Abstract
Background
Allergy to ash pollen is common in some parts of Europe. Sensitization is overlooked if Oleaceae pollen allergens are not included in screening tests.
Methods
Between 1983 and 2007, sensitization to aeroallergens was systematically investigated using serological methods in 15-year-old school children (Immuno-CAP [carrier polymer] test). Samples from 1986 and 2006 were also tested using the immuno-solid-phase allergen chip (ISAC) assay. School children with sensitizations in 1986 were retested in 2010. Airborne pollen concentrations were determined by the Swiss pollen measuring network.
Results
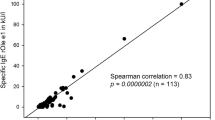
Sensitization (>0.7 kU/l) to ash pollen (Fraxinus americana t15)—16.3% (102/627)—was more frequent than to birch pollen (Betula verrucosa t3): 15.3% (96/627). ISAC assays performed in children in 1986 and 2006 revealed higher molecular seroprevalence for nOle e 1 (15%; 15/100) compared to rBet v 1 (12%; 12/100). Followed-up subjects (age, 39) showed an increase in sensitizations to ash pollen. IgE levels to pollen from indigenous ash (Fraxinus excelsior t25) were higher than to pollen from American ash (Fraxinus americana t15). Low ash pollen emission levels were recorded at all measuring sites in Switzerland every 2–4 years. The infection of ashes by Chalara fraxinea resulted in increased emission of ash pollen.
Conclusion
Symptoms in individuals sensitized to ash pollen vary according to the pollen count and may be masked by pollen from other trees that flower at the same time of year. Sensitization to ash/Ole e 1 can be higher than to birch/Bet v 1. The determination of IgE to common ash (Fraxinus excelsior) is more sensitive than to American ash (Fraxinus americana). Ash dieback due to Chalara appears to increase pollen emission. Allergies to ash pollen can be significantly underestimated due to a failure to (correctly) identify them; they can also be masked by other pollen families (birch). Harmful organisms such as Chalara can intensify pollen emissions at least temporarily.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aerobiology is the interdisciplinary study of airborne particles of biological origin, of their sources, emission, dispersion, and effects, particularly on human environmental health. This article is intended to illustrate interrelationships using the model “ash pollen allergy.” Harmful organisms and commercial interests have an impact on planting in urban areas. They interfere with indigenous plants, as well as geographical, climatological, and meteorological fundamentals.
In order to correctly diagnose an allergy, especially with regard to specific immunotherapy, a correlation between a patient’s symptoms and exposure, as well as qualitatively optimal identification of the corresponding sensitization, is crucial.
Fagaceae (beech family) in Europe
In Europe, pollen is the most frequent cause of symptoms of immunoglobulin E (IgE)-triggered sensitization to an aeroallergen, most commonly grass pollen, followed by tree pollen [1]. A variety of symptoms resulting from sensitization to the botanical order Fagales (Betulaceae: major pollen birch) have been best investigated. Allergic cross-reactions are common within the botanical family. This applies in particular to allergies to hazel, alder, and birch pollen.
In urban areas where people live and work, nonindigenous varieties and species of trees that also release pollen with a high risk for causing allergies are most commonly planted. Exposure to alder pollen begins in Central Europe with a cultivated neophyte, Alnus x spaethii (Spaeth’s alder), in December [2, 3]. This tree was planted in numerous towns due to its characteristics. The flowering period ends with Alnus viridis (green alder), which releases pollen in May and June. There is very little awareness of this symptomatically relevant exposure. Due to alpine pastures that are no longer cultivated, this shrub has doubled in terms of surface area within 70 years in some Alpine regions, where it causes landslides by supplanting the stabilizing vegetation [4]. Its pollen is relevant not only to hikers, but also to inhabitants of urban areas further away at the edge of the Alps. Horticultural companies recommend planting these green alders in northern Germany—even to stabilize slopes. Thus, allergen exposure to alder lasts 6 months in Central Europe and has been prolonged in an anthropogenic manner via the trade in plants, independent of climate change caused by anthropogenic effects.
Fagaceae allergens
Fagales pollen contains rBet v 1 as its “major molecular allergen,” with its various cross-reactions to other PR-10 proteins (pathogenesis related protein family 10). These are commonly found in foods (fruit, nuts) and frequently cause perioral contact urticaria in central Europe.
Oleaceae (the olive family) in Europe
Oleaceae are found worldwide and are rich in species. The olive tree is one of the oldest cultivated plants in the Mediterranean region. Pollen from cultivated olive trees plays an important role in terms of allergy in Southern Europe [5, 6], where they flower in May and June.
Ashes in the genus Fraxinus have been growing in Europe for centuries—from the Mediterranean to southern parts of Scandinavia. The common ash (Fraxinus excelsior) is the only, but very widespread, indigenous tree in this botanical group in Central Europe.
