Abstract
Purpose of Review
Sleep disturbance is common in TD. However, our understanding of the pathophysiological mechanisms involved is preliminary. This review summarizes findings from neuroimaging, genetic, and animal studies to elucidate potential underlying mechanisms of sleep disruption in TD.
Recent Findings
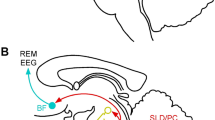
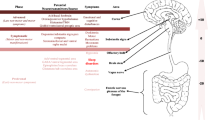
Preliminary neuroimaging research indicates increased activity in the premotor cortex, and decreased activity in the prefrontal cortex is associated with NREM sleep in TD. Striatal dopamine exhibits a circadian rhythm and is influenced by the suprachiasmatic nucleus via multiple molecular mechanisms. Conversely, dopamine receptors regulate circadian function and striatal expression of circadian genes. The association of TD with restless legs syndrome and periodic limb movements indicates shared pathophysiology, including iron deficiency, and variants in the BTDB9 gene. A mutation in the L-Histidine Decarboxylase gene in TD suggests the involvement of the histaminergic system, implicated in arousal, in TD.
Summary
These biological markers have implications for application of novel, targeted interventions, including noninvasive neuromodulation, iron supplementation, histamine receptor antagonists, and circadian-based therapies for tic symptoms and/or sleep and circadian rhythms in TD.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Psychiatric Association. 5th ed. Diagnostic and statistical manual for mental disorders. Washington, DC: 2013.
Tinker SC, Bitsko RH, Danielson ML, Newsome K, Kaminski JW. Estimating the number of people with Tourette syndrome and persistent tic disorder in the United States. Psychiatry Res. 2022;114684.
Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, et al. Gilles de la Tourette syndrome. Nat Rev Dis Primers. 2017;3(1):1–20.
Bloch MH, Leckman JF. Clinical course of Tourette syndrome. J Psychosom Res. 2009;67(6):497–501.
Evans J, Seri S, Cavanna AE. The effects of Gilles de la Tourette syndrome and other chronic tic disorders on quality of life across the lifespan: a systematic review. Eur Child Adolesc Psychiatry. 2016;25(9):939–48. https://doi.org/10.1007/s00787-016-0823-8.
• Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72(4):325–33. This comprehensive study provides clinical and epidemiological data for Tourette’s disorder using a controlled design.
Jiménez-Jiménez FJ, Alonso-Navarro H, Garcia-Martin E, Agundez JA. Sleep disorders in Tourette syndrome. Sleep Med Rev. 2020;53:101335.
Groth C, Debes NM, Rask CU, Lange T, Skov L. Course of Tourette syndrome and comorbidities in a large prospective clinical study. J Am Acad Child Adolesc Psychiatry. 2017;56(4):304–12.
Sambrani T, Jakubovski E, Müller-Vahl KR. New insights into clinical characteristics of Gilles de la Tourette syndrome: findings in 1032 patients from a single German center. Front Neurosci. 2016;10:415. https://doi.org/10.3389/fnins.2016.00415.
Ricketts EJ, Rozenman MR, Choy C, Goldberg HB, Kim JS, Colwell CS, et al. Sleep sufficiency in pediatric and adolescent Tourette’s disorder: national survey of children’s health. J Dev Behav Pediatr. 2018;39(1):72.
Isomura K, Sidorchuk A, Sevilla-Cermeño L, Åkerstedt T, Silverberg-Morse M, Larsson H, et al. Insomnia in Tourette syndrome and chronic tic disorder. Mov Disord. 2022;37(2):392–400.
Ghosh D, Rajan PV, Das D, Datta P, Rothner AD, Erenberg G. Sleep disorders in children with Tourette syndrome. Pediatr Neurol. 2014;51(1):31–5. https://doi.org/10.1016/j.pediatrneurol.2014.03.017.
Ricketts EJ, Montalbano GE, Burgess HJ, McMakin DL, Coles ME, Piacentini J, et al. Sleep and chronotype in adults with persistent tic disorders. J Clin Psychol. 2022;78(7):1516–39. https://doi.org/10.1002/jclp.23323.
