Abstract
Background
The pharmacological management of hyperkalemia traditionally considered calcium or sodium polystyrene sulfonate and, since recently, the novel binders patiromer and sodium zirconium cyclosilicate. We evaluated their patterns of use, duration of treatment and relative effectiveness/safety in Swedish routine care.
Methods
Observational study of adults initiating therapy with sodium polystyrene sulfonate or a novel binder (sodium zirconium cyclosilicate or patiromer) in Stockholm 2019–2021. We quantified treatment duration by repeated dispensations, compared mean achieved potassium concentration within 60 days, and potential adverse events between treatments.
Results
A total of 1879 adults started treatment with sodium polystyrene sulfonate, and 147 with novel binders (n = 41 patiromer and n = 106 sodium zirconium cyclosilicate). Potassium at baseline for all treatments was 5.7 mmol/L. Sodium polystyrene sulfonate patients stayed on treatment a mean of 61 days (14% filled ≥3 consecutive prescriptions) compared to 109 days on treatment (49% filled ≥3 prescriptions) for novel binders. After 15 days of treatment, potassium similarly decreased to 4.6 (SD 0.6) and 4.8 (SD 0.6) mmol/L in the sodium polystyrene sulfonate and novel binder groups, respectively, and was maintained over the 60 days post-treatment. In multivariable regression, the odds ratio for novel binders (vs sodium polystyrene sulfonate) in reaching potassium ≤ 5.0 mmol/L after 15 days was 0.65 (95% CI 0.38–1.10) and after 60 days 0.89 (95% CI 0.45–1.76). Hypocalcemia, hypokalemia, and initiation of anti-diarrheal/constipation medications were the most-commonly detected adverse events. In multivariable analyses, the OR for these events did not differ between groups.
Conclusion
We observed similar short-term effectiveness and safety for all potassium binders. However, treatment duration was longer for novel binders than for sodium polystyrene sulfonate.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperkalemia is a common and potentially fatal electrolyte abnormality attributed to excess dietary potassium intake, comorbidity burden, metabolic disorders, or intake of medications that interfere with potassium excretion [1, 2]. These conditions often coincide in people with chronic kidney disease (CKD), compounding the risk of hyperkalemia.
Sodium and calcium polystyrene sulfonate have been available for 70 years for the management of hyperkalemia. Recently, two additional novel cation exchangers, patiromer sorbitex calcium (patiromer) and sodium zirconium cyclosilicate, received regulatory approval. All agents have shown short- and mid-term efficacy in clinical trials treating acute hyperkalemia against the non-pharmacological standard-of-care [3,4,5,6,7,8,9]. No trial to date has performed head-to-head comparisons of all three agents. However, a small randomized cross-over trial in patients on dialysis recently suggested sodium polystyrene sulfonate to be more effective than patiromer in reducing mean weekly potassium [10]. In their pivotal trials, all agents consistently reported the occurrence of minor gastrointestinal disorders and low electrolyte levels [3,4,5,6,7,8,9, 11]. A network meta-analysis of these trials[12] suggested a similar rate of minor adverse effects across all three agents and a higher probability of nausea and constipation among patiromer users compared to users of sodium zirconium cyclosilicate or sodium polystyrene sulfonate.
Clinical trials are carefully conducted experiments with strict monitoring routines in highly selected patients. Phase IV post-marketing studies are needed to confirm safety and efficacy in the heterogeneous clinical practice, where therapies are given to patients with higher levels of comorbidity and where there is usually greater variability in clinical monitoring or compliance with therapy. Some observational studies have been published to date, evaluating the introduction of single potassium binders.[13,14,15,16,17,18,19,20,21]. Currently, no routine-care studies have compared all three agents.
Healthcare workers in Sweden have prescribed sodium polystyrene sulfonate routinely for decades; sodium zirconium cyclosilicate and patiromer became available in Sweden in late 2018. In this study, we describe the treatment practice of potassium binders after the introduction of the novel binders and compare the use, safety, and effectiveness of all three agents in contemporary routine care in a single-provider health-care system in Stockholm.