South of the Alps, Fraxinus ornus (manna ash) is also frequently found, cultivated in part to obtain the sweetening agent mannitol. Manna ashes were already grown over 100 years ago north of the Alps as ornamental trees (flowering ash; e.g., Sihlfeld Cemetery, Zurich, Switzerland). They bloom a few weeks later than Fraxinus excelsior. The Oleaceae family also includes a number of frequently grown ornamental shrubs, e.g., lilac, jasmine, and forsythia, which are pollinated primarily by insects. Ligustrum (privet tree, multiple varieties) causes pollen emissions in northern Spain. Both the shrubs and the trees are popular as fencing plants and flower in that region in June and July [6].
Ash pollen allergens
Oleaceae (olive family) pollen allergy is common. Allergy to ash pollen, for example, is well known in Switzerland [7,8,9,10,11]. In the past, epidemiological studies rarely measured sensitization to ash pollen. An allergen mix was often used for screening (Phadiatop, sx1, tx1–9), as well as for testing in practice. Only positive sera were tested further for the allergens they contained. These commonly used mixes did not contain any ash pollen allergens [9, 10]. It was not until the introduction of the tx10 test that Oleaceae-specific allergens (tx15) were tested. For this reason, etiology remained unexplained in patients with symptomatic allergy to ash pollen alone and monovalent sensitization to ash pollen.
Initially, only Fraxinus americana (t15) was commercially available for serological testing using the CAP assay, with the reagent Fraxinus excelsior (t25) being available only from 2006 onwards [8].
Still today, no molecular ash pollen allergens are available for commercial tests. The ISAC test determines olive allergens (rOle e 1, nOle e 7, and rOle e 9). It is simply noted that rOle e 1 is a marker for ash pollen sensitization.
Hymenoscyphus fraxineus (Hymenoscyphus pseudoalbidus, Chalara fraxinea, ash dieback) epidemic
In recent decades, a new fungal disease has spread across Europe and threatened the existence of the ashes (ash dieback). The disease, which was first observed in Poland 20 years ago, went on to spread across Europe at an alarming rate [12]. It was first discovered in Switzerland in 2008 in the Basel area. The disease has also been evident in Eastern Switzerland since 2011 (pollen measuring stations: Buchs [St. Gallen] and Münsterlingen). It reached Valais (Visp) in 2013 and Ticino (south of the Alps: Lugano, Locarno) in 2014 [13].
This epidemic is highly significant in terms of forestry. How ash dieback will evolve in Europe remains unclear. Both young and old ash trees belonging to the Fraxinus excelsior species are affected. The disease is easily identified in summer: young ash trees have wilted leaves, while older ashes have dead branches in their crown, as well as bushy shoots (Fig. 1).
The fungus Chalara fraxinea, or a genotype variant (Hymenoscyphus pseudoalbidus) of the pathogenic fungus (Hymenoscyphus albidus) indigenous to this part of this world, has been identified as the pathogen [12]. It is remarkable that only the sexually produced spores of imported genotypes of this inconspicuous ground fungus produce a phytoxin (viridol). Only these spores, which are airborne transmitted, are highly pathogenic for common ash trees (Fraxinus excelsior). Ashe trees from the Japan/Siberia region (Fraxinus mandshurica), where these pathogenic fungal variants are commonly found, are resistant [14]. It is assumed, therefore, that the epidemic started there. How ash dieback affects ash pollen immission levels is important from an allergy perspective.
The risk of disease in common ash (Fraxinus excelsior) is extremely high due its susceptibility to Hymenoscyphus fraxineus. Other species like Fraxinus mandshurica, Fraxinus americana, and Fraxinus ornus are at least more resistant. In line with this, genetic investigations (associative transcriptomics) revealed identical characteristics in species known to be resistant (Fraxinus americana, Fraxinus ornus), and now also to a certain extent in surviving resistant types of ash tree in Denmark (Fraxinus excelsior) [15]. These nonindigenous types from North America (Fraxinus americana, Fraxinus pennsylvanica) and Asia are likely to be preferred for planting in the future due to their resistance.
Methods
Seroepidemiology
Population (cross-sectional studies)
Between 1983 and 2007, all 2033 pupils in their eighth school year (age 15) in Grabs, Switzerland, were interviewed as part of the compulsory pupil health checks and serologically tested with the consent of their parents. From 1992–2000, these check-ups were integrated in the SCARPOL national project (Swiss Surveillance Program of Childhood Allergy and Respiratory Symptoms with respect to Air Pollution and Climate) [10].
