Abstract
Background
Advanced component-resolved diagnostics (CRD) in Hymenoptera venom allergy (HVA) has improved the precise description of individual sensitization profiles. However, diagnostic gaps, peptide-based cross-reactivity, early identification of severe reactors and diagnosis of patients with a clear history of sting reactions but negative specific IgE and skin tests, remain challenging.
Methods
Systematic literature search in PubMed and critical analysis of recently published studies on insect venom allergy diagnostics.
Results and discussion
CRD has increased the sensitivity of IgE testing and improved the discrimination of primary sensitization from irrelevant cross-reactivity, ultimately providing a better rationale for therapeutic decisions. Despite these major advances, there is still room for improvement in routine HVA diagnostics. Peptide based cross-reactivity among homologous allergens from Vespinae and Polistinae venoms as well as still existing diagnostic gaps are particularly challenging. No marker allergens are currently available to differentiate Vespula and Polistes sensitizations. Several strategies including clinical setting of basophil activation test (BAT) for routine diagnostics, venomic analysis for the identification of novel allergens and characterization of the molecular basis of cross-reactivity could be used to address major limitations and unresolved issues in molecular diagnostics of HVA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hymenoptera venom allergic individuals experience a wide diversity of clinical manifestations including local symptoms and/or mild to severe systemic reactions. Mild systemic reactions such as generalized urticaria, angioedema and pruritus are limited to the skin. In contrast, potentially fatal severe systemic reactions often involve vascular and respiratory systems and can cause multiorgan failure [1]. Hymenoptera stings are among the most frequent causes of severe anaphylaxis in adults in Europe [2] and account for approximately 20% of the anaphylaxis-related fatalities worldwide [3]. The prevalence of systemic reactions ranges from 0.3–7.5% in European population [4] and from 0.5–3.3% in the United States [5], while in Latin America, Hymenoptera stings elicit about 15% of the severe allergic reactions annually reported [6].
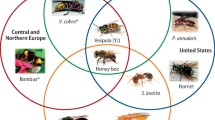
Venom immunotherapy (VIT) is the only long-term curative treatment available. Clinical data suggest that VIT prevents subsequent systemic sting reactions in 77–84%, 91–96% and 97–98% of patients treated with honeybee venom (HBV), yellow jacket venom (YJV) and ant venom, respectively [7]. The safety profile and efficacy of VIT critically relies on the unequivocal identification of the culprit insect which in Central and Northern Europe are predominantly honeybee and yellow jacket, while in the Mediterranean region, honeybee, Vespinae (Vespula, Vespa) and Polistinae (Polistes dominula) are the most frequent elicitors. Honeybee, yellow jacket, Polistes exclamans, Polistes annularis and Solenopsis invicta (fire ants) [8] account for most sensitizations in the US, whereas in Southern America other genera from Polistinae (Polybia, Apoica, Agelaia) and fire ant are clinically relevant ([9]; Fig. 1).
Major elicitors of Hymenoptera venom allergy in different geographical regions. Minor elicitors are also indicated (Asterisk). (Photos of A. mellifera, P. paulista, A. pallipes, A. pallens and S. invicta [kindly provided by Prof. M.S. Palma], P. annularis [kindly provided by Salvador Vitanza], Vespula [reproduced with permission from Peter Firus/Flagstaffotos]; V. crabro [PiccoloNamek, CC BY-SA 3.0], P. dominula and Bombus [J. Alves Gaspar, CCBY-SA 3.0] as well as Dolichovespula [F. Geller-Grimm, CC-BY-SA-2.5], are reproduced from http://commons.wikimedia.org, under the referred licenses)
Routine diagnostic of insect venom allergy is based on the clinical history of allergic sting reactions, skin testing and laboratory diagnostics for the identification of venom specific IgE (sIgE) directed against whole venom preparations or individual venom allergens [10].
Over the last decade, venomic analysis using classical as well as novel proteomic and transcriptomic approaches have allowed the characterization of individual allergens in several insect venoms [11, 12]. To date, 76 Hymenoptera venom components have been officially listed as allergens (www.allergen.org). The molecular characterization and heterologous production of some of these venom components allowed the rational design of panels of individual allergens for component-resolved diagnostics (CRD) of Hymenoptera venom allergy. The identification of a number of marker allergens in apian, Vespinae and Polistinae venoms has improved the precision of the sIgE diagnostics, which can now be used to discriminate, in most cases, genuine HBV and YJV sensitizations.
Despite the outstanding advances in diagnostics of HVA, there are several unresolved issues that should be addressed in the immediate future to improve the precise identification of the relevant sensitizations. We conducted a systematic review of the recent literature on HVA diagnostics using mainly the NCBI’s PubMed database (2010–2019). For some topics and relevant studies, the timeframe was expanded. Based in this search, we discuss the current situation, major challenges and suggest strategies for further improvement of HVA diagnostics.
Venom extract-based diagnostics: a gold standard with limitations
Detection of sIgE using unfractionated venom preparations has represented the gold standard for diagnostics of insect venom allergy for decades [13]. Despite being widely used, this approach is often hampered by a number of issues.
The composition of native Hymenoptera venom is complex. In HBV more than 100 different protein/peptide components have been described [14], some of which are present in high quantities and some of which are present only in trace amounts. Among the recognized allergens in HBV only phospholipase A2 (Api m 1) and melittin peptide (Api m 4) are highly abundant components, accounting for 12% and 50% of the venom dry weight, respectively, while the hyaluronidase Api m 2 (1–3%), and a number of other allergens such as Api m 3, Api m 5, Api m 6 and Api m 10 are only present in low abundance (1% or below). Variability in allergen content and/or stability [15] may thus influence the outcome of diagnostic testing, particularly in patients with sensitizations to low abundance allergens.
Similarly, in YJV only phospholipase A1 (PLA1) (Ves v 1; 6–14%) and antigen 5 (Ves v 5; 5–10%) are present in higher quantities, while the other allergens (Ves v 2, Ves v 3, Ves v 6) are of lower abundance. Even though antigen 5 belongs to the allergens of high abundance, a lack of Ves v 5 IgE immunoreactivity was reported for YJV preparations, as compared to sIgE reactivity to recombinant Ves v 5 (rVes v 5) [14]. In this study the sensitivity of YJV extract to detect YJV allergic patients was as low as 83%, while testing with rVes v 5 alone allowed detection of 90% of the patients. Spiking of YJV preparation with recombinant Ves v 5 significantly improved the detection of YJV sensitizations and resulted in the detection of 97% of the patients [16].
