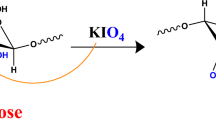
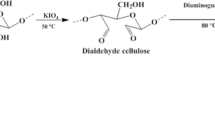
Abstract
Developing cheap and affordable materials for the removal of toxic heavy metal ions such as Pb (II) ions from water is a challenge. In response to this, this work evaluated the synthesis and use of hydroxamic acid modified Adansonia digitata cellulose (ADHX) as a useful resource for the removal of Pb (II) ions from water. ADHX was characterized using Fourier Transform Infrared spectrometry (FTIR), Thermogravimetric analysis (TGA), X-ray Diffraction analysis (XRD), zeta potential, Particle Size Dispersion (PSD), Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS). The sorption of Pb (II) ions on ADHX follows the pseudo-second order model, intra-particle diffusion and Liquid film diffusion kinetic models. The adsorption capacity of ADHX was found to be 18.00 mg g−1, which fitted well for Langmuir, Temkin and Freundlich isotherms. PSD revealed ADHX to be monomodal while Gibb’s free energy change (∆Go) suggests a non-spontaneous process. The negative nature of enthalpy change (∆Ho, −69.774 kJ mol−1) shows that the process is exothermic while entropy change (∆So, −0.214 kJ mol−1) suggests a stable configuration of Pb (II) ions on the surface of ADHX. However, the desorption studies revealed a possible regeneration of ADHX with a desorption capacity of 68.75% in 0.01 M NaNO3 while quantum chemical computation using Density Functional Theory (DFT) revealed the mechanism of sorption to be via ionic interaction. This study revealed ADHX to be a promising resource for removing Pb (II) ions from water.
Graphical abstract











Similar content being viewed by others
References
Abdeen Z (2015) Enhanced recovery of Pb2+ ions from aquatic media by using polyurethane composite as adsorbent. Environ Process 2:189–203
Adewuyi A, Oderinde RA (2019) Chemically modified vermiculite clay: a means to removing emerging contaminant from polluted water system in developing nation. Polym Bull 76:4967–4989
Adewuyi A, Oderinde RA, Fayemi SO (2019) Fatty hydroxamic acid mixture from underutilized Adansonia digitata seed oil: A potential means for scavenging free radicals and combating drug resistant microorganisms. La Riv Ital Delle Sost Grasse XCVI:269–284
Adewuyi A, Oderinde RA, Rao BVSK, Prasad RBN, Anjaneyulu B (2012) Blighia unijugata and Luffa cylindrica seed oils: renewable sources of energy for sustainable development in rural Africa. BioEner Res 5:713–718
Adewuyi A, Pereira FV (2017) Underutilized Luffa cylindrica sponge: a local bio-adsorbent for the removal of Pb (II) pollutant from water system. Beni-Suef Uni J Basic Appl Sci 6:118–126
Åkerholm M, Hinterstoisser B, Salmén L (2004) Characterization of the crystalline structure of cellulose using static and dynamic FT-IR spectroscopy. Carbohydr Res 339:569–578
Anyanwu BO, Ezejiofor AN, Igweze ZN, Orisakwe OE (2018) Heavy metal mixture exposure and effects in developing nations: an update. Toxics 6:65
Ayawei N, Ekubo AT, Wankasi D, Dikio ED (2015) Adsorption of Congo red by Ni/Al-CO3: equilibrium, thermodynamic and kinetic studies. Orien J Chem 31:1307–1318
Babu AN, Mohan GVK, Ravindhranath K (2016) Removal of chromium (VI) from polluted waters using adsorbents derived from Chenopodium album and Eclipta prostrate plant materials. Int J ChemTech Res 9:506–516
Batmaz R, Mohammed N, Zaman M, Minhas G, Berry R, Tam K (2014) Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 21:1655–1665
Bolisetty S, Peydayesh M, Mezzenga R (2019) Sustainable technologies for water purification from heavy metals: review and analysis. Chem Soc Rev 48:463–487
Buvaneswaria N, Kannanb C (2011) Plant toxic and non-toxic nature of organic dyes through adsorption mechanism on cellulose surface. J Hazard Mater 189:294–300
Chauhan GS, Bhatt S, Kaur I, Singha AS, Kaith BS (1999) Modification of natural polymers: graft copolymers of methyl methacrylate onto rayon fibre initiated by ceric ions- a study in the swelling and thermal properties. J Polym Mat 16:245–252
Chen G, Shi L (2017) Removal of cd(II) and Pb(II) ions from natural water using a low-cost synthetic mineral: behavior and mechanisms. RSC Adv 7:43445–43454