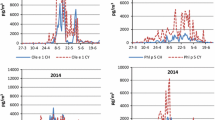
A total of 1951 school children (96%) completed a detailed questionnaire and 1535 (76%) agreed to have a blood sample taken. Every year, IgE antibodies to the following major allergens were determined using current methods (RAST/CAP): timothy grass (g6), birch (t3), house dust mite (d1), and cat (e1). In the studies on school children in Grabs, these four major allergens were continuously investigated independently of sx1 and supplemented by other allergens (Fig. 2). In 1993–1996, 1999, and 2003–2007, sensitizations to ash pollen (Fraxinus americana t15) were also determined. The test for Fraxinus excelsior (t25) only became available in 2006 [8].
Trend in seroprevalence mapped on the basis of school children in Grabs, Switzerland, between 1983 and 2007. RAST/CAP tests: IgE > 0.7 kU/l; ISAC > 3 ISU; sx1: at least one positive RAST/CAP or positive screening test; timothy grass: (g6); birch: (t3); house dust mite, Dermatophagoides pteronyssinus: (d1); ash, Fraxinus americana: (t15) (ISAC: > 3 ISU: at least one positive test, rBet v 1, major birch pollen allergen, rPhl p 1, major grass pollen allergen, nOle e 1, major allergen of Oleaceae pollen [olive tree, ash])
RAST/CAP class >2 (>0.7 kU/l) was used as the cut-off. All reagents were obtained from the same company (albeit with changing names: Pharmacia, Phadia, Thermo Fisher) and tested by the same laboratory technicians in the same laboratory. Several panels of each serum were frozen at minus 20 °C for additional microbial and allergological testing.
In 2009, 54 preserved sera from 1986 and 46 sera from 2006 were tested for specific IgE to 103 (at that time) molecular allergens using the ISAC test (Phadia). In accordance with the manufacturer, >3 ISU (ISAC standardized units) was used as the cut-off. The manufacturer uses exclusively olive tree allergens (nOle e 1 as a surrogate for nFra e 1) to determine sensitization to ash pollen. Thus, only the 2006 ISAC findings could be compared with the CAP test (t15) for ash pollen (Fig. 2).
Population (cohort 1986/2010)
In all, 12 former students who had reacted positively to one of the four major allergens in the 1986 CAP test were invited to repeat serology testing in 2010 using the ISAC test (cohort study). Only one of these students declined serological follow-up. No person received immunotherapy. One person was examined incidentally at the same time, since one of her children suffered from rhinoconjunctivitis. She herself had exhibited sensitization only to plane pollen (Pla a 2) in the ISAC test in 1996. The students were followed-up for Fraxinus excelsior (t25), and seven additionally for Fraxinus americana (t15).
Pollen data
Since 1969, airborne pollen immission levels in Switzerland have increasingly been measured in a systematic and standardized manner at a variety of locations (Fig. 3). Airborne particles were collected volumetrically (10 l/min) using a Hirst pollen trap and the pollen types identified and counted microscopically in a laboratory. The results were reported as daily average concentrations (pollen/m3 air). The annual pollen integral (APIn) is the sum of these daily values over a calendar year (previously named pollen index).
Pollen counts were started in Buchs (St. Gallen)/Grabs on 1 April 1984 for the seroepidemiological studies mentioned above. The measuring station is located on the roof of the Interstate University of Applied Sciences and Technology (NTB), 12 m above ground level and 445 m above sea level, in the middle of the north–south Rhine valley. It was never moved and always evaluated using the same method (equipment, counting).
Results
Serology
Cross-sectional studies
A total of 627 pupils from the 1993–2007 study were additionally tested for ash pollen (Fraxinus americana t15) from 1993 onwards. Of these, 102 (16.3%) showed specific IgE (>0.7 kU/l). In comparison, only 96 (15.3%) reacted to birch pollen (t3). The sensitization trend over the period 1983–2007 is shown as percentages in Fig. 2.
Of the 100 school children in the years 1986 and 2006 who were investigated for molecular allergens, 15% tested positive to nOle e 1 (common olive group 1), 9% to nOle e 2 (profilin), 12% to birch rBet v 1, 9% to rBet v 2, as well as 21% to rPhl p 1 and 9% to rPhl p 5.
Cohort
In 2010, sensitizations to Fraxinus were also measured: seven subjects tested positive to nOle e 1: four with high titers (>1.4 ISU) in both study years, while three first showed sensitization in 2010 (3.9; 1; 0.4 ISU). Three had values below the cut-off (0.02–0.3 kU/l). Four subjects reacted to nOle e 2 (profilin) in 1986 (15.42–0.39 ISU). Only the subject with the highest titer still had a titer of 1.6 ISU in 2010 (not shown in detail here).
In the CAP test, 5 students had positive values to Fraxinus excelsior (t25) (>0.7 kU/l) of between 0.97 and 13.9 kU/l and four had values below the cut-off of 0.35 kU/l (0.04–0.23).
Seven of these individuals were investigated with CAP tests for both ash allergens (tx15 and tx25). All seven reacted more weakly to Fraxinus americana (tx15), some with marked differences. Three individuals had t25 values above the cut-off of 0.7 kU/l and t15 values below this level: thus, a positive CAP test with Fraxinus excelsior allergen and a negative test with Fraxinus americana (details not shown here).
