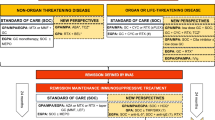
Abstract
Purpose of review
This review summarises the evidence for the use of therapeutic plasma exchange (TPE) in anti-neutrophil cytoplasm antibody (ANCA)–associated vasculitis. TPE is an extra-corporeal treatment which efficiently removes IgG and other pathogenic small molecules from the blood. There are several mechanistic reasons why this should be of benefit in AAV including the well-described pathogenicity of ANCA.
Recent findings
The recently published PEXIVAS trial is the largest study of TPE in AAV to date. It did not show a benefit for adjunctive TPE on a primary end point of ESRD or death. There was no difference in serious adverse events between those treated with TPE and those treated with immunosuppressive drugs alone.
Conclusions
Based on the results of PEXIVAS, most patients with AAV should not be treated with adjunctive TPE. However, there are subgroups of patients with AAV for whom TPE may still be of benefit, including those with double positivity for anti-GBM antibodies and ANCA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Therapeutic plasma exchange (TPE) is an extra-corporeal treatment which removes plasma from whole blood allowing it to be replaced with donor plasma or human albumin solution (HAS). TPE is effective at removing large molecules such as immunoglobulins and as such is an essential component of treating many antibody-mediated conditions including neurological, haematological and renal diseases [1]. The use of TPE in patients with renal disease was first described in 1975 in a patient with Goodpasture’s syndrome who recovered from both renal failure and pulmonary haemorrhage [2]. Subsequently, a series of nine patients with crescentic glomerulonephritis (GN) not due to anti-GBM disease was described in 1977, five of whom rapidly recovered renal function [3]. This led to the use of TPE in severe, rapidly progressive glomerulonephritis (RPGN) in the absence of anti-GBM antibodies, of which many cases were likely due to ANCA-associated vasculitis (AAV).
There are two available techniques for separating plasma from the cellular components of the blood, centrifugal and membrane-based apheresis [4, 5]. In centrifugal apheresis, cells are separated from plasma based on density; the cells are then mixed with replacement fluid and infused back to the patient. Membrane-based apheresis uses filtration based on a molecular weight cut off. The principle difference between these techniques is that with centrifugal apheresis there is no upper limit for the size of molecule removed from the plasma whereas membrane-based apheresis has an upper limit of around 3 million daltons. This means that in membrane-based apheresis, IgM is cleared less well than IgG, and it also may not adequately remove large cryoglobulins or immune complexes. Which method of apheresis is used is largely down to the experience of individual units. There are regional differences in choice of TPE method with centrifugal apheresis the most commonly used technique in the USA and membrane apheresis most commonly used in Japan and Korea [6]. Method of TPE is generally not specified in clinical trial protocols.
Both centrifugal and membrane TPE require the replacement of the patient’s blood volume with colloid solution to prevent circulatory collapse. This dilution of the patient’s plasma leads to an exponential decrease in the amount of immunoglobulin or other factors removed with each plasma volume exchanged. Therefore, the volume used for each session of TPE is usually limited to one plasma volume as the benefit of removing additional small amounts of antibody with increasing volumes is likely not outweighed by the risks to the patient of a longer exchange [1, 7]. Another consideration for the removal of IgG from the circulation is that 30–45% of total IgG is found in the extravascular space meaning that following each exchange there will be redistribution of IgG to the intravascular compartment. As such, multiple sessions of TPE are required to successfully clear IgG from the patient’s circulation even in the absence of ongoing antibody production [7].