Lesperance P, Djerroud N, Diaz Anzaldua A, Rouleau GA, Chouinard S, Richer F, et al. Restless legs in Tourette syndrome. Mov Disord. 2004;19(9):1084–7. https://doi.org/10.1002/mds.20100.
Modafferi S, Stornelli M, Chiarotti F, Cardona F, Bruni O. Sleep, anxiety and psychiatric symptoms in children with Tourette syndrome and tic disorders. Eur J Paediatr Neurol. 2016;20(5):696–703. https://doi.org/10.1016/j.ejpn.2016.05.003.
Voderholzer U, Muller N, Haag C, Riemann D, Straube A. Periodic limb movements during sleep are a frequent finding in patients with Gilles de la Tourette’s syndrome. J Neurol. 1997;244(8):521–6. https://doi.org/10.1007/s004150050136.
Mendelson WB, Caine ED, Goyer P, Ebert M, Gillin JC. Sleep in Gilles de la Tourette syndrome. Biol Psychiatry. 1980;15:339–43.
Cohrs S, Rasch T, Altmeyer S, Kinkelbur J, Kostanecka T, Rothenberger A, et al. Decreased sleep quality and increased sleep related movements in patients with Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 2001;70(2):192–7.
Glaze DG, Frost JD, Jankovic J. Sleep in Gilles de la Tourette’s syndrome: disorder of arousal. Neurology. 1983;33(5):586.
Silvestri R, Raffaele M, De Domenico P, Tisano A, Mento G, Casella C, Tripoli MC, Serra S, Di Perri R. Sleep features in Tourette’s syndrome, neurocanthocytosis and Huntington’s chorea. Neuropysiological Clinics. 1995;25:66–77.
Drake ME Jr, Hietter SA, Bogner JE, Andrews JM. Cassette EEG sleep recordings in Gilles de la Tourette syndrome. Clin Electroencephalogr. 1992;23(3):142–6.
Kirov R, Brand S, Banaschewski T, Rothenberger A. Opposite impact of REM sleep on neurobehavioral functioning in children with common psychiatric disorders compared to typically developing children. Front Psychol. 2017;7:2059.
Kostanecka-Endress T, Banaschewski T, Kinkelbur J, Wüllner I, Lichtblau S, Cohrs S, et al. Disturbed sleep in children with Tourette syndrome: a polysomnographic study. J Psychosom Res. 2003;55(1):23–9.
Tulen JH, Niers T, Vegt R, Cath D, Hengeveld MW. Sleep quality and motor activity during sleep in adults with ADHD or Tourette’s disorder. Sleep-Wake Res Netherlands. 2007;18:119–22.
Pringsheim T, Nosratmirshekarlou E, Doja A, Martino D. Physical activity, sleep and neuropsychiatric symptom severity in children with Tourette syndrome. Eur Child Adolesc Psychiatry. 2021;30(5):711–9.
Allen RP, Singer HS, Brown JE, Salam MM. Sleep disorders in Tourette syndrome: a primary or unrelated problem? Pediatr Neurol. 1992;8(4):275–80.
Hibberd C, Charman T, Bhatoa RS, Tekes S, Hedderly T, Gringras P, et al. Sleep difficulties in children with Tourette syndrome and chronic tic disorders: a systematic review of characteristics and associated factors. Sleep. 2020;43(6):zsz308.
Quezada J, Coffman KA. Current approaches and new developments in the pharmacological management of Tourette syndrome. CNS Drugs. 2018;32(1):33–45.
Bruni O, Angriman M, Melegari MG, Ferri R. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257–71.
Keenan L, Sherlock C, Bramham J, Downes M. Overlapping sleep disturbances in persistent tic disorders and attention-deficit hyperactivity disorder: a systematic review and meta-analysis of polysomnographic findings. Neurosci Biobehav Rev. 2021;126:194–212.
Romano A, Cundari G, Bruni O, Cardona F. Tic disorders and arousal dysfunction: clinical evaluation of 49 children and adolescents. Minerva Pediatr. 2004;56(3):327–34.
Godar SC, Bortolato M. What makes you tic? Translational approaches to study the role of stress and contextual triggers in Tourette syndrome. Neurosci Biobehav Rev. 2017;76(Pt A):123–33. https://doi.org/10.1016/j.neubiorev.2016.10.003.