Ash pollen (Immission levels)
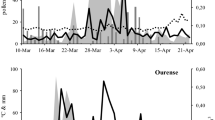
Fig. 3 shows the APIn of ash pollen at all sites in Switzerland for the years 1993–2018. The measurements reveal extremely large annual fluctuations in APIn. There are years with virtually no measureable pollen level, followed by 4 years with some extremely high pollen levels. It is not possible to detect any regularity in pollen levels either in general or for individual measuring stations.
The years with low levels have been occurring at all locations in Switzerland in a coordinated manner for 20 years, whereas there are significant regional fluctuations for years with high levels.
An APIn level of 10,568 was measured in Basel in 2013—not until 5 years after “ash dieback” was first recorded. However, even higher levels were measured as early on as 2006 (10,880) and 2003 (12,632). At a level of 17,320, the highest pollen level (APIn) was measured in Buchs (St. Gallen) in 2011, 1 year after an above-average high level had already been recorded. The highest levels were measured in Geneva and Lausanne in 2013 and 2019, in Visp in 2015 (canton in Valais), and in Lugano and Locarno (Ticino) in 2017. No ash trees grow in Davos (1600 m above sea level). Nevertheless, pollen is carried upwards from lower areas by the wind.
The highest APIn (15,641) in 2018 was recorded in Geneva, followed by Lausanne (14,914), and less pronounced levels in Zurich (10,122), Basel (9990), Bern (9652), Neuchâtel (8816), La Chaux-de-Fonds (8496), and Münsterlingen (7111). The decrease in Ticino is striking: Lugano (6489), Locarno (5777), Lucerne (6461), Buchs (St. Gallen) (5372), Visp (5147), and Davos (289).
The highest ash pollen levels were usually seen at the respective measuring stations 1–2 years following the detection of ash dieback in the region [13].
Discussion
Frequency of ash pollen sensitization
The data from this long-term seroepidemiological study in adolescent school children investigated in a nonselective manner reveal the high rate of seroprevalence to ash pollen. As mentioned above, this was often not recorded in epidemiological studies. The frequency of sensitizations to ash pollen is higher with tx15, measured in school children in Grabs (16%), than to birch pollen (15%). More subjects in the cohort reacted to Fraxinus excelsior (t25) in the CAP test than to Fraxinus americana (t15). Therefore, the rate of seroprevalence would have been even higher in the cross-sectional studies in 1983–2007 if allergens from indigenous ash (t25) had been used.
Different allergens
The number of school-age individuals in Grabs is too low to obtain frequency data on subgroups with sensitization to ash pollen. It is nevertheless surprising that, even in this small study, one can discern different sensitization patterns.
Cohort studies in nonselectively investigated school children, including spontaneous follow-up all the way into late adulthood, are rare. This small comparable group (n = 12) followed for over 24 years revealed that the majority have kept the same sensitization pattern (pollen or house dust mites, animal danders) from the time prior to adolescence. This even applies to the subgroup with sensitization to ash pollen. However, one of 12 individuals clearly showed new sensitization to the major allergen nOle e 1.
IgE antibodies to a sum of allergens from the corresponding pollen were investigated in the CAP tests. The molecular structures of some of these ash and olive allergens are known. Determination of rOle e 1, the major allergen in Oleaceae pollen, has become established for the molecular diagnosis of sensitization to ash pollen. Strong cross-reactions between this allergen from ash pollen (nFra e 1) and nOle e 1 from olive pollen have been described [16]. For this reason, they are used as a surrogate for sensitizations to ash pollen.
It remains unclear to what extent the use of olive allergens alone (investigated here: rOle e 1) is relevant in the molecular diagnosis of all sensitizations to ash pollen, even though the molecular similarity (ash: rFra e 1) is extremely strong. After all, CAP tests, which contain numerous molecular allergens, show clear differences. However, there are no recombinant molecular ash pollen allergens available for serology. The differing sensitization pattern to pollen allergens from Fraxinus americana (t15) and Fraxinus excelsior (t25) seen in school children in Grabs demonstrates that different varieties can cause different relevant sensitizations.
As with birch pollen, ash pollen also contains numerous accompanying or minor allergens, such as polcalcins (Fra e 3) and profilins (Fra e 2), which are widespread in nature and also share high molecular similarity. Like the profilins and polcalcins in birch pollen, they are probably of less relevance in terms of respiratory symptoms.
However, from a scientific point of view, it is problematic to use individual molecular allergens as surrogates for sensitization to one pollen species. This also applies to rBet v 1. Although this molecule is important when assessing the efficacy of hyposensitization, it does not exclude sensitization or allergy due to other allergens from these pollen—and even less so sensitization due to all other Fagales pollen.