Plasma exchange as a therapeutic strategy in AAV
There are several mechanisms by which TPE may be an effective treatment for AAV. ANCA have been shown in several clinical and pre-clinical studies to be directly pathogenic and so removing the antibodies should be of benefit [8]. In vitro studies have shown the ability of ANCA to bind to and activate both neutrophils and monocytes, leading to cell degranulation and release of reactive oxygen species and adhesion of cells to endothelial cells in culture [9,10,11]. There is also much evidence from in vivo studies; passive transfer of anti-MPO antibody has shown it to be pathogenic, causing glomerulonephritis, independent of T cell responses [8, 12]. However, the relationship between ANCA and active vasculitis in human disease is complex and many studies have shown that ANCA are not always pathogenic. ANCA can persist in remission and natural antibodies have been identified in healthy individuals [13, 14]. A proportion of patients have the clinical syndrome of AAV but without detectable ANCA, particularly those with limited disease or without renal involvement [15, 16]. Titre of ANCA has been shown to correlate with disease severity and risk of relapse, particularly in those with renal disease although this has not been confirmed in all studies [17, 18].
In addition to removing potentially pathogenic antibodies, TPE may have additional benefits by removing other pathogenic small molecules in the plasma such as adhesion molecules, cytokines, or complement components [19]. One study, in patients with AAV undergoing TPE identified lower serum levels of adhesion molecules post TPE but no effect on pro-inflammatory cytokines despite their presence in the discarded plasma [20]. It is possible that this is in part due to increased cytokine production due to contact of the cells with the apheresis device. TPE is also thought to have an immunomodulatory effect. No studies have been carried out in patients with AAV; however, studies in lupus have theorised that TPE can lead to an increase in regulatory T cells and a skew in T cell balance to a TH1 phenotype [21, 22].
Complications of plasma exchange
In addition to the removal of pathogenic antibodies and other soluble factors, TPE will also remove normal plasma components such as clotting factors which can result in adverse events. Coagulopathy and bleeding can result, particularly if human albumin solution (HAS) is used as replacement fluid. In patients with high bleeding risk or active bleeding such as lung haemorrhage, fresh frozen plasma (FFP) should be used as the replacement solution to minimise this risk. Despite the potential for coagulopathy, the incidence of bleeding complications is low and death is extremely rare at 0.05% in one study [23, 24]. The most frequent complications of TPE are symptomatic hypovolaemia, hypocalcaemia and anaphylactoid reactions to FFP.
If testing for other autoantibodies or antibodies to infectious agents is to be carried out, samples should be collected prior to TPE, as these antibodies will be removed by TPE leading to false negative results. Drug dosing also needs to be considered as some drugs, particularly those which are protein bound, will be removed by TPE. Rituximab is one example of this with particular relevance in AAV; it is an IgG antibody and so is efficiently removed by TPE. Studies have shown that peak and trough rituximab levels are lower in patients receiving TPE compared with those treated with rituximab alone [25, 26]. Despite this, one study has shown peripheral B cell depletion to be equivalent between those receiving TPE and those not, although tissue B cell depletion was not measured [25].
Historical use of plasma exchange in AAV and the rationale for PEXIVAS
Following the report by Lockwood et al. in 1977, there were several small case series and non-randomised trials reporting of the use of TPE in RPGN without evidence of anti-GBM antibodies, with varying results [3, 27, 28]. In 1991, Pusey et al. reported a randomised controlled trial of 48 cases with renal vasculitis treated with TPE in addition to cyclophosphamide and steroids, or with drug treatment only [29]. There was no difference in outcome in patients with non-dialysis requiring disease but those who were with dialysis requiring at presentation were more likely to recover renal function if treated with TPE [29] This trial began in 1978 and spanned the discovery of ANCA in 1982; the majority of cases had clinical disease compatible with a diagnosis of AAV despite the lack of ANCA testing [29, 30]. In a subgroup who were tested for ANCA, 13 of 16 patients with ANCA positivity and dialysis requiring disease, treatment with TPE resulted in short-term improvement in renal function.