Kirov R, Becker A, Rothenberger A. Sleep in Tourette syndrome. Curr Dev Disord Rep. 2014;1(4):252–9. https://doi.org/10.1007/s40474-014-0028-0.
Comings DE, Comings BG. A controlled study of Tourette syndrome. I. Attention-deficit disorder, learning disorders, and school problems. Am J Hum Genet. 1987;41(5):701.
Ricketts EJ, Burgess HJ, Montalbano GE, Coles ME, McGuire JF, Thamrin H, et al. Morning light therapy in adults with Tourette’s disorder. J Neurol. 2022;269(1):399–410. https://doi.org/10.1007/s00415-021-10645-z.
Barabas G, Matthews WS, Ferrari M. Somnambulism in children with Tourette syndrome. Dev Med Child Neurol. 1984;26(4):457–60.
Młodzikowska-Albrecht J, Żarowski M, Steinborn B. Sleep disorders in Tourette syndrome in children and adolescents. Neurologia Dziecięca. 2010;11:10–4.
Wand R, Matazow G, Shady G, Furer P, Staley D. Tourette syndrome: associated symptoms and most disabling features. Neurosci Biobehav Rev. 1993;17(3):271–5.
Barabas G, Matthews WS, Ferrari M. Disorders of arousal in Gilles de la Tourette’s syndrome. Neurology. 1984;34(6):815-.
Jankovic J, Rohaidy H. Motor, behavioral and pharmacologic findings in Tourette’s syndrome. Can J Neurol Sci. 1987;14(S3):541–6.
Erenberg G. Sleep disorders in Gilles de la Tourette’s syndrome. 1985.
• Lerner A, Bagic A, Boudreau EA, Hanakawa T, Pagan F, Mari Z, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007;68(23):1979–87. https://doi.org/10.1212/01.wnl.0000264417.18604.12. This is the only study to date to perform imaging during sleep in Tourette’s disorder.
•• Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–8. This imaging study was the first study to directly support the hyperarousal model of insomnia.
Huang Z, Liang P, Jia X, Zhan S, Li N, Ding Y, et al. Abnormal amygdala connectivity in patients with primary insomnia: evidence from resting state fMRI. Eur J Radiol. 2012;81(6):1288–95.
Polyanska L, Critchley HD, Rae CL. Centrality of prefrontal and motor preparation cortices to Tourette syndrome revealed by meta-analysis of task-based neuroimaging studies. NeuroImage: Clinical. 2017;16:257–67.
Müller N, Voderholzer U, Kurtz G, Straube A. Tourette’s syndrome associated with restless legs syndrome and akathisia in a family. Acta Neurol Scand. 1994;89(6):429–32.
Rivière J-B, Xiong L, Levchenko A, St-Onge J, Gaspar C, Dion Y, et al. Association of intronic variants of the BTBD9 gene with Tourette syndrome. Arch Neurol. 2009;66(10):1267–72.
Earley C, Jones BC, Ferré S. Brain-iron deficiency models of restless legs syndrome. Exp Neurol. 2022;114158.
Cortese S, Lecendreux M, Dalla Bernardina B, Mouren MC, Sbarbati A, Konofal E. Attention-deficit/hyperactivity disorder, Tourette’s syndrome, and restless legs syndrome: the iron hypothesis. Med Hypotheses. 2008;70(6):1128–32.
• Harduf EV, Matzner A, Belelovsky K, Bar-Gad I. Dissociation of tic generation from tic expression during the sleep-wake cycle. Iscience. 2021;24(4):102380. In a mouse model of Tourette’s disorder, study findings showed that during sleep tic expression was blocked by striatal neural activity, suggesting a potential mechanism by which tics may be attenuated during sleep in humans.
Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4(2):121–30.
•• Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O'Roak BJ, Mason CE, et al. L-histidine decarboxylase and Tourette’s syndrome. N Engl J Med. 2010;362(20):1901–8. https://doi.org/10.1056/NEJMoa0907006. This study identified a rare mutation in the L-histidine decarboxylase gene present in all affected family members with Tourette’s disorder across two generations, suggesting involvement of the histaminergic system in Tourette’s disorder.
