies (e.g., Chen et al. 2009; Hall and Beaulieu 2013; Yan et al. 2018; Hung et al. 2020). However, organic matter associated with WWTP effluents (e.g., higher DOC concentrations at D3.0) can be an important energy input exceeding in-situ GPP (Gücker et al. 2006; Aristi et al. 2015). Additionally, GPP is directly controlled by light availability (Griffiths et al. 2013; Ferreira et al. 2020). Therefore, the increase for ER and GPP in reaches with elevated nutrients are not always consistent (Bernhardt et al. 2018), which explains the inconsistency between the increased significance of GPP and ER at D3.0 in our study. Furthermore, changes of light availability led to the diurnal variation of GPP as we observed that the GPP was the highest between 11:00 am and 1:00 pm, then decreased to 0 in the night-time (Fig. 7). Reach-scale riparian vegetation is also a key factor affecting GPP because abundant riparian vegetation blocks the light transmission into the river, as the case at U0.2, resulting in lower GPP values at U0.2 in our study (Wondzell et al. 2019; Tan et al. 2021; Table 6).
ER was positively correlated with water temperatures (r2 = 0.889, p < 0.001) due to temperature-related enzyme activity involved in respiration (Beaulieu et al. 2013; Jauhiainen et al. 2014). The SEM model also showed that biological activity was significantly affected by water temperature, which in turn affected ER. As a result, due to the warm WWTP effluent (Table 7), ER at D3.0 was significantly higher than at U0.2 (p = 0.020). However, ER will not increase indefinitely with increasing temperature due to factors like substrate limitation and/or protein degradation (Demars et al. 2011; Manning et al. 2018). As a result, with the continuous DO consumption by ER and decomposition, and the potentially limited carbon supply due to the metabolism of carbon compounds, the maximum DO deficit was reached before sunrise and the initiation of photosynthesis (Hill et al. 2018). In some systems, this can produce anoxic or hypoxic periods (Reid et al. 2019), but this was not the case during our study. By studying the decomposition of organic matter in the five tributaries of the Thames River in London, Lavelle (2018) proposed that water temperature can also promote the decomposition process, thus accelerating the DO consumption, which likely caused biological activity (e.g., invertebrates and microbes) at D3.0 with elevated water temperature and nutrients to be stimulated (Woodward et al. 2012), resulting in higher DO consumption, which needs to be further explored in our study river.
River respiration is more sensitive to temperatures and more vulnerable to climate change when higher activation energy (Ea) is required (Perkins et al. 2012); however, similar Ea was observed between both sites (U0.2 = 0.97 eV, D3.0 = 0.95 eV) in our study, which could be applied to other rivers in the region for making temperature corrections to metabolic processes (Hill et al. 2018). However, these two results are higher than the average activation energy (0.65 eV) of river respiration proposed by Yvon-Durocher et al. (2010), which is likely due to the narrow reaches and dense distribution of aquatic vegetation (e.g., phytoplankton) in our study compared to most rivers, resulting in higher ER and required activation energy (Gillooly et al. 2001; Hill et al. 2018).
The balance of GPP and ER plays an important role in the carbon cycle, which are affected by anthropogenic factors, such as WWTP effluents and dissolved organic matter (DOM) inputs (Kaushal et al. 2014). As we found, WWTP effluent significantly increased ER which has implications for CO2 emissions. According to the respiratory quotient of 0.85 (Grace and Imberger 2006), U0.2 and D3.0 on average released 8.228 g/m3 and 13.948 g/m3 of CO2, respectively, during the study period. Strategies to reduce nutrient loads in WWTP effluents via improved WWTP operations are paramount to reducing overall ER in affected systems, thereby lowering the associated CO2 emissions (de la Barrera and Hooda 2016).