Double positive results (e.g., to HBV and YJV) are common findings in venom extract-based allergy diagnostics. Previous studies conducted in Central Europe demonstrated that up to 47% of patients with anaphylactic sting reactions (n = 530) display double positivity to HBV and YJV [17]. Unfortunately, often this result does not reflect true sensitizations to different insects but is rather associated with the detection of clinically irrelevant cross-reactive IgE [10]. Cross-reactivity can be caused by IgE directed against cross-reactive carbohydrate determinants (CCDs) which are defined as an α‑1,3‑linked core fucose, and/or IgE directed against common peptide epitopes in homologous venom allergens from different insects. Several native allergens in HBV and YJV preparations are CCD carrying proteins that can be recognized by irrelevant CCD-sIgE. Venom preparations also contain homologous cross-reactive allergens shared by insects from different genus, family and even superfamily (Apoidea and Vespoidea), which also contribute to cross-reactivity, overall hampering the reliable detection of the primary sensitizer.
Component-resolved diagnostics
In Europe, cross-reactivity problems associated with the use of whole venom preparations have been partially solved with the development of CRD. This novel approach has been particularly explored for the discrimination of HBV and YJV sensitizations. Twelve different allergens (Api m 1—Api m 12) have been identified in HBV while five different allergens have been described in YJV (Ves v 1–3, Ves v 5, Ves v 6) and European paper wasp venom (Pol d 1–5) (Fig. 2a). Particularly, Api m 1, Api m 3–4 and Api m 10 as well as Ves v 1 and Ves v 5 represent apian and yellow jacket marker allergens that allow differential diagnosis of most HBV and YJV sensitizations. Similarly, the use of the apian marker allergens and Pol d 1/Pol d 5 allows the differential diagnosis of HBV and Polistes sensitizations (Fig. 2b). Unfortunately, Ves v 1 and Pol d 1 as well as Ves v 5 and Pol d 5 are significantly cross-reactive, partially hampering their use for unambiguous discrimination of Vespula or Polistes sensitizations.
Graphical representation of the electrophoretic profile (SDS-PAGE) of the HBV, YJV and European paper wasp venom (a). The marker allergens for differentiation of HBV/YJV and HBV/Polistes (upper panel, green lines), the cross-reactive allergens from YJV and Polistes venom (upper panel, red line) and cross-reactive shared by these insects (lower panel, red line) are shown (b)
Six HBV allergens, namely Api m 1–5 and Api 10 as well as two from the YJV (Ves v 1 and Ves v 5) and the European paper wasp P. dominula (Pol d 1, Pol d 5) provided by different manufacturers [10], are now available as recombinant components for routine diagnostic workup. Remarkably, the use of recombinant venom allergens produced in bacterial and Sf9 insect cells as proteins devoid of CCD reactivity helps to circumvent carbohydrate-based cross reactivity. The routine diagnostic settings for HBV and YJV allergies currently include the use of the venom based-skin testing and in vitro tests followed by the application of marker allergens to solve double positivity outcome potentially caused by cross-reactivity (Fig. 3). Detection of IgE to a unique marker allergen for the respective specie is sufficient for correct diagnosis.
Two-step routine diagnostic setting for detection of HBV and YJV sensitizations. Asterisk Due to potential protein based cross-reactivity with the hyaluronidase of YJV, Ves v 2, a positive result to Api m 2 does not formally exclude a YJV sensitization. However, due to the frequent sensitization to Api m 2 in HBV allergic patients and the low prevalence of sensitization to Ves v 2 in YJV allergic patients, this is unlikely (for details see text)
Early studies with recombinant Api m 1 (rApi m 1) in a cohort of confirmed HBV and YJV allergic patients, reported levels of sensitization of 97% [18]. Sensitization frequencies of rVes v 1 and rVes v 5 in a similar cohort range from 33–54% and 84.5–90%, respectively [10]. Remarkably, CRD using these marker allergens on routine diagnostic test systems such as the ImmunoCap (Thermo Fisher Scientific, Uppsala, Sweden) has significantly improved the identification of the culprit insect in patients previously diagnosed with HBV and YJV sensitizations. The initial studies using rApi m 1 and rVes v 5 allowed the unambiguous discrimination of HBV or YJV monosensitization in 37/64 (57.8%) [19] and 27/29 (93%) [20] of the patients with double positivity to HBV and YJV extracts.
Similarly, the application of rApi m 1, rVes v 5 and rVes v 1 allowed the identification of HBV (n = 9, 11.8%) and YJV (n = 30, 39.5%) monosensitized in a cohort of patients (n = 76) previously diagnosed with double positivity to venom extracts [21]. Several additional studies have reported the diagnostic value of these marker allergens for precise discrimination of YJV or HBV sensitizations and true double positivity from irrelevant cross-reactivity [22, 23].
Despite its major contribution to increased diagnostic precision, the use of recombinant allergens still shows important limitations. In contrast to early reports (97%) [21], recent studies reported lower sensitivity for rApi m 1 [24, 25] and even for the whole panel (78–93%) of HBV allergens currently available for the detection of HBV sensitization [25, 26]. From the clinical point of view this means that HBV allergy cannot be ruled out reliably after an apian component-based negative result. In contrast to the situation in YJV allergy, patients with HBV allergy display a much broader spectrum of sensitization profiles, which may account for the limited sensitivity of the existing allergen panel. Here, additional marker allergens may be required to improve the diagnostic sensitivity in the detection of HBV sensitization.
A second major limitation of CRD in HVA is the lack of components that allow an unequivocal differentiation of Vespinae and Polistinae sensitization. Currently, no marker allergen exists that serves this purpose. One interesting candidate, Pol d 4, which was identified in P. dominula venom but not in Vespula venom, failed as marker allergen due to low sensitization prevalence in Polistes sensitized individuals (unpublished data). All other Polistes allergens that have been described so far, have a homologous counterpart in Vespinae venom and display a high degree of peptide-based cross-reactivities [27], making them unsuitable for precise discrimination of YJV or Polistes sensitizations.
Component-resolved diagnostics and peptide based cross-reactivity
Until recently, IgE binding to CCDs was suggested to be the major cause of cross-reactivity (75%) and double positive results during differential diagnosis of HBV and YJV allergy [28]. However, more recent studies challenged this assumption by demonstrating that 55–70% of patients with CCD reactivity display true double sensitization as determined by IgE reactivity to CCD-free recombinant marker allergens [29, 30]. Thus, CCDs do not seem to play the major role in cross-reactivity and double positive results. In addition, venoms from European and Neotropical paper wasps were shown to lack CCD, implying that diagnosis of paper wasp allergies is only hampered by peptide-based cross-reactivity [31, 32]. Overall, these findings suggest that peptide based cross-reactivity plays a more important role in the differential diagnosis of HVA than previously anticipated.