Choi S, Jeong Y (2008) The removal of heavy metals in aqueous solution by hydroxyapatite/cellulose composite. Fiber Polym 9:267–270
Ciolacu D, Ciolacu F, Popa VI (2011) Amorphous cellulose-structure and characterization. Cellulose Chem Technol 45:13–21
Coelho GF, Gonçalves AC, Schwantes D, Rodríguez EA, Tarley CRT, Dragunski D, Junior EC (2018) Removal of cd(II), Pb(II) and Cr(III) from water using modified residues of Anacardium occidentale L. Appl Water Sci 8:96
Cruz-Olivares J, Martínez-Barrera G, Pérez-Alonso C, Barrera-Díaz CE, Chaparro-Mercado MC, Ureña-Núñez F (2016) Adsorption of lead ions from aqueous solutions using gamma irradiated minerals. J Chem 2016:1–7
de Andrade JR, Oliveira MF, da Silva MGC, Vieira MGA (2018) Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: A Review. Ind Eng Chem Res 57:3103–3127
Daniel AB, Zahir E, Hussain I, Naz S, Asghar MA (2020) Citric acid modified cellulose: a cost effective adsorbent for the immobilization of Cr (III) ions from the aqueous phase. Ener Sourc part a: Recov Utiliz environ effects 1–13. https://doi.org/10.1080/15567036.2020.1773963
Ekpete OA, Horsfall M Jnr, Spiff AI (2012) Kinetics of chlorophenol adsorption onto commercial and fluted pumpkin activated carbon in aqueous systems. Asian J Nat Appl Sci 1:106–117
Escudero-Oñate C, Poch J, Villaescusa I (2017) Adsorption of cu(II), Ni(II), Pb(II) and cd(II) from ternary mixtures: Modelling competitive breakthrough curves and assessment of sensitivity. Environ Process 4:833–849
Flauzino Neto WP, Silvério HA, Dantas NO, Pasquini D (2013) Extraction and characterization of cellulose nanocrystals from agro-industrial residue – soy hulls. Ind Crop Prod 42:480–488
Fox SC, Li B, Xu D, Edgar KJ (2011) Regioselective esterification and etherification of cellulose: a review. Biomacromolecules 12:1956–1972
Galadima A, Garba ZN (2012) Heavy metals pollution in Nigeria: causes and consequences. Elixir Pollut 45:7917–7922
Galadima A, Muhammad NU, Garba ZN (2010) Spectroscopic investigation of heavy metals in waste water from university students’ halls of residence. Int J Chem 20:239–244
Goldberg S (2005) Equations and models describing adsorption processes in soils. Soil science Society of America, USA. Chemical Processes in Soils. SSSA Book Series
Gusain R, Kumar N, Fosso-Kankeu E, Ray SS (2019) Efficient removal of Pb(II) and cd(II) from industrial mine water by a hierarchical MoS2/SH-MWCNT nanocomposite. ACS Omega 4:13922–13935
Hanaor DAH, Michelazzi M, Leonelli C, Sorrell CC (2012) The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J Eur Cer Soc 32:235–244
He Y, Wu P, Xiao W, Li G, Yi J, He Y, Chen C, Ding P, Duan Y (2019) Efficient removal of Pb(II) from aqueous solution by a novel ion imprinted magnetic biosorbent: adsorption kinetics and mechanisms. PLoS One 14:e0213377
Hokkanen S, Bhatnagar A, Sillanpaa M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173
Imran M, Anwar K, Akram M, Shah GM, Ahmad I, Shah NS, Khan ZUH, Rashid MI, Akhtar MN, Ahmad S, Nawaz M, Schotting RJ (2019) Biosorption of Pb(II) from contaminated water onto Moringa oleifera biomass: kinetics and equilibrium studies. Int J Phytoremed 21:777–789
Joseph L, MoonJun B, Flora JRV, Park CM, Yoon Y (2019) Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere 229:142–159
Kakoi B, Kaluli JW, Ndiba P, Thiong’o G (2016) Removal of lead (II) from aqueous solution using natural materials: a kinetic and equilibrium study. J Sust Res Eng 3:53–62
Koesmawati TA, Moelyo M, Rizqiani A, Tanuwidjaja S (2017) Pre-concentration of Pb, cd, and Ni in river water using back extraction method. IOP Conf. Ser.: earth. Environ Sci 60:012021
Liu X, Xu X, Dong X, Park J (2020) Competitive adsorption of heavy metal ions from aqueous solutions onto activated carbon and agricultural waste materials. Pol J Environ Stud 29:749–761
Lu H, Gui Y, Zheng L, Liu X (2013) Morphological, crystalline, thermal and physicochemical properties of cellulose nanocrystals obtained from sweet potato residue. Food Res Int 50:121–128
Mengistie AA, Rao TS, Rao AVP, Singanan M (2008) Removal of lead (II) ions from aqueous solutions using activated carbon from Militia ferruginea plant leaves. Bull Chem Soc Ethiop 22:349–360