Accordingly, each molecular selection worsens the sensitivity of a sensitization.
Immunotherapy
The symptoms to ash pollen can be effectively treated with hyposensitization, much like those to Fagales pollen (major allergen, rBet v 1). Patients sensitized to birch and ash pollen often respond less well to hyposensitization with birch pollen alone [8]. The symptoms to ash pollen become unmasked, given that the simultaneous relevance of ash pollen was previously not taken into account due to the symptoms caused by Fagales pollen. Time-consuming and costly hyposensitization is then inadequately effective not for immunological reasons, but due to the insufficient diagnostic work-up or allergen selection, seeing as extracts without ash pollen allergens were used. As such, the recommendation in the current European guideline [17] not to use or mix these allergens is incorrect and requires revision in clinical routine. Mixed extracts of relevant pollens are difficult to obtain commercially today (e.g., birch–ash).
The sensitization pattern according to molecular allergens is important in patients from a migrant background. Many children in Spain suffer from a relevant allergy to grass and olive pollen [18]. In Switzerland, a large number of children are polysensitized to grass, birch, and ash pollen.
Although ash pollen is wind-pollinated and ash flowers do not produce any nectar, bees also collect wind pollinated pollen, especially from ash. Among the Oleaceae, pollen from Fraxinus ornus (manna ash) is popular [19]. Depending on the time of year and the location of a beehive, the content of this pollen in honey changes. Peroral immunotherapy uses aeroallergens as an active substance. Local honey is considered an effective phytotherapeutic agent in aeroallergen allergies. The relevance of these aeroallergens in foods in terms of sensitization, adaptation, or pathogenesis in other diseases is unclear, e.g., in eosinophilic esophagitis [20].
The role of the “major and minor allergens,” e.g., IgE to profilins, polcalcins, and in particular nonspecific lipid transfer proteins (nsLTPs) in most tree pollen (Fagales, Oleaceae, Platanaceae, Ailanthus, Castanea, etc.) with the cross-reactive food allergens is poorly investigated.
Exposure (ash pollen level)
Clinical relevance
Individuals with ash pollen allergy are often symptom-free in years with low levels. A comparison of their symptoms between years with a low ash pollen count and mast years makes it possible to assess the relevance of ash sensitization, much like a pollen exposure chamber. It is very difficult to judge in polysensitized individuals which pollen levels cause which symptoms, since the most important trees in terms of allergy—birch and ash—bloom almost simultaneously in Switzerland: the ashes usually only for a few days, but sometimes up to 2 weeks before or after birch trees [21]. Therefore, it is virtually impossible to determine pollen thresholds responsible for symtoms in polysensitized individuals (two concomitant allergies). The same problem arises as a result of the simultaneous blooming of olive and grass in Spain [6, 18].
Since annual fluctuations in ash pollen levels are considerable yet unpredictable, standardized pollen data over decades are needed (Fig. 3). Only in this way is it possible to assess secondary phenomena, in particular the effects of climate conditions and harmful organisms, such as the epidemic caused here by an imported mutant fungus Hymenoscyphus fraxineus.
Ashes were problematic in the forest 20 years ago due to their rapid propagation and also due to storm damage; they were considered the “weed of the forest.” Back then, an “ashification” of the forest was feared [22]. The mast years 2003, 2005, 2007, 2009, 2013, 2015, and 2018, which were the same at all measuring stations, likely correspond to the natural rhythm of these trees. The fungus was first recorded in 2008 in Basel, along the Jura, as well as in Zurich. The published forestry data are often subject to regional variation. Damaged ashes were registered in central Switzerland and in particular eastern Switzerland in 2010, in western Switzerland in 2011, in Valais in 2013, and in Ticino in 2015, at first primarily in the Maggia Valley (north of Locarno) and later in southern Ticino (Lugano). This means that the disparate changes in regional peak levels of ash pollen (APIn) occurred approximately 2 years after the tree disease was recorded in forestry data. This paradoxical peak pollen production was identified in Buchs in 2011. Effects of this kind (surges in stress) are known to occur in the case of pollutant exposure in the form of excessive seed and cone production, as seen in forest degradation of the 1980s (forest dieback).
Knowledge of the remarkably synchronous inactive periods every 2–3 years in which ash trees release very little pollen (Fig. 3) are important when assessing clinical relevance.
Thus, the lack of pollen production (emission) in 2014 and 2016 was not an effect of ash dieback, but evidently a natural inactive period.
A large number of sick ash trees were felled in the winter of 2017/2018. In 2018, ash trees at all measuring stations in Switzerland (with the exception of Ticino) once again produced more pollen, despite the epidemic caused by Hymenoscyphus. This is a further indication that not all indigenous ashes (Fraxinus excelsior) simply “die off,” but are also able to recover and adapt (Fig. 1). According to the rhythm of mast years hitherto, an inactive phase would have been more likely. In fact, however, pollen levels in west Switzerland and urban centers were remarkably high in 2018, with a moderate rise in east Switzerland and rural regions. In Ticino, on the other hand, both measuring sites recorded a decline. This trend is difficult to interpret and, as a result, prognostic calculations are becoming ever more speculative. In contrast to the Fagales, the ash influorescences are virtually impossible to assess phenologically prior to flowering.