Peptide based cross-reactivity relies on the presence of common linear and/or discontinuous protein epitopes shared by homologous allergens present in different insect venoms. In the case of HBV and YJV, this has been demonstrated for relevant allergens such as dipeptidyl peptidase IV (DPPIV) (Api m 5 and Ves v 3) and vitellogenins (Api m 12, Ves v 6) (Fig. 2). Despite displaying certain degree of sequence identity, cross-reactivity between HBV (Api m 2) and YJV (Ves v 2) hyaluronidases is mostly based on IgE reactivity to CCD epitopes, as demonstrated using CCD-lacking Ves v 2 [33]. While sensitization to Api m 2 independent of CCD reactivity is quite common in HBV allergic patients (42–52%) [17], little or no IgE reactivity to CCD-free rVes v 2 has been reported in YJV allergic patients [33]. One explanation for the lack of peptide based cross-reactivity between YJ and HB hyaluronidases could be differences in their three-dimensional structures and surface epitopes, which only display a low degree of similarity when analyzed by computational modeling [34, 35].
Vespinae and Polistinae venoms display a similar proteome and allergome profile with a limited set of allergens currently identified. As noted, venom PLA1 and antigen 5 are the most prominent allergens in these insect venoms and share high levels of primary sequence and 3‑D structure identity (Fig. 4). Due to these structural similarities, venom PLA1 and antigen 5 have limited diagnostic value for discrimination of Vespula and Polistes sensitizations [13, 17]. A recent study showed significant levels of peptide-based cross-reactivity between antigen 5 from several members of Vespinae and Polistinae [27]. Venom PLA1 were shown to cross-react to a lesser extent [10, 36].
Overlay of the three-dimensional (3-D) models of the venom antigen 5 (a) and PLA1 (b) from clinically relevant Hymenoptera. Percentages of sequence identity (red triangles) and levels of 3‑D structure similarities (green triangles) expressed by the root-mean square deviation (RMSD) of the atomic positions on the 3‑D models are provided. RMSD values between 0–2 Å indicate a high-quality model with lower values indicating higher similarity to the template
To date, cross-reactivity among Vespinae and Polistinae venoms is relevant in Mediterranean regions whereas in Central and Northern Europe Polistes sensitization is rare. Nonetheless, in Central Europe Polistes allergy could be underdiagnosed as testing for Polistes sensitizations is not part of the clinical routine. Moreover, the increased spreading of P. dominula over new areas in Europe represents a potential challenge for allergists. The lack of biomarkers for unequivocal differentiation of Vespinae and Polistinae sensitizations could be addressed by applying high dynamic range techniques including shotgun proteomics to identify novel low abundant allergens differentially present in wasp venoms [14].
Interestingly, few studies for whole venome and allergome profiling of European Vespinae and Polistinae species are currently available, hampering the identification of novel allergens which can be used as potential subfamily-specific biomarkers. Previous systemic analysis from venome profiling of the Neotropical paper wasp [37] and the Asian hornet Vespa affinis [38] resulted in the detection of several novel proteins which were recognized by IgE in the sera of sensitized patients. Unfortunately, the potential role of these proteins as relevant allergens remains largely unexplored. As in the case of Api m 10 in HBV [39], the newly identified low abundant wasp venom components could represent novel Vespinae and Polistinae marker allergens. This however still needs to be investigated. To date the identification of novel subfamily biomarkers seems not very likely as the most important Vespinae and Polistinae allergens are cross-reactive [27].
Characterization of linear and conformational IgE epitopes in allergens could be exploited as an alternative for improved the differential diagnosis of wasp allergies. Although, structural analysis of Vespinae venom PLA1 and antigen 5 showed low variations in their primary sequence and tertiary structures, differential epitopes could be potentially identified on the allergen surfaces. Continuous and discontinuous IgE-epitopes settled in zones of low conservation among these allergens represent potential candidates for the differential diagnosis of allergy. In addition to PLA1, epitope mapping of hyaluronidase could result in the identification novel molecular markers for Polistinae sensitization. Unlike Ves v 2, hyaluronidase from Polistinae members have been suggested to display high sensitization frequencies [10].
Diagnostic gaps
Despite the remarkable improvement in differential identification of the culprit venom, in some cases CRD fails to detect relevant sensitizations. The remaining diagnostic gap is clinically relevant for clinicians as undetected sensitizations could result in severe reactions after the next insult. In contrast to the initial findings (97%) [18], diagnostic sensitivity of Api m 1 were reported to be limited, ranging from 58–80% [24]. A recently published study reported lower values (54.5%) in a population of HBV allergic patients (n = 189) using the CAP system (cut-off IgE >0.35 kUA/L) [25]. Several factors including the selected population, regional differences and sensitivity of the test system have been suggested to cause this wide range of sensitization frequencies [10].
The inclusion of the other HBV proteinaceous marker allergens (Api m 3 and Api m 10) resulted in a partial increase of the sensitization frequencies (87%) [26]. Higher sensitivities (91–93%) were obtained by combining the recombinant forms of the marker and cross-reactive allergens [26, 30]. However, a follow-up study [25] using the same cut-off (>0.35 kU/L), reported lower sensitivity values for the same panel of allergens (71.6%) in a cohort of HBV monosensitized patients. Lowering the cut-off (>0.1 kUA/L) resulted in a limited increased sensitivity (84.3%). Remarkably and in line with our previously published observations [26], higher sensitization frequencies (92.7%) to the whole panel of HBV allergens were found in the cohort of double sensitized patients (n = 55), suggesting that the sensitization frequency in the CRD of HBV allergy strongly depends on the number of double-sensitized patients included in the studied population. As we suggested [26], this result might be related to a more advanced state of atopic immune deviation in double-sensitized population.
The use of recombinant YJV allergens significantly increased the sensitivity of the systems currently used for allergy diagnostics. A study with YJV allergic patients (n = 308) showed that only 83.4% of the patients displayed sensitization to venom extracts. Shortage in Ves v 5 or lack of immunoreactivity of Ves v 5 in the YJV preparations was suggested to cause this limited sensitization frequency. Ves v 5-spiked YJV venom increases test sensitivity to 96.8% [16] and is currently available on the ImmunoCAP (Thermo Fisher Scientific) platform for routine YJV allergy diagnostics. Moreover, the combined use of Ves v 1 and Ves v 5 in CRD of YJV allergy has consistently allowed the unequivocal identification of 92–98% of the YJV sensitizations [40, 41].
A limited set of additional relevant YJV allergens (Ves v 2, Ves v 3) is available at research level and has been suggested to increase the detection rate of YJV sensitization in patients with a clear history of insect sting anaphylaxis but negative sIgE to YJV [42]. This observation, however could not be confirmed in a subsequent study analyzing sIgE to the same allergens on a routine sIgE detections system [43]. Particularly, the prevalence of sIgE to CCD-free Ves v 2 sIgE is low (10%) [44], questioning its value to augment diagnostic sensitivity. Higher prevalence of sIgE to the cross-reactive allergens Ves v 3 (57%) [12] and Ves v 6 (39%) [45] were detected in vitro. Nonetheless, further analyses in a larger cohort of YJV allergic patients to determine the relevance of these allergens as well as their potential contribution to increased sensitivity, are required.