4.4 Factors Driving Reaeration
Significantly increased night-time reaeration was mainly due to the lack of oxygen supply from photosynthesis. Also, the lower water temperature at night promoted oxygen solubility (Roberts et al. 2007). There was no correlation between diurnal reaeration rates and ER, but a positive correlation was found between night-time reaeration rates and ER (r2 = 0.013), indicating that the night-time reaeration replenished the DO consumed by ER, and GPP contributed to daytime DO replenishment in addition to reaeration. The higher reaeration rate at D3.0 was closely linked to higher ER caused by the effluent (Fig. 9). As a result, positive values of reaeration rate were observed at night, representing the oxygen entering the river from the atmosphere. In the daytime, the reaeration rate was sometimes negative, representing the saturated DO escaped from the river to the atmosphere. Conversely, U0.2 was estimated by a significantly increased reaeration coefficient k, which we relate mainly to river features and substrate. The U0.2 reach is wide and shallow with natural substrates (e.g., cobbles) when compared to D3.0. These features provide a large frictional contact area for oxygen disturbance to water flow and oxygen-water mixing even under baseflow conditions (Zappa et al. 2007; Alin et al. 2011). Additionally, abundant submerged vegetation at U0.2 affected hydraulic roughness and increased small-scale turbulence leading to increased reaeration coefficients (Nifong et al. 2019). As k is primarily physically driven, in our study, k is a power function of discharge Q (Fig. 5b), which is consistent with the results of Hill et al. (2018).
Both GPP and reaeration play a key role in increasing DO content, and many riverine ecological restoration practices are based on increasing GPP or reaeration to avoid anoxia (Levi and McIntyre 2020). With the River Wandle, the river was re-meandered by constructing berms, riffles and marginal wetlands to encourage aquatic vegetation habitats and enhance GPP (Pretty et al. 2003; Bukaveckas 2007; Lorenz et al. 2013). For other urban rivers, increasing biotic habitat through natural channel design (Reisinger et al. 2019) or providing benthic habitat through rock deployment in the channel (Levi and McIntyre 2020) are also ways to enhance GPP. Regarding the improvement of reaeration, enhanced oxygen exchange by flow sinuosity can be achieved by deploying wood, spoil or stones in the channel, such as the Pool River (River Restoration Centre 2012) and the Hogsmill River (South East Rivers Trust 2013) in London, UK. Improving GPP and reaeration should therefore be goals of further river restoration practice. However, elevated ER can result in a reduced NEP as in the case of D3.0 in our study, indicating a net loss of energy and an increased risk of anoxia. We suggest that REM estimates could therefore be used as benchmarks for both river restoration success and overall river health.
5 Conclusions
Our results showed that significant increased ecosystem respiration (ER) downstream of the WWTP effluent was associated with both elevated water temperatures and nutrients. Increased ER resulted in elevated oxygen deficit and associated reaeration rate elucidated by our structural equation modelling exercise. During stable river discharge, we found that both sites were heterotrophic and no significant difference in gross primary production (GPP) was observed. When compared to other studies, our results support the finding that urban rivers affected by WWTP effluents (or with elevated nutrient loads) have comparable levels of river ecosystem metabolism (REM) with similar ranges of GPP and ER. Future work should focus on evaluating the roles of storm flows and seasonal climate environments in affecting GPP, ER and NEP in urban rivers and to what extend restoration effort can be used to modify REM. For example, improving instream conditions by promoting reaeration and aquatic primary production should be considered and REM measurements could become robust benchmarks for evaluating restoration success and overall river ecosystem health. Finally, we suggest that utilizing WWTP effluents across a wide range of rivers as a natural experiment is a powerful approach for understanding how river ecosystems will be affected by future climate change and eutrophication scenarios.
Data Availability
All data are provided within the paper and Supplementary Material.