APIn values above 10,000, as shown in Fig. 3, are extremely high by international standards and have been exceeded at various measuring sites since 2003. In the past, this applied only to 1991 and 1992. Models that predict annual ash pollen levels on the basis of meteorological data [23, 24] are of secondary clinical relevance, since mast years cannot be predicted. Nevertheless, optimal retrospective measurement data are important when assessing an indication for or efficacy of hyposensitization. This information should be made available at the end of a season via open access as meteorological data.
Ash trees in urban areas
Trees are generally extremely important for the health of urban populations, and they improve the climate in urban areas considerably. On the other hand, they cost the taxpayer a lot of money. Due to dense construction, private gardens are becoming smaller and room for large trees is lacking. Local environmental conditions significantly limit the survival time of urban trees.
When planting the ideal tree along an urban road, not only does one need to consider species-specific susceptibility to genetically altered harmful organisms, but also dozens of other mainly site-specific criteria, such as quantitative resistance to frost, road salt, heat, water shortage, etc. Therefore, all nurseries publish data on the suitability of their trees to these conditions. Irrigation systems, fertilizers, protective coatings against heat and parasites, as well as pruning are not feasible for forest trees. Trees in urban areas are less able to withstand storm damage. Ultimately, the survival of an individual tree depends on the sum of these conditions where it is located. Resistant varieties are also important for forestry nurseries [24]. The same applies to specific forest uses (protective forests).
When selecting a tree, landscape architects are concerned with practical and aesthetic criteria, such as shape, color, and leaf color in autumn, rather than with flowers and fruits. An essential aspect is price, which varies depending on the species, variety, and height of the tree. The geographic origin of a tree (the microbiome of the soil with which the tree is delivered) is barely taken into account. In terms of actual planting, a new plant is cultivated nowadays from seeds of international provenance; this plant is raised from cuttings, transported to climatologically and economically favorable regions for faster rearing, transported back, and sometimes “stored temporarily” at a regional nursery. The roots are packed in jute and transported on palettes made from international wood. What remains completely unclear is which microbiomes and insects with known and unknown harmful organisms are transposed in this way and planted in urban areas.
Emissions of molecular allergens from these plants represent the essential criterion of quality for the health of allergy sufferers. The example of Spaeth’s alder (Alnus x spaethii) [2] very clearly illustrates the relevance of these interdisciplinary networks. The small cross-sectional study on 100 school children in Grabs at a 20-year interval showed a marked increase in sensitization to the molecular PR-10 allergens, in particular the alders (rAln g 1). The search for a new allergen source revealed the extremely high pollen count along the regional promenade between Christmas and New Year due to Spaeth’s alders, 1–2 months before the release of pollen from indigenous alders (Alnus glutinosa, Alnus incana). These trees are aesthetically pleasing, but did not even survive 20 years. They were cultivated and distributed exclusively. They were felled due to the shade they created after growing too rapidly and profusely—allergies were merely a secondary argument. As a result, they are no longer planted in Zurich and Bern, in contrast to Germany.
From an allergy perspective, the damage potential of these ashes is also problematic, since the investigated school children also reacted to the cross-reacting PR-10 allergen of apple (rMal d 1) [26]. This dents confidence in recommendations for a healthy diet (fruit and vegetables). Targeted selection of more resistant and better sustainable plants and livestock in agriculture is essential for food production.
Using the same methods to also ascertain the allergen content of different pollen and fruits would represent the basis of primary allergy prophylaxis.
Allergen exposure in urban areas is of great importance from an epidemiological point of view, but extremely difficult to measure. Tree registers only record public spaces in larger urban areas, and no shrubs. It is impossible to determine how often Alnus viridis or Ligustrum vulgare and other varieties [6] are planted as hedges or ornamental shrubs. Likewise, these anthropogenically planted trees in urban areas cannot be recorded using individual, distant pollen measuring sites [3]. It is virtually impossible to reliably determine individual trees using current remote sensing data. Individual roads are not always planted with the same tree species, which makes sense. The diversity of species used in urban development, with different varieties of organisms, both indigenous as well as new varieties and neophytes, hinders a standardized flowering forecast. This makes it all the more important for patients to assume responsibility for themselves. They should be able to recognize trees and shrubs in a natural habitat and particularly in their environment. “Pollen Apps” could be helpful here; however, these are no substitute for—and could possibly even hinder—a person’s own observations.