Similarly, additional studies to investigate the prevalence of IgE to venom PLA1, are needed. Sensitization frequencies obtained with native venom PLA1 appear to be significantly higher than the values reported with rVes v 1 and rPol d 1 [46]. This is particularly important for Polistes-sensitized patients, as lower sensitization frequencies (69–72%) [8, 46] have been reported for rPol d 5. Finally, the DPPIV from the European paper wasp (Pol d 3) was shown to be recognized by a high number of Polistes-allergic patients (66%) and can be explored as a candidate to increase sensitivity and overall, for further improvement of CRD.
Diagnostic gaps might be also reduced by using a lower cut-off value (0.1 kUA/l) in laboratory tests [47]. Although previous studies reported limited increased sensitivity after lowering the cut-off [25], this strategy can be particularly valuable for patients with low total IgE [10]. In addition, direct quantitative comparison of IgE levels to homologous cross-reactive HBV and YJV might prove useful towards identifying the primary sensitizer in patients with negative results to HBV and YJV marker allergens. This approach may be also applied for the identification of Vespula and Polistes venom sensitization. Recently, we showed significant variations in the levels of peptide-based cross-reactivity among PLA1 from Vespinae venoms, even in members of the same genera (Polistinae) [36]. Levels of sIgE against the primary sensitizer were significantly higher. Our results suggested that quantitative comparison of sIgE levels to the primary sensitizer represent a feasible tool to rank the probability of culprit species. The diagnostic value of this approach was also suggested in early attempts to differentiate between Polistes and Vespula sensitizations [46].
Overall, these data show that a number of diagnostic gaps still remain in molecular diagnostics of insect venom allergy. This is particularly true in the case of HBV allergy, due to the wider sensitization profile and the low rates of sensitization to individual allergens. Currently, in some particular patient groups, a large number of sensitizations (15.7%) remains undetected. Further allergome profiling of HBV and wasp venoms as well as characterization of low abundant clinically relevant components that could be included in the panel of recombinant allergens may help to improve sensitivity and overall the outcome of the CRD.
Potential novel elicitors of Hymenoptera venom allergy in European regions
Climate change, habitat destruction and accidental introduction of invasive species are drastically changing the distribution of some clinically relevant insects among different geographical regions [48]. In Europe, P. dominula has expanded its habitat toward Central and Northern Europe [49]. In addition, Vespa velutina (Asian hornet), a highly invasive insect from South-East Asia has continuously spread over different European countries since its original introduction in France. Recent studies reported the presence of the Asian hornet in Italy, Belgium, Spain and United Kingdom [50].
Although in Central Europe, P. dominula is less aggressive and has shown a different nesting behavior, its increased presence represents a potential new challenge to routine diagnostic in this area. As noted, until recently the vast majority of HVA cases in Central Europe were caused by Vespinae members and honey bees. Potential sensitization caused by the European paper wasp could create a new scenario for diagnostics as novel systems for differentiation of Vespinae and Polistes sensitizations will be needed. Failing to detect the P. dominula monosensitization could led to systemic hypersensitivity reactions after the next field sting. In addition, failing to distinguish Vespinae and Polistes sensitizations due to cross-reactivity could led to de novo sensitization and severe reactions in patients wrongly diagnosed and treated with Vespinae venom preparations.
Despite not being considered a major medical problem, Vespa velutina also represents a potential novel cause of allergy in Europe. Recently, Vespa velutina was identified as the elicitor of anaphylaxis in a 71-year-old beekeeper from Spain [51]. Moreover, a previous study showed a high prevalence of systemic reactions (81.2%) in patients sensitized to venom of the taxonomically close European hornet, V. crabro [52]. This value was found to be around three times higher than the prevalence obtained with Vespula spp (27.8%) and HBV (24%). Since European and Asian hornets are members of the same genus and display a similar venom composition, V. velutina sting could potentially cause not only de novo sensitization but also triggers hypersensitivity reactions in V. crabro- or Vespula-sensitized patients due to cross-reactivity.
Diagnostic challenges in Northern and Southern America
In addition to honeybees, hornets, yellow jackets and paper wasps are major elicitors of allergy in the US [5]. These group of wasps are highly aggressive and due to their scavenger behavior cause a large number of sting accidents. S. invicta is also clinically relevant and has been reported to cause most cases of hypersensitivity reactions to Hymenoptera venoms in endemic areas [53]. The standard method for insect allergy diagnostic in the US is skin testing using venom extracts from HB, YJ, hornet and Polistes and/or whole-body extracts of imported fire ants. Remarkably, several studies have shown that a large proportion (30%) of severe reactors have negative skin tests [54]. Moreover, double positive results to Vespula and Polistes venom are common (50%) in Vespula-sensitized patients [55]. Overall these findings suggest the need for further improvement in the strategies currently applied for venom allergy diagnosis in the US.
Despite hosting an outstanding diversity of clinically relevant Hymenoptera which include roughly 33% of the social wasp species currently identified worldwide, HVA is a neglected human health problem in Brazil [9, 56]. Epidemiologic surveys as well as studies for the evaluation of the diagnostic systems currently applied are scarce. Standardized venom preparations or individual allergens derived from the venom of the major allergy elicitors Polybia paulista, Apoica pallens, and fire ants are not available [36, 56]. Routine diagnostic tests in Brazil are based on manufactured crude venom extracts thus increasing the incidence of cross-reactivity and therefore causing diagnostic failure to detect the culprit insect. Variations of manufactured venom extract composition also cause inconsistent results when analyzing sensitization frequencies.
During the last few decades, several efforts have been made to overcome this situation. Venomic analysis of P. paulista allowed the identification of three major components that were officially annotated as insect venom allergens (Poly p 1, Poly p 2 and Poly p 5) [56]. Recombinant forms of these allergens were obtained in bacterial and yeast cells and are under evaluation for the rational design of CRD [57, 58]. Moreover, PLA1 and antigen 5 from of A. pallens were recently identified and sequenced by our group (unpublished data). The purified native venom components were recognized by sIgE in the sera of allergic patients and could represent two novel allergens and potential candidates for further improvement of HVA diagnostics in Brazil.
Predictors of severe reactors
Risk factors for severe allergic reactions include elevated baseline serum tryptase concentration (BTC), senior age, insect type, male sex, concomitant cardiovascular diseases and presence of mast cell disorders [59]. To date, measurement of serum tryptase concentration is the only test broadly available in routine diagnostics that can be used as a potential indicator of severe reactors. Several studies had shown that a high BTC is associated with severe symptoms [60]. Moreover, an increase in the tryptase levels on the first day of the VIT has been associated to higher risk of systemic allergic reactions during treatment [61]. Nonetheless, elevated baseline tryptase cannot be considered as an unequivocal biomarker but as a potential indicator of severe symptoms after the next sting.