The methylprednisolone vs plasma exchange in vasculitis (MEPEX) trial was published in 2007. It included 137 patients with severe renal disease (creatinine > 500 μmol/L or dialysis requiring at presentation) who were randomised to receive either seven plasma exchanges or IV methylprednisolone in addition to oral cyclophosphamide and prednisolone. There was improved renal survival in the TPE group at 3 months and decreased requirement for dialysis at 12 months [31]. There was no impact on mortality at 3 months or 1 year although overall mortality was high, 26% at 3 months, largely due to infectious complications. A retrospective analysis of the 69 patients in the trial who were dialysis dependent at trial entry found that adjunctive TPE was a predictor of dialysis independence at 12 months on multivariate analysis [32]. Long-term follow-up of the trial (median follow-up of 3.95 years) showed no difference in a primary composite endpoint of death or end-stage renal disease (ESRD). The benefit of TPE on preventing ESRD seen at 12 months was preserved; however, there was a non-significant increase in the risk of death, largely related to increased infectious complications [33]. Again mortality was high, with only 50% of patients surviving beyond 5 years.
Many case series and trials of TPE in AAV have focussed on patients with severe renal impairment (creatinine > 500 μmol/L or dialysis requiring at presentation). However, there is one RCT of TPE in 32 patients with granulomatosis with polyangiitis (GPA) with less severe renal impairment (median creatinine 240 μmol/L) showing improved renal survival at 1, 3 and 12 months and 5 years [34]. Multivariate analysis of this study identified patients with a starting creatinine > 250 μmol/L were those most likely to benefit from the addition of TPE.
Historically, there are limited numbers of studies which addressed the benefit of TPE for those with AAV and lung haemorrhage. Several case series have reported good outcomes in TPE in patients with pulmonary haemorrhage; however, it is not clear from retrospective cohort studies that adjunctive TPE is of benefit [35,36,37]. The MEPEX trial excluded patients with severe lung haemorrhage and long-term follow-up of the trial demonstrated low mortality from pulmonary haemorrhage in both groups [31, 33].
A Cochrane review (December 2019) and a meta-analysis (April 2011) have both reviewed the evidence for TPE in AAV (Table 1) [38, 39]. The Cochrane review concluded that there may be evidence for TPE preventing ESRD at 3 and 12 months but there was no effect on mortality, duration of remission or total number of adverse events [39]. Similarly, the 2011 meta-analysis by Walsh et al. found plasma exchange may reduce a composite end point of ESRD or death at 1 year. This was due to decreased risk of ESRD in the TPE group and no reduction in the risk of death [38]. This disparity between the risk of ESRD and mortality is surprising given the strong link between renal disease and death. Both analyses concluded that the overall quality of the evidence was low and there was considerable heterogeneity between the studies. Despite the variable quality of the evidence, both the KDIGO and EULAR/ERA-EDTA guidelines recommend the use of TPE in patients with severe renal disease or pulmonary haemorrhage [40, 41].
PEXIVAS
The paucity of high-quality evidence for the benefit of TPE and the need for studies in patients with moderate renal involvement or lung haemorrhage led to the development of the trial of plasma exchange and glucocorticoid dosing in the treatment of AAV (PEXIVAS). PEXIVAS used a 2 × 2 factorial design to assess TPE vs no TPE in addition to standard treatment, and high- vs low-dose steroid treatment. Inclusion criteria were positive ANCA testing with antibodies to myeloperoxidase (MPO) or proteinase-3 (PR3), eGFR < 50 ml/min and either haematuria and proteinuria or renal biopsy showing focal necrotising GN. Patients with pulmonary haemorrhage were included if they had radiographic evidence with no alternative diagnosis plus one of (i) broncho-alveolar lavage confirming the diagnosis, (ii) frank hemoptysis, (iii) elevated diffusing capacity of carbon monoxide or (iv) drop in haemoglobin ≥ 2 g/dL without other cause [42]. All patients were treated with IV methylprednisolone and either cyclophosphamide (oral or IV) or rituximab (at the discretion of the local investigators). Patients treated with cyclophosphamide induction received azathioprine as remission maintenance. Patients treated with TPE received 7 exchanges over 14 days and the two options for steroid taper are shown in Table 2. The trial recruited 704 patients with a median follow-up of 2.9 years. The median creatinine at enrolment was 330 μmol/L, 20% of patients required haemodialysis, and 27% of patients had evidence of pulmonary haemorrhage [43]. More patients received cyclophosphamide than rituximab as induction therapy (85%).