References
Alin S, Fátima F, Rasera M, Salimon C, Richey J, Holtgrieve G, Krusche A, Snidvongs A (2011) Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. Journal of Geophysical Research Biogeosciences 116(G1): https://doi.org/10.1029/2010JG001398
Aristi I, von Schiller D, Arroita M, Barceló D, Ponsatí L, García-Galán M, Sabater S, Elosegi A, Acuña V (2015) Mixed effects of effluents from a wastewater treatment plant on river ecosystem metabolism: subsidy or stress? Freshwater Biology 60(7): 1398–1410. https://doi.org/10.1111/fwb12576
Arroita M, Elosegi A, Hall R (2018) Twenty years of daily metabolism show riverine recovery following sewage abatement. Limnology and Oceanography 64: 77–92. https://doi.org/10.1002/Ino11053
Beaulieu J, Arango C, Balz D, Shuster W (2013) Continuous monitoring reveals multiple controls on ecosystem metabolism in a suburban stream. Freshwater Biology 58(5): 918–937. https://doi.org/10.1111/fwb12097
Bernhardt ES, Heffernan JB, Grimm NB, Stanley EH, Harvey JW, Arroita M, Appling AP, Cohen MJ, McDowell WH, Hall RO, Read JS, Roberts BJ, Stets EG, Yackulic CB (2018) The metabolic regimes of flowing waters. Limnol Oceanogr 63(S1):S99–S118. https://doi.org/10.1002/lno.10726
Bernot MJ, Sobota DJ, Hall RO, Mulholland PJ, Ddodds WK, Webster JR, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Gregogy SV, Grimm NB, Hamilton SK, Johnson SL, Mcdowell WH, Meyer JL, Peterson B, Poole GC, Valett HM, Wilson K (2010) Inter-regional comparison of land-use effects on stream metabolism. Freshw Biol 55(9):1874–1890. https://doi.org/10.1111/j.1365-2427.2010.02422.x
Bristow LA, Mohr W, Ahmerkamp S, Kuypers MM (2017) Nutrients that limit growth in the ocean. Curr Biol 27(11):474–478. https://doi.org/10.1016/j.cub.2017.03.030
Bukaveckas PA (2007) Effects of channel restoration on water velocity, transient storage, and nutrient uptake in a channelized stream. Environ Sci Technol 41(5):1570–1576. https://doi.org/10.1021/es061618x
Chan K, Schillereff D, Baas A, Chadwick M, Main B, Mulligan M, O’Shea F, Pearce R, Smith T, Soesbergen A, Tebbs E, Thompson J (2020) Low-cost electronic sensors for environmental research: pitfalls and opportunities. Prog Phys Geogr 45(3):305–338. https://doi.org/10.1177/0309133320956567
Chen CC, Shiah FK, Chiang KP, Gong GC, Kemp WM (2009) Effects of the Changjiang (Yangtze) River discharge on planktonic community respiration in the East China Sea. J Geophys Research: Oceans 114:1–15. https://doi.org/10.1029/2008JC004891
de la Barrera B, Hooda PS (2016) Greenhouse gas emissions of waste management processes and options: a case study. Waste Manag Res 34(7):658–665. https://doi.org/10.1177/0734242X16649680
Demars BL, Thompson J, Manson JR (2015) Stream metabolism and the open diel oxygen method: Principles, practice, and perspectives. Limnol Oceanogr Methods 13(7):356–374. https://doi.org/10.1002/lom3.10030
Demars BO, Manson JR, Ólafsson JS, Gíslason GM, Friberg N (2011) Stream hydraulics and temperature determine the metabolism of geothermal Icelandic streams. Knowl Manage Aquat Ecosyst 402:1–15. https://doi.org/10.1051/kmae/2011046
Dodds WK (2007) Trophic state, eutrophication and nutrient criteria in streams. Trends Ecol Evol 22(12):669–676. https://doi.org/10.1016/j.tree.2007.07.010
Elosegi A, Gessner MO, Young RG (2017) River doctors: Learning from medicine to improve ecosystem management. Sci Total Environ 595:294–302. https://doi.org/10.1016/j.scitotenv.2017.03.188
Escoffier N, Bensoussan N, Vilmin L, Flipo N, Rocher V, David A, Métivier F, Groleau A (2018) Estimating ecosystem metabolism from continuous multi-sensor measurements in the Seine River. Environ Sci Pollut Res Int 25(24):23451–23467. https://doi.org/10.1007/s11356-016-7096-0