Kucharewicz et al. [62] showed that only 19% (n = 12) of untreated patients with reaction grade III (n = 37) or IV (n = 25) according to Muller scale have BTC (11.9–53.5 µg/L) above the widely used cutoff (≥11.4 µg/L). A more recent analysis for the description of severity predictors showed that an increase in the BTC from 4.25 to 20 µg/L augments the risk of severe systemic reactions by a factor of 3.8 and described a nonlinear continuous association between BTC and insect venom allergic severe reactions [63]. Thus, the authors recommended to consider the whole range of tryptase concentrations rather than define a cutoff for individualized risk assessment [59, 63].
In addition to tryptase levels, the influence of IgE levels in the frequency of insect venom-triggered severe reactions has been explored. A large cohort of allergic patients (n = 758) with a history of systemic symptoms and complete laboratory test showed no significant correlation between grade III and IV reactions and the levels of tIgE and venom-sIgE. Sturm and colleagues [64] showed that untreated allergic patients with low level of tIgE (<50 kU/l) suffered severe reactions including loss of consciousness after a natural sting. A recent study also found no association between IgE/IgG4 ratios to relevant allergens, and the severity of the clinical symptoms. Overall, these data suggest that the degree of sensitization as determined by levels of sIgE to individual venom components has no value for the prediction of symptoms severity and anaphylaxis.
Advances in flow cytometry techniques and omic approaches may be useful for the identification of novel biomarkers of severe reactions. Over the last few decades, several molecules such as chymase, carboxypeptidase A3 and the serum CCL‑2 have been investigated as indicators of anaphylaxis in different types of allergies [65]. Particularly, increased levels of serum CCL‑2 were reported during anaphylaxis as compared to convalescent samples. Nonetheless, most of these molecules are useful for diagnosis of anaphylaxis rather than for prediction of the severity of symptoms to a future field sting. Thus, further studies are required for the identification of reliable predictors of severe reactors and risk assessment.
Perspective for basophil activation test
Negative skin and in vitro tests in patients with a clear history of severe sting reactions represent a major challenge for allergy clinicians. Often, these patients do not receive VIT and consequently are at risk of systemic life-threatening reactions after a subsequent sting. It has been reported that 30% of the insect sting-related fatal anaphylaxis occurred in patients with low or undetectable levels of sIgE [66]. The basophil activation test (BAT) using whole venom preparations has been suggested as a valuable tool to overcome this diagnostic problem. Previous studies showed that BAT detected sensitizations in 17/21 (81%) of severe reactors with previous negative sIgE test using the cut off level of 0.35 KU/L [67]. These reports however have two limitations: since sIgE detection on semiautomated routine platforms is FDA approved down to levels of 0.1 KU/L, detection of sIgE levels between 0.1 and 0.35 KU/L should have also been considered in these studies, particularly in patients with low total IgE levels. In addition, the introduction of the Ves v 5 spiked YJV extract for routine diagnostics [16] mostly likely reduces the number of sIgE-negative patients with a clear history of Hymenoptera sting anaphylaxis, thus reducing the number of patients who would benefit from the use of the BAT even further.
BAT has also been suggested as a novel alternative for improved discrimination of true double sensitization from cross-reactivity by allowing quantitative detection of the stronger basophil activation elicitor. Although BAT reliability for unequivocal identification of the primary sensitizing species is hampered by the presence of CCDs, at least one study showed that recombinant allergen-based BAT testing improved the identification of the culprit venom as compared to classical in vitro tests for sIgE detection [22]. Particularly, a more recent study with a panel of YJV individual allergens showed that BAT-based diagnostic increased specificity as compared to routine diagnostic test [68]. The high specificity of this approach could result in the detailed description of the component-resolved sensitization profile in allergic patients potentially leading to the rational design of a stratified and personalized VIT.
To date, most reports on the diagnostic value of BAT have been conducted in advanced research setting. Only one study with a limited number of patients (n = 21) was conducted in a routine clinical practice [67]. Several factors including increased costs, higher expertise requirement for the implementation of the method as well as the interpretation of the biological data has partially hampered its application in routine diagnostic of allergy. This would be in particular true for the use of recombinant venom allergens in the BAT diagnostics that are so far only available for research purpose and not yet in a standardized way that would be required for routine diagnostics in a clinical setting. Additional studies for standardization of the test involving larger cohorts of patients and better standardized allergens, are required. If these prerequisites would be available, the BAT would most likely be a valuable addition for component resolved diagnostics in Hymenoptera venom allergy. Considering that allergens of European paper wasps are devoid of CCD, BAT could also be a valuable tool to increase diagnostic precision for the detection of primary sensitization to P. dominula venom and to discriminate between Vespula and Polistes sensitization.
Concluding remarks
In vitro diagnostics of insect allergy has experienced outstanding advances during the last decade. Analysis of sIgE reactivity to panels of defined venom allergens now allows an improved differentiation of the relevant sensitization particularly in HBV and YJV allergy. In addition, it enables us to characterize individual sensitization profiles, particularly in HBV allergy that may be of relevance for the treatment outcome of VIT. Based on this, we are tempted to assume that the improved precision of CRD results in an improved safety and efficacy of VIT; however, data confirming this are still lacking. Nonetheless, some issues remain unresolved. There are still diagnostic gaps in the CRD of HVA that should be closed either by improving assay sensitivities and/or by expanding the range of allergens available for routine diagnostics. In addition, marker allergens or other diagnostic markers are still required that allow a reliable discrimination between sensitization to Vespinae and Polistinae venoms. Here, profiling of peptide specific IgE reactivity may be a promising approach. Finally, more studies are required for the identification and evaluation of prognostic biomarkers for risk assessment and proper selection of patients for venom immunotherapy.
Abbreviations
- BAT:
-
Basophil activation test
- BTC:
-
Baseline serum tryptase concentration
- CCDs:
-
Cross-reactive carbohydrate determinants
- CRD:
-
Component-resolved diagnostics
- DPPIV:
-
Dipeptidyl peptidase IV
- HBV:
-
Honeybee venom
- PLA1:
-
Phospholipase A1
- RMSD:
-
Root-mean square deviation
- sIgE:
-
Specific IgE
- VIT:
-
Venom immunotherapy
- YJV:
-
Yellow jacket venom
References
Demain JG, Minaei AA, Tracy JM. Anaphylaxis and insect allergy. Curr Opin Allergy Clin Immunol. 2010;10:318–22. https://doi.org/10.1097/ACI.0b013e32833a6c72.
Worm M, Eckermann O, Dolle S, Aberer W, Beyer K, Hawranek T, et al. Triggers and treatment of anaphylaxis: an analysis of 4,000 cases from Germany, Austria and Switzerland. Dtsch Arztebl Int. 2014;111:367–75. https://doi.org/10.3238/arztebl.2014.0367.