There was no effect of plasma exchange on the composite primary outcome of ESRD or death (HR 0.86; 95% CI 0.65–1.13, p = 0.27), and no differences in secondary outcomes including serious adverse events. A reduced-dose steroid regimen was shown to be non-inferior (ARR 2.3%; 95% CI − 4.5 to 9.1) with significantly fewer serious infections in the first year in the reduced-dose group (incidence rate ratio 0.69, 95% CI 0.52–0.93) [43]. There are several strengths to PEXIVAS; it is a large trial and included patients from 95 centres in 16 countries. Along with the broad enrolment criteria, this allows for generalizable results. Numbers lost to follow-up were small and assigned treatment adherence was good despite the open-label design.
PEXIVAS and steroids
The PEXIVAS findings regarding steroid dosing warrant consideration, as it is been increasingly recognised that steroids are major contributors to drug toxicity and adverse events in AAV [44]. In recent controlled trials, attempts to reduce or eliminate exposure to cyclophosphamide with drugs such as rituximab and MMF have not been associated with a reduction in adverse events [45, 46]. This might indicate that glucocorticoids, used at high dose in most studies, are a major contributor to drug toxicity and many current studies in AAV are investigating a low-dose steroid approach. Open-label cohort studies have shown favourable rates of remission induction and relapse with a combination treatment approach using low-dose intravenous cyclophosphamide and rituximab, along with a rapid oral glucocorticoid taper similar to the low-dose arm of PEXIVAS [47, 48]. An RCT in patients over the age of 65 has shown a remission induction regimen combining low-dose cyclophosphamide and low-dose steroids to be effective in comparison with conventional treatment [49]. However, relapses were common both in patients treated with the low-dose and standard regimens. In some patients, an even more rapid glucocorticoid taper may be possible. A two centre cohort study has reported 49 patients who received combination treatment with rituximab and low-dose IV cyclophosphamide and a rapid glucocorticoid taper, approximating a total steroid dose of 1 g administered over 2 weeks. Ninety percent of patients were in sustained remission at 12 months without requiring additional glucocorticoids, and there were reduced rates of serious infections and new onset diabetes compared with historical EUVAS controls [50].
There is also potential for novel therapeutics to replace steroids entirely. For example, an early-phase clinical study showed blocking C5aR with avacopan was non-inferior to prednisolone for remission induction [51]. A subsequent phase 3 study (ADVOCATE; NCT02994927) has randomised patients to receive either avacopan or glucocorticoids during remission induction with either cyclophosphamide or rituximab. Top-line data from late 2019 suggests non-inferiority of avacopan at 26 weeks and superiority over glucocorticoids at 52 weeks, with a similar safety profile at this time point.
Reducing steroid exposure is a central goal of many ongoing studies in AAV and patients treated with low or no steroids require careful monitoring. In particular, caution may be required if a low-dose steroid approach is used in patients treated with rituximab as induction therapy; in this subgroup of patients in PEXIVAS, there was a trend towards benefit of the higher dose regimen, although this did not reach statistical significance.