Fan Y, Chen J, Shirkey G, John R, Wu SR, Park H, Shao C (2016) Applications of structural equation modelling (SEM) in ecological studies: an updated review. Ecol Processes 5(1):1–12. https://doi.org/10.1186/s13717-016-0063-3
Fellows CS, Clapcott JE, Udy JW, Bunn SE, Harch BD, Smith MJ, Davies PM (2006) Benthic metabolism as an indicator of stream ecosystem health. Hydrobiologia 572(1):71–87. https://doi.org/10.1007/s10750-005-9001-6
Ferreira V, Elosegi A, Tiegs SD, Schiller DV, Young R (2020) Organic matter decomposition and ecosystem metabolism as tools to assess the functional integrity of streams and rivers—A systematic review. Water (Basel) 12(3523):3523. https://doi.org/10.3390/w12123523
Finlay JC (2011) Stream size and human influences on ecosystem production in river networks. Ecosphere 2(8):1–21. https://doi.org/10.1890/ES11-00071.1
Fuß T, Behounek B, Ulseth AJ, Singer GA (2017) Land use controls stream ecosystem metabolism by shifting dissolved organic matter and nutrient regimes. Freshw Biol 62(3):582–599. https://doi.org/10.1111/fwb.12887
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293(5538):2248–2251. https://doi.org/10.1126/science.1061967
González-Pinzón R, Peipoch M, Haggerty R, Martí E, Fleckenstein JH (2016) Nighttime and daytime respiration in a headwater stream. Ecohydrology 1:93–100. https://doi.org/10.1002/eco.1615
Grace M, Imberger S (2006) Stream metabolism: performing & interpreting measurements. Water Studies Centre Monash University, Murray Darling Basin Commission and New South Wales Department of Environment and Climate Change 204: 1–204
Griffiths NA, Tank JL, Royer TV, Roley SS, Rosi-Marshall EJ, Whiles MR, Beaulieu JJ, Johnson LT (2013) Agricultural land use alters the seasonality and magnitude of stream metabolism. Limnol Oceanogr 58(4):1513–1529. https://doi.org/10.4319/lo.2013.58.4.1513
Gücker B, Brauns M, Pusch MT (2006) Effects of wastewater treatment plant discharge on ecosystem structure and function of lowland streams. J North Am Benthological Soc 25(2):313–329. https://doi.org/10.1899/0887-3593(2006)25[313:EOWTPD]2.0.CO;2
Hall RO, Beaulieu JJ (2013) Estimating autotrophic respiration in streams using daily metabolism data. Freshw Sci 32(2):507–516. https://doi.org/10.1899/12-147.1
Heffernan JB, Cohen MJ (2010) Direct and indirect coupling of primary production and diel nitrate dynamics in a subtropical spring-fed river. Limnol Oceanogr 55(2):677–688. https://doi.org/10.4319/lo.2010.55.2.0677
Hill NB, Riha SJ, Walter MT (2018) Temperature dependence of daily respiration and reaeration rates during baseflow conditions in a northeastern U.S. stream. J Hydrology Reg Stud 19:250–264. https://doi.org/10.1016/j.ejrh.2018.09.006
Holmes RM, Aminot A, Kerouel R, Hooker BA, Peterson BJ (1999) A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56(10):1801–1808. https://doi.org/10.1139/cjfas-56-10-1801
Hung JJ, Wang YJ, Tseng CM, Lee Chen YL (2020) Controlling mechanisms and cross linkages of ecosystem metabolism and atmospheric CO2 flux in the northern South China Sea. Deep-Sea Research. Part I. Oceanogr Res Papers 157:103205. https://doi.org/10.1016/j.dsr.2019.103205
Jankowski KJ, Mejia FH, Blaszczak JR, Holtgrieve GW (2021) Aquatic ecosystem metabolism as a tool in environmental management. Wiley Interdisciplinary Reviews: Water 11–27. https://doi.org/10.1002/wat2.1521
Jauhiainen J, Kerojoki O, Silvennoinen H, Limin S, Vasander H (2014) Heterotrophic respiration in drained tropical peat is greatly affected by temperature-a passive ecosystem cooling experiment. Environ Res Lett 9(10):105013. https://doi.org/10.1088/1748-9326/9/10/105013