In the case of the ashes, it is important to have more knowledge on the molecular allergen content in the pollen of the different species and genotypes (organisms: subgroup of the same taxonomic unit). Variety-specific differences have also been described in olive pollen [25]. From an allergy perspective, it is not advisable to plant nonindigenous ashes, the allergy potential of which is wholly unclear, in urban areas. It stands to reason that the suffering of patients due to allergy to allergens common to ash and Oleaceae pollen is prolonged when late-flowering species are planted in urban areas. The choice of species or organisms in the context of town planning is even more important in the case of alders (Fagales). From a health policy perspective, it is counterproductive to plant allergens that could be avoided in a selection.
Farm children suffer from allergies less frequently, even though they are the group most exposed to the commonest allergens, e.g., grass pollen. The same is likely to also apply to indigenous tree pollen. They are also more exposed to photochemical oxidants (predominantly composed of ozone) [9, 25]. Children in urban centers grow up under increasing exposure to particulate and chemical industrial pollutants. Their immunological response is different [27]. Due to anthropogenic effects, they are also exposed for longer and more intensively to new allergens from tree pollen.
On the other hand, the risk of allergy must not limit the planting of trees in urban areas in general. Therefore, when selecting trees, it is important to put the risk/benefit ratio into perspective based on the molecular aspects of relevant allergens and to adapt to local conditions. As such, molecular allergens should be accepted as a quality criterion in tree cultivation and trade.
Migration
Due to migration and growing mobility, the spectrum of molecular sensitization is becoming ever more important to patients with Oleaceae pollen allergy. Many people travel for business or spend their holidays in Mediterranean countries; others seek and find work in the region. In northern Spain, Fraxinus excelsior flowers as early on as January [6], whereas other widespread Oleaceae, such as manna ash (Fraxinus ornus) and the olive trees, flower weeks to months later, at the same time as grasses. Patients that have become monovalently sensitized to ash report symptoms in that region in May and June. This creates an unexpected paradoxical situation in terms of time, since relevant allergen exposure in the south occurs later [5].
Conclusions
Some allergens cause symptoms of the respiratory tract as well as symptoms of the gastrointestinal tract and skin via cross-reactions with fruits—even anaphylaxis, particularly in the case of simultaneous exertion. Therefore, not only the concept of “united airways,” but also of “united surfaces” is apt to describe the adaptation of allergy patients to their environment.
The spread of molecular allergens represents a quality characteristic for human life. They cause numerous diseases and, hence, considerable costs for health systems. This should be taken into account not only when selecting plants (trees and shrubs) for urban areas, but also fruits, if only due to pollen sensitizations.
Allergies to Oleaceae are common. When investigating pollen allergies, correct testing for Oleaceae, in this case the ashes, is important in terms of diagnosis and treatment (hyposensitization).
Knowledge of molecular cross-reactions (in this case ashes–olives) is important for migrants and travelers.
Research into allergies should not be promoted merely on the basis of algorithmic criteria. Interdisciplinary (interfaculty) networks are becoming ever more important.
Abbreviations
- APIn:
-
Annual pollen integral (annual sum of daily pollen average concentrations)
- CAP:
-
ImmunoCAP Carrier polymer (test)
- IgE:
-
Immunoglobulin E
- ISAC:
-
Immuno-solid-phase allergen chip
- nsLTP:
-
Nonspecific lipid transfer protein
- PR-10:
-
Pathogenesis-related protein family 10
- RAST:
-
Radioallergosorbent test
- SCARPOL:
-
Swiss Surveillance Program of Childhood Allergy and Respiratory Symptoms with respect to Air Pollution and Climate
- kU:
-
Kilounit
References
D’Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, et al. Allergenic pollen and pollen allergy in Europe. Arerugi. 2007;62:976–90.
Gassner M, Gehrig R, Schmid-Grendelmeier P. Hay fever as a christmas gift. N Engl J Med. 2013;368:393–4.
Gehrig R, Gassner M, Schmid-Grendelmeier P. Alnus x spaethii pollen can cause allergies already at Christmas. Aerobiologia. 2015;31:239–47.
Huber B, Frehner M. Die Entwicklung der Grünerlenbestände in der Ostschweiz: Die Grünerle breitet sich aus. WaldHolz. 2013;94:47–50.
Feliziani V. Pollini di interesse allergologico. Guida al loro riconoscimento. Italia: Milano: Ed. Masson; 1986.
Vara A, Fernández-Gonzáles M, Aira MJ, Rodríguez-Rajo FJ. Oleaceae cross-reactions as potential pollinosis cause in urban areas. Sci Total Environ. 2016;524(Pt A):435–40.
Schmid-Grendelmeier P, Peeters AG, Wahl R, Wüthrich B. Zur Bedeutung der Eschenpollenallergie. Allergologie. 1994;11:535–42.
Wüthrich B. Esche ist nicht Esche. Allergologie. 2006;29:231–5.