PEXIVAS and TPE
That reduced-dose steroids were non-inferior to higher doses has been accepted without the controversy that the lack of benefit for TPE has generated. This possibly reflects how committed many physicians are to the belief that TPE is of benefit in those with severe disease, and potentially different findings compared with MEPEX, the largest study to evaluate adjunctive therapy with TPE in patients with AAV prior to PEXIVAS. There are several differences between the two trials which may underlie this. Firstly, there are differences in the severity of renal disease; in patients enrolled in MEPEX, the average creatinine was 735 μmol/L, BVAS 21 and CRP 93 mg/dL compared with 330 μmol/L, 9 and 45 mg/dL respectively in PEXIVAS. The numbers requiring dialysis were also significantly different; 70% of MEPEX patients compared with 20% in PEXIVAS [31, 43]. However, despite these differences in the overall cohort of patients, when the subgroup of patients with creatinine > 500 μmol/L or requiring dialysis in PEXIVAS was analysed, there was a trend to benefit for TPE on the primary outcome but this was non-significant with a confidence interval spanning 1 (HR 0.77, 95% CI 0.53–1.11). Due to the large number of patients enrolled in PEXIVAS, this subgroup analysis is of similar size (n = 205) to the patient numbers enrolled in MEPEX (n = 173).
A further difference in enrolment criteria is that renal biopsy was not a requirement for entry into PEXIVAS. Patients enrolled in MEPEX had a mean of 56% crescents on biopsy representing significant disease activity. It is well described that some patients may present late with renal limited disease which has been progressing for some time leading to significant renal fibrosis which may not respond to treatment [52]. It is not known what proportion of patients in PEXIVAS had fibrotic disease and it would be useful to identify if the subgroup of patients with active crescents and minimal scarring on biopsy derived benefit from TPE.
There were also differences in immunosuppressive drug treatment between the two studies. In MEPEX, all patients were treated with oral cyclophosphamide compared with only a third of patients in PEXIVAS. Additionally, all patients in PEXIVAS were treated with IV methylprednisolone, whereas in MEPEX, TPE was compared with IV methylprednisolone. These differences could account in part for the differing trial results, perhaps implying there is only a benefit for TPE when it is used as a replacement for IV steroids; a potential for benefit from TPE should be kept in mind as more radical steroid-minimising protocols are developed. The two trials were also carried out in different eras; there is a 15-year interval between the two recruitment windows. It is well described that there have been decade on decade improvements in outcomes in AAV and so it is plausible that in the current era, with improved immunosuppression and general medical care, a benefit for TPE has been lost. In keeping with this, the event rate in PEXIVAS was lower than expected and recruitment needed to be increased from 500 to 700 patients to meet the pre-specified event rate.
The duration of follow-up was also different between the two studies. MEPEX reported its primary outcome at 1 year whereas median follow-up in PEXIVAS was 2.9 years. It may be that the heterogeneity of how patients were treated over a long-term follow-up period reduces the capacity of the trial to detect a benefit for TPE. At 1 year in PEXIVAS, there was a numerical but not statistically significant trend to benefit for TPE on the primary outcome (HR 0.77; 95% CI 0.53–1.11). However, 1-year data are not reported for the subgroup of patients with severe renal impairment or requiring dialysis.
The future of TPE in AAV
Despite PEXIVAS showing no benefit for routine use of TPE in patients with AAV, there are some groups of patients we feel should still be treated with TPE. Firstly, and the clearest indication, are those who are ‘double positive’ for ANCA and anti-GBM antibodies. Patients with double positivity present initially with aggressive pulmonary and renal disease similar to those with single anti-GBM positivity. As such, they should be treated in the same way as patients with anti-GBM disease, with immunosuppression and adjunctive TPE [53, 54]. These patients may show greater improvement with treatment than those single positive for anti-GBM. In one large case series, double positive patients were more likely to recover independent renal function; 35% of patients with double positivity who presented dialysis dependent had renal recovery at 1 year compared with only 10% of patients with anti-GBM disease [53].