Jin HS, White DS, Ramsey JB, Kipphut GW (2012) Mixed Tracer Injection Method to Measure Reaeration Coefficients in Small Streams. Water Air Soil Pollut 223(8):5297–5306. https://doi.org/10.1007/s11270-012-1280-8
Kaushal SS, McDowell WH, Wollheim WM (2014) Tracking evolution of urban biogeochemical cycles: past, present, and future. Biogeochemistry 121(1):1–21. https://doi.org/10.1007/s10533-014-0014-y
Lavelle A (2018) Stream ecosystem responses to restoration across urban tributaries of the River Thames, London. Ph.D. thesis. King’s College London, United Kingdom, London
Lavelle A, Bury NR, O’Shea FT, Chadwick MA (2019) Influence of urban river restoration on nitrogen dynamics at the sediment-water interface. PLoS ONE 14(3):e0212690. https://doi.org/10.1371/journal.pone.0212690
Levi P, McIntyre P (2020) Ecosystem responses to channel restoration decline with stream size in urban river networks. Ecological Applications 30(5): p.e02107. https://doi.org/10.1002/eap.2107
Lorenz AW, Stoll S, Sundermann A, Haase P (2013) Do adult and YOY fish benefit from river restoration measures? Ecol Eng 61:174–181. https://doi.org/10.1016/j.ecoleng.2013.09.027
Manning DP, Rosemond AD, Gulis V, Benstead JP, Kominoski JS (2018) Nutrients and temperature additively increase stream microbial respiration. Glob Change Biol 24(1):233–247. https://doi.org/10.1111/gcb.13906
Meyer JL, Paul MJ, Taulbee WK (2005) Stream ecosystem function in urbanizing landscapes. J North Am Benthological Soc 24(3):602–612. https://doi.org/10.1899/04-021.1
Millier HK, Hooda PS, Downward SR (2010) The impact of treated sewage wastewater discharges on the phosphorus levels and hydrology of two second order rivers flowing into the Thames. J Environ Monit 12(6):1307–1314. https://doi.org/10.1039/b923263j
Mulholland PJ, Helon AM, Dodds WK, Findlay SG, Gergogy SV, Grimm NB, Johnson SL, Mcdowell WH, Meyer JL, Valett HM, Webster JR, Arango CP, Poole GC, Beaulieu JJ, Bernor MJ, Burgin AJ, Crenshaw CL, Johnson LT, Niederlehner BR, Dahm CN (2008) Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452(7184):202–205. https://doi.org/10.1038/nature06686
Nifong RL, Taylor JM, Yasarer L (2019) To model or measure: Estimating gas exchange to measure metabolism in shallow, low-gradient stream habitats. Freshw Sci 39(1):70–85. https://doi.org/10.1086/707460
Noormets A (2009) Phenology of ecosystem processes: Applications in global change research. Springer New York. https://doi.org/10.1007/978-1-4419-0026-5
Omole DO, Longe EO, Musa AG (2013) An approach to reaeration coefficient modeling in local surface water quality monitoring. Environ Model Assess 18(1):85–94. https://doi.org/10.1007/s10666-012-9328-0
Pei S, Laws EA, Zhang H, Ye S, Kemper MT, Yuan H, Xu G, Yang S, Liu H, Zhu Y (2018) Study on chemical hydrography, Chlorophyll-a and primary productivity in Liaodong Bay, China. Estuarine. Coastal Shelf Sci 202:103–113. https://doi.org/10.1016/j.ecss.2017.12.012
Perkins DM, Yvon-Durocher G, Demars BO, Reiss J, Pichler DE, Friberg N, Trimmer M, Woodward G (2012) Consistent temperature dependence of respiration across ecosystems contrasting in thermal history. Glob Change Biol 18(4):1300–1311. https://doi.org/10.1111/j.1365-2486.2011.02597.x
Pollock MS, Clarke MJ, Dubé MG (2007) The effects of hypoxia on fishes: From ecological relevance to physiological effects. Environ Reviews 15:1–14. https://doi.org/10.1139/a06-006
Preisner M (2020) Surface water pollution by untreated municipal wastewater discharge due to a sewer failure. Environ Processes 7(3):767–780. https://doi.org/10.1007/s40710-020-00452-5