Materials and methods
Data sources
The study population is derived from the Stockholm CREAtinine Measurements (SCREAM) project, a health care utilization cohort that includes all citizens of the region of Stockholm, Sweden [22]. Laboratory data were linked with regional and national administrative databases for complete information on healthcare utilization, diagnoses and procedures, dispensed drugs, and follow-up. The Stockholm Ethics Review Board approved the study with a waiver of consent.
Study population
For this study, we included all adults (> 18 years) who newly initiated sodium polystyrene sulfonate, sodium zirconium cyclosilicate, or patiromer during 2019–2021. New initiations were defined as a pharmacy dispensation with at least 6 preceding months without any other recorded potassium binder use. The date of the first dispensation was the index date (baseline), at which point all covariates were assessed, and follow-up started. We followed patients for up to 365 days or until death, emigration from the region, or end of data collection, whichever occurred first.
Study exposure
The study exposures were the different potassium binders started, identified by filled dispensations in Swedish pharmacies with the following ATC codes: V03AE01 (for sodium polystyrene sulfonate), V03AE09 (patiromer), and V03AE10 (sodium zirconium cyclosilicate). Calcium polystyrene sulfonate is not commercialized in Sweden. The indication for sodium polystyrene sulfonate in Sweden is to treat hyperkalemia in subjects with CKD; however, there is a long-standing clinical practice of using low doses of sodium polystyrene sulfonate for hyperkalemia prevention in patients with a history of elevated potassium. [23] Both patiromer and sodium zirconium cyclosilicate are approved in Sweden for treating hyperkalemia in adults, but costs are subsidized by the Swedish Government only when prescribed to patients with heart failure or patients with CKD G3 to 5 for whom treatment with sodium polystyrene sulfonate is not appropriate/sufficient/tolerated. [24]
Study covariates
We calculated study covariates at the index date, extracting data on age, gender, laboratory tests, comorbidities, and medications. Comorbidities were defined by the presence of relevant diagnostic codes [International Classification of Diseases (ICD)] prior to index date (Supplementary Table 1). Medications were assumed concomitant if there was a pharmacy dispensation at the time of, or within, the previous 6 months from the index date (Supplementary Table 2). Out-patient plasma creatinine was used to estimate glomerular filtration rate (eGFR) with the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [25] without adjusting for race; Swedish law prohibits collection of race information. Chronic kidney disease was categorized into KDIGO categories [26] as follows: ≥ 60 ml/min/1.73 m2, 45–59 ml/min/1.73 m2, 30–44 ml/min/1.73 m2; 15–29 ml/min/1.73 m2, and < 15 ml/min/1.73 m2 including those undergoing kidney replacement therapy (KRT). Plasma and serum potassium levels from all types of care, in-patient and out-patient, were extracted during the 90 days before the index date to evaluate indications for therapy. The highest potassium within that period was categorized into four mutually exclusive groups: normokalemia (≤ 5.0 mmol/L), mild (5.1–5.49 mmol/L), moderate (5.5–5.9 mmol/L) and severe hyperkalemia (≥ 6.0 mmol/L).
Study outcomes
Persistence on therapy
The duration of treatment was ascertained during the 365 days after initiation by consecutive fills and the time (in days) between them. The expected duration of the fill (i.e., the number of days that one package of medication was expected to cover) was that stipulated in the product label, which tended to be around 30 days for all three agents. Discontinuation of therapy was defined by the absence of a new dispensation during additional 30 days after the end of the last estimated pill supply.
Achieved potassium concentrations
There is no consensus recommendation for the frequency of potassium monitoring [1, 27, 28], and in routine care, this varies by patient and practitioner. As in previous studies [14, 16], we evaluated mean achieved potassium levels, if measured, and within the 0–15 days, 16–30 days, 31–45 days, and 46–60 days after treatment initiation. For each period, we considered all patients with at least one potassium measurement. In our primary analyses, we modeled the potassium value closest to the end of each time interval. As a sensitivity analysis, we averaged all the potassium values per patient during each follow-up interval.