Gassner-Bachmann M, Wüthrich B. Bauernkinder leiden selten an Heuschnupfen und Asthma. Dtsch Med Wochenschr. 2000;125:924–31.
Braun-Fahrländer C, Gassner M, Grize L, Takken-Sahli K, Neu U, Stricker T, et al. Swiss Study on Childhood Allergy an Respiratory symptoms; Air Pollution (SCARPOL team). No further increase in asthma, hay fever and atopic sensitisation in adolescents living in Switzerland. Eur Respir J. 2004;23:407–13.
Wüthrich B, Schmid-Grendelmeier P, Schindler C, Imboden M, Bircher A, Zemp E, et al. Prevalence of atopy and respiratory allergic diseases in elderly SAPALDIA population. Int Arch Allergy Immunol. 2013;162:143–8.
Gross A, Holdenrieder O, Pautasso M, Queloz V, Sieber TN. Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol Plant Pathol. 2014;15:5–21.
Engesser R, Meier F. Eschenwelke wird bedrohlicher. Aktuelle Verbreitung und neuer Infektionsweg. WaldHolz. 2012;12:35–9.
Cleary MR, Andersson PF, Broberg A, Elfstrand M, Daniel G, Stenlid J. Genotypes of Fraxinus excelsior with different susceptibility to the ash dieback pathogen Hymenoscyphus pseudoalbidus and their response to the phytotoxin viridol—a metabolomic and microscopic study. Phytochemistry. 2014;102:115–25.
Harper AL, McKinney LV, Nielsen LR, Havlickova L, Li Y, Trick M, et al. Molecular markers for tolerance of European ash (Fraxinus excelsior) to dieback disease identified using Associative Transcriptomics. Sci Rep. 2016;6:19335.
Imhof K, Probst E, Seifert B, Regenass S, Schmid-Grendelmeier P. Ash pollen allergy: reliable detection of sensitization on the basis of IgE to Ole e 1. Allergo J Int. 2014;23:78–83.
Pfaar O, Bachert C, Bufe A, Buhl R, Ebner C, Eng P, et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23:282–319.
Martínez-Cañavate Burgos A, Torres-Borrego J, Molina Terán AB, Corzo JL, García BE, Rodríguez Pacheco R, et al. Molecular sensitization patterns and influence of molecular diagnosis in immunotherapy prescription in children sensitized to both grass and olive pollen. Pediatr Allergy Immunol. 2018;29:369–74.
Bucher E, Kofler V, Vorwohl G, Zieger E. Das Pollenbild der Südtiroler Honige. Bozen: Biologisches Labor der Landesagentur für Umwelt und Artenschutz; 2004.
Simon D, Straumann A, Dahinden C, Simon HU. Frequent sensitization to Candida albicans and profilins in adult eosinophilic esophagitis. Allergy. 2013;68:945–8.
Peeters AG. Frost periods and beginning of the ash (Fraxinus excelsior L.) pollen season in Basel (Switzerland). Aerobiologia. 2000;16:353.
Kubik-Komar A, Piotrowska-Weryszko K, Weryszko-Chmielewska E, Kaszewski BM. Analysis of Fraxinus pollen seasoons and forecast models based on meteorological factors. Ann Agric Environ Med. 2018;25:285–91.
Vara A, Fernández-Gonzáles M, Aira MJ, Rodríguez-Rajo FJ. Fraxinus pollen and allergen concentrations in Ourense (South-western Europe). Environ Res. 2016;147:241–8.
Heinze B, Tiefenbacher H, Litschauer R, Kirisits T. Ash dieback in Austria—history, current situation and outlook. In: Vasaitis R, Enderle R, editors. Dieback of European Ash (Fraxinus spp.)—Consequeces and Guidlines of sustainable management. Uppsala: SLU Service/Repro; 2017. pp. 33–52.
Conde Hernández J, Conde Hernández P, Gónzales Quevedo Tejerína MT, Conde Alcañiz MA, Conde Alcañiz EM, Crespo Moreira P, et al. Antigenic and allergenic differences between 16 different cultivars of Olea europaea. Arerugi. 2002;57(Suppl 71):60–5.
Farmers GM, Environment T. Protective influences of the farming environment against the development of allergies. In: Bergmann KC, Ring J, editors. History of allergy. Chem Immunol allergy. Basel: Karger; 2014. pp. 278–86.
Martikainen MV, Rönkkö TJ, Schaub B, Täubel M, Gu C, Wong GW, et al. Integrating farm and air pollution studies in search for immunoregulatory mechanisms operating in protective and high-risk environments. Pediatr Allergy Immunol. 2018;29:815–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Gassner, P. Schmid-Grendelmeier and B. Clot declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gassner, M., Schmid-Grendelmeier, P. & Clot, B. Ash pollen allergy and aerobiology. Allergo J Int 28, 289–298 (2019). https://doi.org/10.1007/s40629-019-00105-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-019-00105-6