Bilò MB. Anaphylaxis caused by hymenoptera stings: from epidemiology to treatment. Allergy. 2011;66:35–7. https://doi.org/10.1111/j.1398-9995.2011.02630.x.
Bilò BM, Bonifazi F. Epidemiology of insect-venom anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8:330–7. https://doi.org/10.1097/ACI.0b013e32830638c5.
Golden DBK, Demain J, Freeman T, Graft D, Tankersley M, Tracy J, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28–54. https://doi.org/10.1016/j.anai.2016.10.031.
Sole D, Ivancevich JC, Borges MS, Coelho MA, Rosario NA, Ardusso LRF, et al. Anaphylaxis in latin America: a report of the online latin American survey on anaphylaxis (OLASA). Clinics. 2011;66:943–7. https://doi.org/10.1590/S1807-59322011000600004
Sturm GJ, Varga EM, Roberts G, Mosbech H, Bilò MB, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. 2018;73:744–64. https://doi.org/10.1111/all.13262.
Bilò MB, Ollert M, Blank S. The role of component-resolved diagnosis in hymenoptera venom allergy. Curr Opin Allergy Clin Immunol. 2019;19(6):614–22. https://doi.org/10.1097/aci.0000000000000574.
Guimarães M. Death Swarm. Pesquisa FAPESP, 2009. Available online: http://revistapesquisa.fapesp.br/en/2008/11/01/death-swarm-2/ (accessed on 01 September 2019)
Jakob T, Müller U, Helbling A, Spillner E. Component resolved diagnostics for hymenoptera venom allergy. Curr Opin Allergy Clin Immunol. 2017;17:363–72. https://doi.org/10.1097/ACI.0000000000000390.
Spillner E, Blank S, Jakob T. Hymenoptera allergens: from venom to “venome. Front Immunol. 2014;5:1–7. https://doi.org/10.3389/fimmu.2014.00077.
Blank S, Seismann H, Bockisch B, Braren I, Cifuentes L, McIntyre M, et al. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight hymenoptera venom allergens Api m 5 and Ves v 3. J Immunol. 2010;184:5403–13. https://doi.org/10.4049/jimmunol.0803709.
Jakob T, Blank S, Spillner E. Benefits and limitations of recombinant allergens in diagnostics of insect venom allergy. Molecular allergy diagnostics: innovation for a better patient management. 2017. pp. 341–62. https://doi.org/10.1007/978-3-319-42499-6.
Van Vaerenbergh M, Debyser G, Devreese B, de Graaf DC. Exploring the hidden honeybee (Apis mellifera) venom proteome by integrating a combinatorial peptide ligand library approach with FTMS. J Proteomics. 2014;99:169–78. https://doi.org/10.1016/j.jprot.2013.04.039.
Blank S, Etzold S, Darsow U, Schiener M, Eberlein B, Russkamp D, et al. Component-resolved evaluation of the content of major allergens in therapeutic extracts for specific immunotherapy of honeybee venom allergy. Hum Vaccin Immunother. 2017;13:2482–9. https://doi.org/10.1080/21645515.2017.1323603.
Vos B, Köhler J, Müller S, Stretz E, Ruëff F, Jakob T. Spiking venom with rVes v 5 improves sensitivity of IgE detection in patients with allergy to vespula venom. J Allergy Clin Immunol. 2013;131:1225–7. https://doi.org/10.1016/j.jaci.2012.07.041.
Jakob T, Rafei-Shamsabadi D, Spillner E, Sabine S. Diagnostics in hymenoptera venom allergy : current concepts and developments with special focus on molecular allergy diagnostics. Allergo J Int. 2017;26:93–105. https://doi.org/10.1007/s40629-017-0014-2.
Müller UR, Johansen N, Petersen AB, Fromberg-Nielsen J, Haeberli G. Hymenoptera venom allergy: analysis of double positivity to honey bee and vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5. Allergy. 2009;64:543–8. https://doi.org/10.1111/j.1398-9995.2008.01794.x.
Hofmann SC, Pfender N, WeckesserS, Huss-Marp J, Jakob T. Added value of IgE detection to rApi m 1 and rVes v 5 in patients with Hymenoptera venom allergy. J Allergy Clin Immunol. 2011;127:265–7. https://doi.org/10.1016/j.jaci.2010.06.042
Mittermann I, Zidarn M, Silar M, Markovic-Housley Z, Aberer W, Korosec P, et al. Recombinant allergen-based IgE testing to distinguish bee and wasp allergy. J Allergy Clin Immunol. 2010;125:1300–1307.e3. https://doi.org/10.1016/j.jaci.2010.03.017.
Müller U, Schmid-Grendelmeier P, Hausmann O, Helbling A. IgE to recombinant allergens Api m 1, Ves v 1, and Ves v 5 distinguish double sensitization from crossreaction in venom allergy. Allergy. 2012;67:1069–73. https://doi.org/10.1111/j.1398-9995.2012.02847.x.
Eberlein B, Krischan L, Darsow U, Ollert M, Ring J. Double positivity to bee and wasp venom: improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2012;130:155–61. https://doi.org/10.1016/j.jaci.2012.02.008.
Šelb J, Kogovšek R, Šilar M, Košnik M, Korošec, P. Improved recombinant Api m 1‑ and Ves v 5‑based IgE testing to dissect bee and yellow jacket allergy and their correlation with the severity of the sting reaction. Clin Exp Allergy. 2016;46(4):621–30. https://doi.org/10.1111/cea.12639.
Korošec P, Valenta R, Mittermann I, Čelesnik N, Eržen R, Zidarn M, et al. Low sensitivity of commercially available rApi m 1 for diagnosis of honeybee venom allergy. J Allergy Clin Immunol. 2011;128:671–3. https://doi.org/10.1016/j.jaci.2011.03.012.
Arzt L, Bokanovic D, Schrautzer C, Schwarz I, Laipold K, Aberer W, et al. Questionable diagnostic benefit of the commercially available panel of bee venom components. Allergy. 2017;72:1419–22. https://doi.org/10.1111/all.13154.
Köhler J, Blank S, Müller S, Bantleon F, Frick M, Huss-Marp J, et al. Component resolution reveals additional major allergens in patients with honeybee venom allergy. J Allergy Clin Immunol. 2014;133:1383–9. https://doi.org/10.1016/j.jaci.2013.10.060.
Schiener M, Eberlein B, Moreno-Aguilar C, Pietsch G, Serrano P, McIntyre M, et al. Application of recombinant antigen 5 allergens from seven allergy-relevant hymenoptera species in diagnostics. Allergy. 2017;72:98–108. https://doi.org/10.1111/all.13000.
Hemmer W, Focke M, Kolarich D, Wilson IBH, Altmann F, Wöhrl S, et al. Antibody binding to venom carbohydrates is a frequent cause for double positivity to honeybee and yellow jacket venom in patients with stinging-insect allergy. J Allergy Clin Immunol. 2001;108:1045–52. https://doi.org/10.1067/mai.2001.120013.