Baldan LC, Williams KA, Gallezot J-D, Pogorelov V, Rapanelli M, Crowley M, et al. Histidine decarboxylase deficiency causes Tourette syndrome: parallel findings in humans and mice. Neuron. 2014;81(1):77–90.
Hartmann A, Worbe Y, Arnulf I. Increasing histamine neurotransmission in Gilles de la Tourette syndrome. J Neurol. 2012;259(2):375–6.
Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36(3):177–85.
Mendoza J, Challet E. Circadian insights into dopamine mechanisms. Neuroscience. 2014;282:230–42. https://doi.org/10.1016/j.neuroscience.2014.07.081.
Mesgar S, Jameie SB, Aliaghaei A, Parvardeh S, Torabi A, Haghparast A. Dopamine D1 receptor-mediated regulation of Per1, Per2, CLOCK, and BMAL1 expression in the suprachiasmatic nucleus in adult male rats. J Mol Neurosci. 2022;72(3):618–25.
Imbesi M, Yildiz S, Arslan AD, Sharma R, Manev H, Uz T. Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience. 2009;158(2):537–44.
Ganos C. Tics and Tourette’s: update on pathophysiology and tic control. Curr Opin Neurol. 2016;29(4):513–8. https://doi.org/10.1097/wco.0000000000000356.
Ganos C, Roessner V, Munchau A. The functional anatomy of Gilles de la Tourette syndrome. Neurosci Biobehav Rev. 2013;37(6):1050–62. https://doi.org/10.1016/j.neubiorev.2012.11.004.
Braun AR, Stoetter B, Randolph C, Hsiao JK, Vladar K, Gernert J, et al. The functional neuroanatomy of Tourette’s syndrome: an FDG-PET study. I. Regional changes in cerebral glucose metabolism differentiating patients and controls. Neuropsychopharmacology. 1993;9(4):277–91. https://doi.org/10.1038/npp.1993.64.
Eidelberg D, Moeller JR, Antonini A, Kazumata K, Dhawan V, Budman C, et al. The metabolic anatomy of Tourette’s syndrome. Neurology. 1997;48(4):927–34. https://doi.org/10.1212/wnl.48.4.927.
Pourfar M, Feigin A, Tang CC, Carbon-Correll M, Bussa M, Budman C, et al. Abnormal metabolic brain networks in Tourette syndrome. Neurology. 2011;76(11):944–52. https://doi.org/10.1212/WNL.0b013e3182104106.
Ganos C, Kahl U, Brandt V, Schunke O, Bäumer T, Thomalla G, et al. The neural correlates of tic inhibition in Gilles de la Tourette syndrome. Neuropsychologia. 2014;65:297–301.
Neuner I, Werner CJ, Arrubla J, Stöcker T, Ehlen C, Wegener HP, et al. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front Hum Neurosci. 2014;8:362.
Jackson SR, Loayza J, Crighton M, Sigurdsson HP, Dyke K, Jackson GM. The role of the insula in the generation of motor tics and the experience of the premonitory urge-to-tic in Tourette syndrome. Cortex. 2020;126:119–33.
Nofzinger EA, Mintun MA, Price J, Meltzer CC, Townsend D, Buysse DJ, et al. A method for the assessment of the functional neuroanatomy of human sleep using FDG PET. Brain Res Protoc. 1998;2(3):191–8.
Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335–52. https://doi.org/10.1046/j.1365-2869.2000.00225.x.
Wu JC, Gillin JC, Buchsbaum MS, Chen P, Keator DB, Khosla WuN, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31(12):2783–92. https://doi.org/10.1038/sj.npp.1301166.
Taylor BJ, Hasler BP. Chronotype and mental health: recent advances. Curr Psychiatry Rep. 2018;20(8):59. https://doi.org/10.1007/s11920-018-0925-8.
Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018;19(8):453–69.
Buijs RM, Tinoco ECS, Alvarado GH, Escobar C. The circadian system: from clocks to physiology. Handb Clin Neurol. 2021;179:233–47.