Patients who are critically unwell may also warrant consideration of adjunctive TPE. There were low numbers of patients with severe pulmonary haemorrhage included in PEXIVAS; this is possibly due to a selection bias as some investigators may not have enrolled these patients due to concerns they would be randomised to the no TPE plus low-dose steroids group. There was a trend towards benefit from TPE in patients with both non-severe pulmonary haemorrhage (HR 0.64; 95% CI, 0.33 to 1.24) and severe pulmonary haemorrhage (hazard ratio, 0.67; 95% CI, 0.28 to 1.64); due to the small numbers of patients included in these subgroups, confidence intervals are wide. Given there was no increased risk of serious adverse events reported in the TPE group, the addition of TPE could be considered in these patients. Likewise, patients with severe RPGN and little chronic damage on renal biopsy are a group who may derive maximum benefit from adjunctive TPE. Patients who have a very short period of dialysis dependence or are approaching the need for renal replacement therapy may also have much to gain from TPE, particularly if they have a high percentage of glomeruli with cellular crescents on renal biopsy.
There could also be a role for TPE in patients with refractory disease which has not responded to initial treatment with immunosuppression or in patients where there is diagnostic uncertainty such as concerns of infection. In those with potential infection, TPE will not result in significant enduring immunosuppression in the same manner as cyclophosphamide, and initial treatment with TPE pending further investigations may be a useful strategy in this group. PEXIVAS did not report any difference in the number of serious infections at 1 year between the group treated with and without TPE.
Conclusions
PEXIVAS is by far the largest trial of TPE in AAV to date. It did not show benefit for adjunctive TPE on its primary endpoint of a composite of death and ESRD, and based on this evidence, most patients with AAV should not be treated with adjunctive TPE. However, there are subgroups of patients with AAV for whom TPE may still be of benefit and we would still consider using TPE in these cases.
References and Recommended Reading
Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the Writing Committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34(3):171–354.
Lockwood CM, Boulton-Jones JM, Lowenthal RM, Simpson IJ, Peters DK. Recovery from Goodpasture’s syndrome after immunosuppressive treatment and plasmapheresis. Br Med J. 1975;2(5965):252–4.
Lockwood CM, Pinching AJ, Sweny P, Rees AJ, Pussell B, Uff J, et al. Plasma-exchange and immunosuppression in the treatment of fulminating immune-complex crescentic nephritis. Lancet. 1977;1(8002):63–7.
Williams ME, Balogun RA. Principles of separation: indications and therapeutic targets for plasma exchange. Clin J Am Soc Nephrol. 2014;9(1):181–90.
Levy J, Pusey CD. Chapter 95 - plasma exchange. In: Floege J, Johnson RJ, Feehally J, editors. Comprehensive clinical nephrology. 4th ed. Philadelphia: Mosby; 2010. p. 1108–16.
Malchesky PS, Koo AP, Skibinski CI, Hadsell AT, Rybicki LA. Apheresis technologies and clinical applications: the 2007 International Apheresis Registry. Ther Apher Dial. 2010;14(1):52–73.
Winters JL. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines. ASH Educ Program Book. 2012;2012(1):7–12.
Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110(7):955–63.
Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990;87(11):4115–9.
Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20(2):289–98.
Weidner S, Neupert W, Goppelt-Struebe M, Rupprecht HD. Antineutrophil cytoplasmic antibodies induce human monocytes to produce oxygen radicals in vitro. Arthritis Rheum. 2001;44(7):1698–706.
Little MA, Smyth CL, Yadav R, Ambrose L, Cook HT, Nourshargh S, et al. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood. 2005;106(6):2050–8.
Xu PC, Cui Z, Chen M, Hellmark T, Zhao MH. Comparison of characteristics of natural autoantibodies against myeloperoxidase and anti-myeloperoxidase autoantibodies from patients with microscopic polyangiitis. Rheumatology (Oxford). 2011;50(7):1236–43.
Girard T, Mahr A, Noel LH, Cordier JF, Lesavre P, Andre MH, et al. Are antineutrophil cytoplasmic antibodies a marker predictive of relapse in Wegener’s granulomatosis? A prospective study. Rheumatology (Oxford). 2001;40(2):147–51.