Šelb J, Stojković UB, Bajrović N, Kopač P, Eržen R, Zidarn M, et al. Limited ability of recombinant Hymenoptera venom allergens to resolve IgE double sensitization. J Allergy Clin Immunol Pract. 2018;6:2118–20. https://doi.org/10.1016/j.jaip.2018.04.045
Frick M, Müller S, Bantleon F, Huss-Marp J, Lidholm J, Spillner E, et al. RApi m 3 and rApi m 10 improve detection of honey bee sensitization in hymenoptera venom-allergic patients with double sensitization to honey bee and yellow jacket venom. Allergy. 2015;70:1665–8. https://doi.org/10.1111/all.12725.
Blank S, Neu C, Hasche D, Bantleon FI, Jakob T, Spillner E. Polistes species venom is devoid of carbohydrate-based cross-reactivity and allows interference-free diagnostics. J Allergy Clin Immunol. 2013;131:1239. https://doi.org/10.1016/j.jaci.2012.10.047
Perez-Riverol A, Miehe M, Jabs F, Seismman H, Romani Fernandes LG, de Lima Zollner R, et al. Venoms of neotropical wasps lack cross-reactive carbohydrate determinants enabling reliable protein-based specific IgE determination. J Allergy Clin Immunol. 2018;141(5):1917–1919.e1. https://doi.org/10.1016/j.jaci.2017.12.990.
Seismann H, Blank S, Braren I, Greunke K, Cifuentes L, Grunwald T, et al. Dissecting cross-reactivity in hymenoptera venom allergy by circumvention of α‑1,3‑core fucosylation. Mol Immunol. 2010;47:799–808. https://doi.org/10.1016/j.molimm.2009.10.005.
Skov LK, Seppälä U, Coen JJF, Crickmore N, King TP, Monsalve R, et al. Structure of recombinant Ves v 2 at 2.0Å resolution: structural analysis of an allergenic hyaluronidase from wasp venom. Acta Crystallogr D Biol Crystallogr. 2006; 62:595–604. https://doi.org/10.1107/S0907444906010687
Marković-Housley Z, Miglierini G, Soldatova L, Rizkallah PJ, Müller U, Schirmer T. Crystal structure of hyaluronidase, a major allergen of bee venom. Structure. 2000;8:1025–35. https://doi.org/10.1016/S0969-2126(00)00511-6.
Perez-Riverol A, Fernandes LGR, Musacchio Lasa A, Dos Santos-Pinto JRA, Moitinho Abram D, Izuka Moraes GH, et al. Phospholipase A1-based cross-reactivity among venoms of clinically relevant hymenoptera from neotropical and temperate regions. Mol Immunol. 2018;93:87–93. https://doi.org/10.1016/j.molimm.2017.11.007.
dos Santos LD, Santos KS, dos Santos Pinto JRA, Dias NB, de Souza BM, dos Santos MF, et al. Profiling the proteome of the venom from the social wasp polybia paulista: a clue to understand the envenoming mechanism. J Proteome Res. 2010;9:3867–77. https://doi.org/10.1021/pr1000829.
Sookrung N, Wong-Din-Dam S, Tungtrongchitr A, Reamtong O, Indrawattana N, Sakolvaree Y, et al. Proteome and allergenome of Asian wasp, vespa affinis, venom and IgE reactivity of the venom components. J Proteome Res. 2014;13:1336–44. https://doi.org/10.1021/pr4009139.
Blank S, Seismann H, Michel Y, McIntyre M, Cifuentes L, Braren I, et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy. 2011;66:1322–9. https://doi.org/10.1111/j.1398-9995.2011.02667.x.
Korošec P, Valenta R, Mittermann I, Čelesnik N, Šilar M, Zidarn M, et al. High sensitivity of CAP-FEIA rVes v 5 and rVes v 1 for diagnosis of vespula venom allergy. J Allergy Clin Immunol. 2012;129:1406–8. https://doi.org/10.1016/j.jaci.2011.12.975.
Ebo DG, Faber M, Sabato V, Leysen J, Bridts CH, De Clerck LS. Component-resolved diagnosis of wasp ( yellow jacket ) venom allergy. Clin Exp Allergy. 2012;43:255–61. https://doi.org/10.1111/cea.12057.
Cifuentes L, Vosseler S, Blank S, Seismann H, Pennino D, Darsow U, et al. Identification of hymenoptera venom-allergic patients with negative specific IgE to venom extract by using recombinant allergens. J Allergy Clin Immunol. 2014;133:909–10. https://doi.org/10.1016/j.jaci.2013.09.047.
Rafei-Shamsabadi D, Müller S, Pfützner W, Spillner E, Ruëff F, Jakob T. Recombinant allergens rarely allow identification of hymenoptera venom-allergic patients with negative specific IgE to whole venom preparations. J Allergy Clin Immunol. 2014;134:493–5. https://doi.org/10.1016/j.jaci.2014.05.035.
Jin C, Focke M, Léonard R, Jarisch R, Altmann F, Hemmer W. Reassessing the role of hyaluronidase in yellow jacket venom allergy. J Allergy Clin Immunol. 2010; 125:184–90. https://doi.org/10.1016/j.jaci.2009.08.037
Blank S, Seismann H, Mcintyre M, Ollert M, Wolf S, Bantleon FI, et al. Vitellogenins are new high molecular weight components and allergens ( Api m 12 and Ves v 6 ) of apis mellifera and vespula vulgaris venom. PLoS One. 2013;8(4):e62009. https://doi.org/10.1371/journal.pone.0062009.
Monsalve RI, Vega A, Marqués L, Miranda A, Fernández J, Soriano V, et al. Component-resolved diagnosis of vespid venom-allergic individuals: phospholipases and antigen 5s are necessary to identify vespula or polistes sensitization. Allergy. 2012;67:528–36. https://doi.org/10.1111/j.1398-9995.2011.02781.x.
Michel J, Brockow K, Darsow U, Ring J, Grunwald T, Blank S, et al. Added sensitivity of component-resolved diagnosis in hymenoptera venom-allergic patients with elevated serum tryptase and / or mastocytosis. Allergy. 2016;71:651–60. https://doi.org/10.1111/all.12850.
Turillazzi S, Turillazzi F. Climate changes and hymenoptera venom allergy: are there some connections? Curr Opin Allergy Clin Immunol. 2017;17:344–9. https://doi.org/10.1097/ACI.0000000000000388.
Höcherl N, Tauzt J. Nesting behavior of the paper wasp polistes dominula in central Europe—a flexible system for expanding into new areas. Ecosphere. 2015;6:1–11. https://doi.org/10.1890/ES15-00254.1.