Pretty JL, Harrison SC, Shepherd DJ, Smith C, Hildrew AG, Hey RD (2003) River rehabilitation and fish populations: Assessing the benefit of instream structures. J Appl Ecol 40(2):251–265. https://doi.org/10.1046/j.1365-2664.2003.00808.x
Raymond AP, Zappa JC, Butman D, Bott LT, Potter J, Mulholland P, Laursen EA, McDowell HW, Newbold D (2012) Scaling the gas transfer velocity and hydraulic geometry in streams and small rivers. Limnology and Oceanography: Fluids and Environments 2(1): 41–53. https://doi.org/10.1215/21573689-1597669
Raymond PA, Hartmann J, Kortelainen P, Durr H, Meybeck M, Ciais P, Guth P, Lauerwald R, Sobek S, McDonald C, Hoover M, Butman D, Striegl R, Mayorga E, Humborg C (2013) Global carbon dioxide emissions from inland waters. Nature 503(7476):355–359. https://doi.org/10.1038/nature12760
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev Camb Philos Soc 94(3):849–873. https://doi.org/10.1111/brv.12480
Reisinger JA, Doody RT, Groffman MP, Kaushal SS, Rosi JE (2019) Seeing the light: urban stream restoration affects stream metabolism and nitrate uptake via changes in canopy cover. Ecol Appl 29:e01941. https://doi.org/10.1002/eap.1941
River Restoration Centre (2012) River Pool Linear Park Enhancement Project, Lewisham
River Wandle Catchment Plan (2014) Available at: http://www.wandle.trust.org
Roberts BJ, Mulholland PJ, Hill WR (2007) Multiple scales of temporal variability in ecosystem metabolism rates: Results from 2 years of continuous monitoring in a forested headwater stream. Ecosystems 10(4):588–606. https://doi.org/10.1007/s10021-007-9059-2
Rode M, Wade AJ, Cohen MJ, Hensley RT, Bowes MJ, Kirchner JW, Jomaa S (2016) Sensors in the stream: The high-frequency wave of the present. Environ Sci Technol 50(19):10297–10307. https://doi.org/10.1021/acs.est.6b02155
Rodríguez-Castillo T, Estévez E, González-Ferreras AM, Barquín J (2019) Estimating ecosystem metabolism to entire river networks. Ecosystems 22(4):892–911. https://doi.org/10.1007/s10021-018-0311-8
Schnell S, Bawa-Allah K, Otitoloju A, Hogstrand C, Miller TH, Barron LP, Bury NR (2015) Environmental monitoring of urban streams using a primary fish gill cell culture system (FIGCS). Ecotoxicol Environ Saf 120:279–285. https://doi.org/10.1016/j.ecoenv.2015.06.012
Smith B, Chadwick M (2014) Litter decomposition in highly urbanized rivers: influence of restoration on ecosystem function. Fundamental & Applied Limnology 185:7–18. https://doi.org/10.1127/fal/2014/0581
Soares PA, Faht G, Pinheiro A, da Silva MR, Zucco E (2013) Determination of reaeration-rate coefficient by modified tracer gas technique. Hydrol Process 27(19):2710–2720. https://doi.org/10.1002/hyp.9371
Son SH, Campbell J, Dowell M, Yoo S, Noh J (2005) Primary production in the Yellow Sea determined by ocean color remote sensing. Mar Ecol Progress Ser (Halstenbek) 303:91–103. https://doi.org/10.3354/meps303091
South East Rivers Trust (2010) Butter Hill Phase 1 River Wandle. Thames Water, Environment Agency, Wild Trout Trust, Carshalton
South East Rivers Trust (2013) Bourne Hall Weirs River Hogsmill. Defra, Ewell
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J North Am Benthological Soc 29(1):344–358. https://doi.org/10.1899/08-171.1
Tan X, Hou E, Zhang Q, Bunn SE (2021) Benthic metabolism responses to environmental attributes at multiple scales and its linkage to algal community structure in streams. Hydrobiologia 848(21):5067–5085. https://doi.org/10.1007/s10750-021-04693-9
Tank JL, Dodds WK (2003) Nutrient limitation of epilithic and epixylic biofilms in ten North American streams: Nutrient limitation on epilithic versus epixylic biofilms. Freshw Biol 48(6):1031–1049. https://doi.org/10.1046/j.1365-2427.2003.01067.x
Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML (2010) A review of allochthonous organic matter dynamics and metabolism in streams. J North Am Benthological Soc 29(1):118–146. https://doi.org/10.1899/08-170.1
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP (2005) The urban stream syndrome: current knowledge and the search for a cure. J North Am Benthological Soc 24(3):706–723. https://doi.org/10.1899/04-028.1
Wang K, Chen JF, Jin HY, Li HL, Gao SQ, Xu J, Lu Y, Huang DJ, Hao Q, Weng HX (2014) Summer nutrient dynamics and biological carbon uptake rate in the Changjiang River plume inferred using a three end-member mixing model. Cont Shelf Res 91:192–200. https://doi.org/10.1016/j.csr.2014.09.013
Wen F, Sun XX, Zheng S, Luo X, Feng QY, Sun S (2012) Spatial and seasonal variations of Chlorophyll-a and primary productivity in spring and summer in the Yellow Sea and East China Sea. Oceanologia et Limnologia Sinica 43(3):438–444. https://doi.org/10.1007/s11783-011-0280-z
Wondzell SM, Diabat M, Haggerty R (2019) What matters most: are future stream temperatures more sensitive to changing air temperatures, discharge, or riparian vegetation? JAWRA J Am Water Resour Association 55(1):116–132. https://doi.org/10.1111/1752-1688.12707
Woodward G, Gessner MO, Dobson M, Elosegi A, Ferreira V, Graca MS, Fleituch T, Lacoursiere JO, Nistorescu M, Pozo J, Risnoveanu G, Schindler M, Giller PS, Vadineanu A, Vought LM, Chauvet E, Gulis V, Hladyz S, Lecerf A, Carlss H (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336(6087):1438–1440. https://doi.org/10.1126/science.1219534
Yan J, Liu J, You X, Shi X, Zhang L (2018) Simulating the gross primary production and ecosystem respiration of estuarine ecosystem in North China with AQUATOX. Ecol Model 373:1–12. https://doi.org/10.1016/j.ecolmodel.2018.02.004
Young RG, Matthaei CD, Townsend CR (2008) Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health. J North Am Benthological Soc 27(3):605–625. https://doi.org/10.1899/07-121.1
Yvon-Durocher JI, Trimmer M, Woodward G, Montoya JM (2010) Warming alters the metabolic balance of ecosystems. Philosophical Trans Biol Sci 365(1549):2117–2126. https://doi.org/10.1098/rstb.2010.0038
Zappa CJ, McGillis WR, Raymond PA, Edson JB, Hintsa EJ, Zemmelink HJ, Dacey JH, Ho DT (2007) Environmental turbulent mixing controls on air-water gas exchange in marine and aquatic systems. Geophys Res Lett 34(10):L10601–n. https://doi.org/10.1029/2006GL028790. a
Zhang M, Francis RA, Chadwick MA (2021) Nutrient dynamics at the sediment-water interface: I fluence of wastewater effluents. Environ Processes 8:1337–1357. https://doi.org/10.1007/s40710-021-00540-0
Acknowledgements
The authors thank Dr. Francis O’Shea, Dr. Kate Olde and Dr. Bruce Main for providing the laboratory and assisting in the use of experimental equipment. This research was partly funded by the Department of Geography, King’s College London.
Funding
This research was supported in part by the Department of Geography, King’s College London.
Author information
Authors and Affiliations
Contributions
M Zhang and M Chadwick designed the research. M Zhang led the field and lab work and wrote the first draft of the manuscript. All authors contributed in editing and completion of the final draft.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
N/A.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, M., Chadwick, M.A. Influences of Elevated Nutrients and Water Temperature from Wastewater Effluent on River Ecosystem Metabolism. Environ. Process. 9, 43 (2022). https://doi.org/10.1007/s40710-022-00597-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40710-022-00597-5