Damoiseaux J, Csernok E, Rasmussen N, Moosig F, van Paassen P, Baslund B, et al. Detection of antineutrophil cytoplasmic antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen-specific immunoassays. Ann Rheum Dis. 2017;76(4):647–53.
Nölle B, Specks U, Lüdemann J, Rohrbach MS, DeRemee RA, Gross WL. Anticytoplasmic autoantibodies: their immunodiagnostic value in Wegener granulomatosis. Ann Intern Med. 1989;111(1):28–40.
Fussner LA, Hummel AM, Schroeder DR, Silva F, Cartin-Ceba R, Snyder MR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol. 2016;68(7):1700–10.
Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis--a meta-analysis. Rheumatology (Oxford). 2012;51(1):100–9.
Reeves HM, Winters JL. The mechanisms of action of plasma exchange. Br J Haematol. 2014;164(3):342–51.
Tesar V, Jelinkova E, Masek Z, Jirsa M Jr, Zabka J, Bartunkova J, et al. Influence of plasma exchange on serum levels of cytokines and adhesion molecules in ANCA-positive renal vasculitis. Blood Purif. 1998;16(2):72–80.
Soltesz P, Aleksza M, Antal-Szalmas P, Lakos G, Szegedi G, Kiss E. Plasmapheresis modulates Th1/Th2 imbalance in patients with systemic lupus erythematosus according to measurement of intracytoplasmic cytokines. Autoimmunity. 2002;35(1):51–6.
Hanly JG, Hong C, Zayed E, Jones JV, Jones E. Immunomodulating effects of synchronised plasmapheresis and intravenous bolus cyclophosphamide in systemic lupus erythematosus. Lupus. 1995;4(6):457–63.
Mokrzycki MH, Kaplan AA. Therapeutic plasma exchange: complications and management. Am J Kidney Dis. 1994;23(6):817–27.
Pusey C, Dash C, Garrett M, Gascoigne E, Gesinde M, Gillanders K, et al. Experience of using human albumin solution 4·5% in 1195 therapeutic plasma exchange procedures. Transfus Med. 2010;20(4):244–9.
McDonald V, Manns K, Mackie IJ, Machin SJ, Scully MA. Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost. 2010;8(6):1201–8.
Puisset F, White-Koning M, Kamar N, Huart A, Haberer F, Blasco H, et al. Population pharmacokinetics of rituximab with or without plasmapheresis in kidney patients with antibody-mediated disease. Br J Clin Pharmacol. 2013;76(5):734–40.
Thysell H, Bygren P, Bengtsson U, Lindholm T, Norlin M, Brun C, et al. Improved outcome in rapidly progressive glomerulonephritis by plasma exchange treatment. Int J Artif Organs. 1983;6(Suppl 1):11–4.
Bruns FJ, Adler S, Fraley DS, Segel DP. Long-term follow-up of aggressively treated idiopathic rapidly progressive glomerulonephritis. Am J Med. 1989;86(4):400–6.
Pusey CD, Rees AJ, Evans DJ, Peters DK, Lockwood CM. Plasma exchange in focal necrotizing glomerulonephritis without anti-GBM antibodies. Kidney Int. 1991;40(4):757–63.
Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J (Clin Res Ed). 1982;285(6342):606.
Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18(7):2180–8.
de Lind van Wijngaarden RAF, Hauer HA, Wolterbeek R, Jayne DRW, Gaskin G, Rasmussen N, et al. Chances of renal recovery for dialysis-dependent ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2007;18(7):2189–97.
Walsh M, Casian A, Flossmann O, Westman K, Hoglund P, Pusey C, et al. Long-term follow-up of patients with severe ANCA-associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclear. Kidney Int. 2013;84(2):397–402.