Keeling MJ, Franklin DN, Datta S, Brown MA, Budge GE. Predicting the spread of the Asian hornet (vespa velutina) following its incursion into Great Britain. Sci Rep. 2017;7:1–7. https://doi.org/10.1038/s41598-017-06212-0.
Chugo S, Lizaso MT, Alvarez MJ, Arroabaren E, Lizarza S, Tabar AI. Vespa velutina nigritorax: a new causative agent in anaphylaxis. J Investig Allergol Clin Immunol. 2015;25:231–2. https://doi.org/10.1186/2045-7022-5-S3-P43
Antonicelli L, Bilò MB, Napoli G, Farabollini B, Bonifazi F. European hornet (vespa crabro) sting: a new risk factor for life-threatening reaction in hymenoptera allergic patients? Eur Ann Allergy Clin Immunol. 2003;35:199.
Steigelman DA, Freeman TM. Imported fire ant allergy: case presentation and review of incidence, prevalence, diagnosis, and current treatment. Ann Allergy Asthma Immunol. 2013;111:242–5. https://doi.org/10.1016/j.anai.2013.07.006.
Golden DBK. Advances in diagnosis and management of insect sting allergy. Ann Allergy Asthma Immunol. 2013;111:84–9. https://doi.org/10.1016/j.anai.2013.05.026.
Golden DBK. New directions in diagnostic evaluation of insect allergy. Curr Opin Allergy Clin Immunol. 2014;14:334–9. https://doi.org/10.1097/ACI.0000000000000072.
Perez-Riverol A, dos Santos-Pinto JRA, Lasa AM, Palma MS, Brochetto-Braga MR. Wasp venomic: unravelling the toxins arsenal of polybia paulista venom and its potential pharmaceutical applications. J Proteomics. 2017;161:88–103. https://doi.org/10.1016/j.jprot.2017.04.016.
Perez-Riverol A, Campos Pereira FD, Musacchio Lasa A, Romani Fernandes LG, dos Santos-Pinto JRA, Justo-Jacomini DL, et al. Molecular cloning, expression and IgE-immunoreactivity of phospholipase A1, a major allergen from polybia paulista (hymenoptera: vespidae) venom. Toxicon. 2016;124:44–52. https://doi.org/10.1016/j.toxicon.2016.11.006.
Bazon ML, Perez-Riverol A, Dos Santos-Pinto JRA, Fernandes LGR, Lasa AM, Justo-Jacomini DL, et al. Heterologous expression, purification and immunoreactivity of the antigen 5 from polybia paulista wasp venom. Toxins (Basel). 2017; https://doi.org/10.3390/toxins9090259.
Stoevesandt J, Sturm GJ, Bonadonna P, Oude Elberink JNG, Trautmann A. Risk factors and indicators of severe systemic insect sting reactions. Allergy. 2019; https://doi.org/10.1111/all.13945.
Blum S, Gunzinger A, Müller UR, Helbling A. Influence of total and specific IgE, serum tryptase, and age on severity of allergic reactions to hymenoptera stings. Allergy. 2011;66:222–8. https://doi.org/10.1111/j.1398-9995.2010.02470.x.
Vega-Castro A, Alonso-Llamazares A,Cárdenas R,Beitia JM, Mateo B, Alvarez-Twose I, et al. An increase in tryptase on the first day of Hymenoptera venom immunotherapy might be a predictor of future systemic reactions during treatment. J Investig Allergol Clin Immunol. 2018;28:305–11. https://doi.org/10.18176/jiaci.0258
Kucharewicz I, Bodzenta-Lukaszyk A, Szymanski W, Mroczko B, Szmitkowski M. Basal serum tryptase level correlates with severity of hymenoptera sting and age. J Investig Allergol Clin Immunol. 2007;17:65–9.
Ruëff F, Przybilla B, Biló MB, Müller U, Scheipl F, Aberer W, et al. Predictors of severe systemic anaphylactic reactions in patients with hymenoptera venom allergy: importance of baseline serum tryptase‑a study of the European academy of allergology and clinical immunology interest group on insect venom hypersensitivity. J Allergy Clin Immunol. 2009;124:1047–54. https://doi.org/10.1016/j.jaci.2009.08.027.
Sturm GJ, Heinemann A, Schuster C, Wiednig M, Groselj-Strele A, Sturm EM, et al. Influence of total IgE levels on the severity of sting reactions in hymenoptera venom allergy. Allergy. 2007;62:884–9. https://doi.org/10.1111/j.1398-9995.2007.01413.x.
Beck SC, Wilding T, Buka RJ, Baretto RL, Huissoon AP, Krishna MT. Biomarkers in human anaphylaxis: a critical appraisal of current evidence and perspectives. Front Immunol. 2019;10:494. https://doi.org/10.3389/fimmu.2019.00494.
Korosec P, Erzen R, Silar M, Bajrovic N, Kopac P, Kosnik M. Basophil responsiveness in patients with insect sting allergies and negative venom-specific immunoglobulin E and skin prick test results. Clin Exp Allergy. 2009; 39:1730–7. https://doi.org/10.1111/j.1365-2222.2009.03347.x
Korošec P, Šilar M, Eržen R, Čelesnik N, Bajrović N, Zidarn M, et al. Clinical routine utility of basophil activation testing for diagnosis of hymenoptera-allergic patients with emphasis on individuals with negative venom-specific IgE antibodies. Int Arch Allergy Immunol. 2013;161:363–8. https://doi.org/10.1159/000348500.
Balzer L, Pennino D, Blank S, Seismann H, Darsow U, Schnedler M, et al. Basophil activation test using recombinant allergens: highly specific diagnostic method complementing routine tests in wasp venom allergy. PLoS One. 2014;9(10):e108619. https://doi.org/10.1371/journal.pone.0108619.
Acknowledgements
The authors want to thank the financial support from the Sao Paulo Research Foundation (FAPESP) (Grant #2016/16212-5) and INCT/CNPq-iii. APR is a Post-Doctoral Fellow from FAPESP (Grant #2017/22405-3, #2018/24834-1). We acknowledge Prof. Dr. Alexis Musacchio Lasa for the collaboration in the structural modelling analyses. This work was partially presented by T. Jakob as a lecture in the EAACI Allergy School on Insect Venom Allergy and Mastocytosis held in Groningen, The Netherlands, April 11–13, 2019.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Jakob reports grants, personal fees and non-financial support from Novartis, personal fees and non-financial support from Thermo Fisher Scientific, grants and personal fees from ALK-Abello, personal fees from Celgene, personal fees and non-financial support from Bencard/Allergy Terapeutics, personal fees from Allergopharma. A. Perez-Riverol and M.S. Palma declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perez-Riverol, A., Palma, M.S. & Jakob, T. Current challenges in molecular diagnostics of insect venom allergy. Allergo J Int 29, 79–91 (2020). https://doi.org/10.1007/s40629-020-00119-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-020-00119-5