Miyazaki S, Tahara Y, Colwell CS, Block GD, Nakamura W, Nakamura TJ. Chronic methamphetamine uncovers a circadian rhythm in multiple-unit neural activity in the dorsal striatum which is independent of the suprachiasmatic nucleus. Neurobiol Sleep Circadian Rhythms. 2021;11:100070.
Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18(9):678–83.
Ferris MJ, España RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci. 2014;111(26):E2751–9.
Natsubori A, Honma Ki, Honma S. Dual regulation of clock gene Per2 expression in discrete brain areas by the circadian pacemaker and methamphetamine-induced oscillator in rats. Eur J Neurosci. 2014;39(2):229–40.
Fifel K, Meijer JH, Deboer T. Circadian and homeostatic modulation of multi-unit activity in midbrain dopaminergic structures. Sci Rep. 2018;8(1):1–14.
Pradel K, Drwięga G, Chrobok L, Błasiak T. Racing and pacing in the reward system: a multi-clock circadian control over dopaminergic signalling. Front Physiol. 2022;1288.
Grippo RM, Purohit AM, Zhang Q, Zweifel LS, Güler AD. Direct midbrain dopamine input to the suprachiasmatic nucleus accelerates circadian entrainment. Curr Biol. 2017;27(16):2465-75.e3.
Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK: BMAL1. Proceedings of the National Academy of Sciences. 2006;103(16):6386–91.
• Brami-Cherrier K, Lewis RG, Cervantes M, Liu Y, Tognini P, Baldi P, et al. Cocaine-mediated circadian reprogramming in the striatum through dopamine D2R and PPARγ activation. Nature communications. 2020;11(1):1–14. This study showed that dopamine stimulation reprograms the molecular clock found in the striatum. This stuggests the possibility of a similar reprogramming in individuals with Tourette's disorder.
Hood S, Cassidy P, Cossette M-P, Weigl Y, Verwey M, Robinson B, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30(42):14046–58.
Badenoch J, Searle T, Watson I, Cavanna AE. Sensory symptoms in body-focused repetitive behaviors, restless legs syndrome, and Tourette syndrome: an overlap? Neurosci Biobehav Rev. 2020;119:320–32. https://doi.org/10.1016/j.neubiorev.2020.10.008.
Lipinski JF, Sallee FR, Jackson C, Sethuraman G. Dopamine agonist treatment of Tourette disorder in children: results of an open-label trial of pergolide. Mov Disord. 1997;12(3):402–7. https://doi.org/10.1002/mds.870120320.
Walters AS, LeBrocq C, Passi V, Patel S, Hanna PA, Cohen B, et al. A preliminary look at the percentage of patients with restless legs syndrome who also have Parkinson disease, essential tremor or Tourette syndrome in a single practice. J Sleep Res. 2003;12(4):343–5. https://doi.org/10.1046/j.0962-1105.2003.00368.x.
Cortese S, Lecendreux M, Bernardina BD, Mouren MC, Sbarbati A, Konofal E. Attention-deficit/hyperactivity disorder, Tourette’s syndrome, and restless legs syndrome: the iron hypothesis. Med Hypotheses. 2008;70(6):1128–32. https://doi.org/10.1016/j.mehy.2007.10.013.
Avni T, Reich S, Lev N, Gafter-Gvili A. Iron supplementation for restless legs syndrome–a systematic review and meta-analysis. Eur J Intern Med. 2019;63:34–41.
Silber MH, Buchfuhrer MJ, Earley CJ, Koo BB, Manconi M, Winkelman JW, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc: Elsevier; 2021;1921–37.
Ghosh D, Burkman E. Relationship of serum ferritin level and tic severity in children with Tourette syndrome. Childs Nerv Syst. 2017;33(8):1373–8.
Rapanelli M, Pittenger C. Histamine and histamine receptors in Tourette syndrome and other neuropsychiatric conditions. Neuropharmacology. 2016;106:85–90. https://doi.org/10.1016/j.neuropharm.2015.08.019.
Shan L, Dauvilliers Y, Siegel JM. Interactions of the histamine and hypocretin systems in CNS disorders. Nat Rev Neurol. 2015;11(7):401–13. https://doi.org/10.1038/nrneurol.2015.99.