Merganpour AM, Nekuonam G, Tomaj OA, Kor Y, Safari H, Karimi K, Kheirabadi V (2015) Efficiency of lead removal from drinking water using cationic resin purolite. Environ Health Eng Manag 2:41–45
Nacke H, Gonçalves AC Jr, Campagnolo MA, Coelho GF, Schwantes D, dos Santos MG, Briesch DL Jr, Zimmermann J (2016) Adsorption of cu (II) and Zn (II) from water by Jatropha curcas L. as biosorbent. Open Chem 14:103–117
Nagpal UMAMK, Rezaei H (2010) Equilibrium sorption studies for Cu2+ and Pb2+ metal ionson three different biomasses. Curr World Environ 5:243–251
Oke IA, Lukman S, Ismail A, Fehintola EO, Amoko JS (2017) Removal of lead ions from water and wastewaters electrochemically. Water Purif Acad Press:643–691
Onwordi CT, Uche CC, Ameh AE, Petrik LF (2019) Comparative study of the adsorption capacity of lead (II) ions onto bean husk and fish scale from aqueous solution. J Water Reuse Desal 9:249–262
Orosun MM, Tchokossa P, Nwankwo LI, Lawal TO, Bello SA, Ige SO (2016) Assessment of heavy metal pollution in drinking water due to mining and smelting activities in Ajaokuta, Nigeria. Nig J Technol Devel 13:31–39
Overah LC, Odiachi I (2017) Evaluation of Dacryodes edulis (native pear) seed biomass for Pb (II) sorption from aqueous solution. J Appl Sci Environ Manage 21:186–199
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10
Poletto M, Ornaghi HL Jr, Zattera AJ (2014) Native cellulose: structure, characterization and thermal properties. Materials 7:6105–6119
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2009) Adsorption of copper (II), chromium (III), nickel (II) and lead (II) ions from aqueous solutions by meranti sawdust. J Hazard Mater 170:969–977
Ravulapalli S, Kunta R (2017) Defluoridation studies using active carbon derived from the barks of Ficus racemosa plant. J Fluor Chem 193:58–66
Salihi IU, Kutty SRM, Isa MH (2017) Adsorption of lead ions onto activated carbon derived from sugarcane bagasse. IOP Conf. Ser.: mater Sci Eng 201:012034
Sarkar M, Sarkar S (2017) Adsorption of Cr(VI) on iron(III) cellulose nanocomposite bead. Environ Process 4:851–871
Sharma RK (2012) A study in thermal properties of graft copolymers of cellulose and methacrylates. Advan Appl Sci Res 3:3961–3969
Surisetty VR, Kozinski J, Nageswara LR (2013) Biosorption of lead ions from aqueous solution using Ficus benghalensis L. J Eng 2013:1–8
Taşar S, Özer A (2020) Pb(II) ions using peanut (Arachis Hypogaea) shell-based biochar from aqueous media. Pol J Environ Stud 29:293–305
Tenebe IT, Emenike CP, Chukwuka CD (2018) Prevalence of heavy metals and computation of its associated risk in surface water consumed in ado-Odo Ota, south-West Nigeria. Human Ecol Risk Assessment: An Int J 25:882–904
Triantafyllidou S, Edwards M (2012) Lead (Pb) in tap water and in blood: implications for lead exposure in the United States. Crit Rev Environ Sci Technol 42:1297–1352
Wanga G, Zhanga S, Yaoa P, Chena Y, Xua X, Lia T, Gong G (2018) Removal of Pb(II) from aqueous solutions by Phytolacca americana L. biomass as a low cost biosorbent. Arab J Chem 11:99–110
Wasike PW, Nawiri MP, Wanyonyi AA (2019) Levels of heavy metals (Pb, Mn, cu and cd) in water from river Kuywa and the adjacent wells. Environ Ecol Res 7:135–138
World Health Organization (2010) Childhood lead poisoning, printed by the WHO document production services. Switzerland, Geneva
Yu X, Tong S, Ge M, Wu L, Zuo J, Cao C, Song W (2013) Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J Environ Sci 25:933–943
Zhao D, Yu Y, Chen JP (2016) Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA. Water Res 101:564–573
Acknowledgements
Author is grateful to the Department of Chemistry, Federal University of Minas Gerais, Brazil for use of equipment and provision of research space. Author is also grateful to the International Foundation of Science for awarding a research grant (No W/5401-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No Conflict of Interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adewuyi, A. Modification of Adansonia digitata Cellulose by Hydroxamic Acid: a Promising Resource for Removing Pb (II) Ions from Water. Environ. Process. 8, 287–310 (2021). https://doi.org/10.1007/s40710-020-00471-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-020-00471-2