Szpirt WM, Heaf JG, Petersen J. Plasma exchange for induction and cyclosporine A for maintenance of remission in Wegener’s granulomatosis—a clinical randomized controlled trial. Nephrol Dial Transplant. 2010;26(1):206–13.
Cartin-Ceba R, Diaz-Caballero L, Al-Qadi MO, Tryfon S, Fervenza FC, Ytterberg SR, et al. Diffuse alveolar hemorrhage secondary to antineutrophil cytoplasmic antibody-associated vasculitis: predictors of respiratory failure and clinical outcomes. Arthritis Rheumatol. 2016;68(6):1467–76.
Hruskova Z, Casian AL, Konopasek P, Svobodova B, Frausova D, Lanska V, et al. Long-term outcome of severe alveolar haemorrhage in ANCA-associated vasculitis: a retrospective cohort study. Scand J Rheumatol. 2013;42(3):211–4.
Klemmer PJ, Chalermskulrat W, Reif MS, Hogan SL, Henke DC, Falk RJ. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am J Kidney Dis. 2003;42(6):1149–53.
Walsh M, Catapano F, Szpirt W, Thorlund K, Bruchfeld A, Guillevin L, et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta-analysis. Am J Kidney Dis. 2011;57(4):566–74.
Walters GD, Willis NS, Cooper TE, Craig JC. Interventions for renal vasculitis in adults. Cochrane Database Syst Rev. 2020;1.
Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75(9):1583–94.
Rovin BH, Caster DJ, Cattran DC, Gibson KL, Hogan JJ, Moeller MJ, et al. Management and treatment of glomerular diseases (part 2): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95(2):281–95.
Walsh M, Merkel PA, Peh CA, Szpirt W, Guillevin L, Pusey CD, et al. Plasma exchange and glucocorticoid dosing in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis (PEXIVAS): protocol for a randomized controlled trial. Trials. 2013;14(1):73.
Walsh M, Merkel PA, Peh C-A, Szpirt WM, Puéchal X, Fujimoto S, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382(7):622–31.
Little MA, Nightingale P, Verburgh CA, Hauser T, De Groot K, Savage C, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69(6):1036–43.
Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–32.
Jones RB, Hiemstra TF, Ballarin J, Blockmans DE, Brogan P, Bruchfeld A, et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann Rheum Dis. 2019;78(3):399–405.
Cortazar FB, Muhsin SA, Pendergraft WF 3rd, Wallace ZS, Dunbar C, Laliberte K, et al. Combination therapy with rituximab and cyclophosphamide for remission induction in ANCA vasculitis. Kidney Int Rep. 2018;3(2):394–402.
McAdoo SP, Medjeral-Thomas N, Gopaluni S, Tanna A, Mansfield N, Galliford J, et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant. 2018;34(1):63–73.
Pagnoux C, Quéméneur T, Ninet J, Diot E, Kyndt X, de Wazières B, et al. Treatment of systemic necrotizing vasculitides in patients aged sixty-five years or older: results of a multicenter, open-label, randomized controlled trial of corticosteroid and cyclophosphamide-based induction therapy. Arthritis Rheumatol. 2015;67(4):1117–27.
Pepper RJ, McAdoo SP, Moran SM, Kelly D, Scott J, Hamour S, et al. A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody–associated vasculitis. Rheumatology. 2018;58(2):260–8.
Jayne DRW, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28(9):2756–67.
Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21(10):1628–36.
McAdoo SP, Tanna A, Hrušková Z, Holm L, Weiner M, Arulkumaran N, et al. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 2017;92(3):693–702.
Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66(4):1535–40.
Acknowledgements
We acknowledge support from the National Institute for Health Research Imperial Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vasculitis
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prendecki, M., McAdoo, S.P. & Pusey, C.D. Is There a Role for Plasma Exchange in ANCA-Associated Vasculitis?. Curr Treat Options in Rheum 6, 313–324 (2020). https://doi.org/10.1007/s40674-020-00161-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-020-00161-y