Woods DW, Piacentini J, Chang S, Deckersbach T, Ginsburg G, Peterson A, et al. Managing Tourette syndrome: a behavioral intervention for children and adults therapist guide. Oxford University Press; 2008.
Pringsheim T, Okun MS, Müller-Vahl K, Martino D, Jankovic J, Cavanna AE, et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019;92(19):896–906.
Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. 2010;303(19):1929–37.
Wilhelm S, Peterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, et al. Randomized trial of behavior therapy for adults with Tourette syndrome. Arch Gen Psychiatry. 2012;69(8):795–803. https://doi.org/10.1001/archgenpsychiatry.2011.1528.
Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders–efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev. 2013;37(6):1162–71.
Tourette Association of America Impact Survey Working Group. 2022 Impact Survey: Assessing the impact of Tourette syndrome and tic disorders on individuals and families. 2022.
Tourette Association of America Impact Survey Working Group. 2018 Impact Survey: assessing the impact of Tourette syndrome and tic disorders on individuals and families. 2018.
Himle MB, Capriotti MR, Hayes LP, Ramanujam K, Scahill L, Sukhodolsky DG, et al. Variables associated with tic exacerbation in children with chronic tic disorders. Behav Modif. 2014;38(2):163–83.
Blake M, Schwartz O, Waloszek JM, Raniti M, Simmons JG, Murray G, et al. The SENSE study: treatment mechanisms of a cognitive behavioral and mindfulness-based group sleep improvement intervention for at-risk adolescents. Sleep. 2017;40(6).
Hiscock H, Sciberras E, Mensah F, Gerner B, Efron D, Khano S, et al. Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: randomised controlled trial. BMJ. 2015;350.
Murawski B, Wade L, Plotnikoff RC, Lubans DR, Duncan MJ. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Med Rev. 2018;40:160–9.
Reese HE, Vallejo Z, Rasmussen J, Crowe K, Rosenfield E, Wilhelm S. Mindfulness-based stress reduction for Tourette syndrome and chronic tic disorder: a pilot study. J Psychosom Res. 2015;78(3):293–8.
Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255–62.
Cheung JMY, Ji XW, Morin CM. Cognitive behavioral therapies for insomnia and hypnotic medications: considerations and controversies. Sleep Med Clin. 2019;14(2):253–65. https://doi.org/10.1016/j.jsmc.2019.01.006.
Ueda K, Black KJ. A comprehensive review of tic disorders in children. J Clin Med. 2021;10(11):2479.
Li J, Somers VK, Xu H, Lopez-Jimenez F, Covassin N. Trends in use of melatonin supplements among US adults, 1999–2018. JAMA. 2022;327(5):483–5.
Panjwani AA, Cowan AE, Jun S, Bailey RL. Trends in nutrient-and non-nutrient–containing dietary supplement use among US children from 1999 to 2016. J Pediatr. 2021;231(131–40):e2.
Smith BL, Ludlow AK. Patterns of nutritional supplement use in children with Tourette syndrome. J Diet Suppl. 2021;1–16. https://doi.org/10.1080/19390211.2021.1958120.
Salanitro M, Wrigley T, Ghabra H, de Haan E, Hill CM, Solmi M, et al. Efficacy on sleep parameters and tolerability of melatonin in individuals with sleep or mental disorders: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;139:104723. https://doi.org/10.1016/j.neubiorev.2022.104723.
Rzepka-Migut B, Paprocka J. Efficacy and safety of melatonin treatment in children with autism spectrum disorder and attention-deficit/hyperactivity disorder—a review of the literature. Brain Sci. 2020;10(4):219.
Uz T, Arslan AD, Kurtuncu M, Imbesi M, Akhisaroglu M, Dwivedi Y, et al. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res. 2005;136(1–2):45–53. https://doi.org/10.1016/j.molbrainres.2005.01.002.
• Roth T, Mayleben D, Feldman N, Lankford A, Grant T, Nofzinger E. A novel forehead temperature-regulating device for insomnia: a randomized clinical trial. Sleep. 2018;41(5). https://doi.org/10.1093/sleep/zsy045. This clinical trial showed that frontal cerebral thermal therapy was associated with improvement in sleep onset latency in insomnia, aligning with effects found for prescribed hypnotic medications.
Nofzinger E, Miewald J, Price J, Buysse DJ. Frontal cerebral hypothermia: a new approach to the treatment of insomnia. Sleep. 2009;32:A287–8.
Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406(6792):147–50. https://doi.org/10.1038/35018000.
Oroz R, Kung S, Croarkin PE, Cheung J. Transcranial magnetic stimulation therapeutic applications on sleep and insomnia: a review. Sleep Sci Pract. 2021;5(1):1–10.
Grados M, Huselid R, Duque-Serrano L. Transcranial magnetic stimulation in Tourette syndrome: a historical perspective, its current use and the influence of comorbidities in treatment response. Brain Sci. 2018;8(7). https://doi.org/10.3390/brainsci8070129.
Sun N, He Y, Wang Z, Zou W, Liu X. The effect of repetitive transcranial magnetic stimulation for insomnia: a systematic review and meta-analysis. Sleep Med. 2021;77:226–37. https://doi.org/10.1016/j.sleep.2020.05.020.
Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701–15. https://doi.org/10.1016/j.jsmc.2010.08.001.
Burgess HJ, Emens JS. Circadian-based therapies for circadian rhythm sleep-wake disorders. Curr Sleep Med Rep. 2016;2(3):158–65.
Faulkner SM, Bee PE, Meyer N, Dijk D-J, Drake RJ. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: a systematic review and meta-analysis. Sleep Med Rev. 2019;46:108–23.
Blume C, Garbazza C, Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie (Berl). 2019;23(3):147–56. https://doi.org/10.1007/s11818-019-00215-x.
Brinkhuijsen M, Koenegracht F, Meesters Y. Symptoms of seasonal affective disorder and of obsessive-compulsive disorder reduced by light therapy. J Affect Disord. 2003;74(3):307–8.
Korman M, Palm D, Uzoni A, Faltraco F, Tucha O, Thome J, et al. ADHD 24/7: Circadian clock genes, chronotherapy and sleep/wake cycle insufficiencies in ADHD. World J Biol Psychiatr. 2020;21(3):156–71.
Coles ME, Strauss GP. Shedding light on tics. Psychiatry Res. 2015;225(3):743. https://doi.org/10.1016/j.psychres.2014.12.024.
Niederhofter H. Bright light therapy may be a therapeutic option for Tourette’s syndrome. Acta Neuropsychologica. 2009;7(4):283–5.
Funding
Research reported in this publication was supported in part by funding from the National Institute of Mental Health (NIMH) K23MH113884, Tourette Association of America, and Brain and Behavior Research Foundation grants to Dr. Ricketts. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position these funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
Dr. Ricketts reports grants from the Tourette Association of America, National Institute of Mental Health, and Brain and Behavior Research Foundation, relevant to the submitted work. She also reports grants and personal fees from the Tourette Association of America, grants from National Heart, Lung, and Blood Institute: Programs to Increase Diversity among Individuals in Health-Related Research; personal fees from Centers for Disease Control and Prevention, personal fees from Springer Nature, and service on the Tourette Association of America Diversity Committee, outside the submitted work. Ms. Swisher, Dr. Greene, and Dr. Silverman have nothing to disclose. Dr. Nofzinger reports being the Founder, Inventor, and Chief Medical Office of Ebb Therapeutics (formerly known as Cerêve, Inc.), a company that manufactured devices to administer frontal cerebral thermal therapy for insomnia, relevant to the submitted work and outside the submitted work. In addition, Dr. Nofzinger has multiple patents related to the frontal cerebral thermal therapy device, with royalties received on University of Pittsburgh license to Ebb Therapeutics. Dr. Colwell reports unpaid consulting for RealSleep™, outside the submitted work.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Sleep and Neurological Conditions
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ricketts, E.J., Swisher, V., Greene, D.J. et al. Sleep Disturbance in Tourette’s Disorder: Potential Underlying Mechanisms. Curr Sleep Medicine Rep 9, 10–22 (2023). https://doi.org/10.1007/s40675-022-00242-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-022-00242-5