Evaluation of potential adverse events
Likewise, there is no consensus recommendation for the frequency of monitoring for adverse events, and in routine care this also varies. We explored the occurrence of adverse events potentially associated with potassium binder use, as reported in their product labels, within the first 90 days of therapy. These included (definitions detailed in Supplementary Table 3): severe adverse gastrointestinal events (intestinal ischemia or thrombosis, gastrointestinal ulcers, and perforation); minor adverse gastrointestinal events (de novo dispensation of laxatives or anti-diarrheal drugs among those free from those medications at baseline)[23]; abnormal plasma electrolyte levels: hypokalemia (potassium < 3.5 mmol/L), hypophosphatemia (phosphate < 0.81 mmol/L), hypocalcemia (total calcium < 2.15 mmol/L) and hypernatremia (sodium > 145 mmol/L). [29,30,31].
Statistical analysis
Values are expressed as mean and standard deviation (SD) for continuous variables with normal distribution, median (interquartile range, IQR) for non-normal distribution variables and percentage of total for categorical. We studied all novel potassium binders as a group: because of small sample sizes, we were not able to study individual novel potassium binders. We constructed survival curves using the Kaplan–Meier method to show persistence on therapy over time. We compared mean potassium concentration at the different time intervals against pre-treatment potassium with paired t tests or one-way ANOVA and evaluated the difference between proportions of patients with potassium > 5.0 and > 5.5 mmol/L with the Chi square test. Serum potassium variability over the first 60 days after treatment start was assessed as the coefficient of variation (CV; standard deviation/mean) of all available tests. We used logistic regression to calculate a) the odds ratio (OR) and 95% confidence interval (CI) of novel binders for achieving normokalemia (≤ 5.0 mmol/L) during treatment compared to sodium polystyrene sulfonate; and b) the OR of novel binders for the presence of potential adverse events at fixed time intervals compared to sodium polystyrene sulfonate. As a sensitivity analysis, we repeated analyses using the mean of all the potassium values per patient during each follow-up interval. All statistical analyses were conducted using R version 3.5.1 and STATA software (version 17.1; Stata Corp, College Station, TX).
Results
In the Stockholm region during 2019–2021, 2130 subjects initiated potassium binders. After excluding 83 patients younger than 18 years old and 21 who were not residents of Stockholm, the study population consisted of 2026 participants, 33% women, with a mean age of 68 (SD 16) years. Of those, 1879 initiated treatment with sodium polystyrene sulfonate, and 147 with novel binders (41 with patiromer and 106 with sodium zirconium cyclosilicate).
Baseline characteristics by treatment are presented in Table 1. New users of sodium polystyrene sulfonate had a mean age of 68 (SD 16) years, mean eGFR 34 (SD 20) ml/min/1.73 m2, and 32% were women. New users of novel binders had a mean age of 63 (SD 17) years, mean eGFR 36 (SD 21) ml/min/1.73 m2, and 44% were women. Hypertension n = 1768 (87%), diabetes n = 918 (45%), and heart failure n = 683 (34%) were the three most common comorbidities in both groups, with common use of angiotensin converting enzyme/angiotensin receptor blockers, beta-blockers and diuretics. A history of hyperkalemia or previous history of sodium polystyrene sulfonate use was more common among novel binder initiators. Patients starting patiromer were older and had a higher eGFR and a larger proportion of baseline heart failure than patients on sodium zirconium cyclosilicate. Median potassium concentration at the start of treatment was 5.7 mmol/L, similar across therapies (5.7 mmol/L in the sodium polystyrene sulfonate group and 5.8 mmol/L in the novel binder group). The last potassium value before treatment initiation was < 5.0 in 15% of patients starting sodium polystyrene sulfonate and 9% in patients starting novel binders.
Treatment duration was longer for novel binders than sodium polystyrene sulfonate (Fig. 1). Patients on sodium polystyrene sulfonate stayed on treatment for a mean of 61 days, and 14% filled three or more consecutive prescriptions suggesting chronic use. Patients on novel binders stayed on treatment for a mean of 109 days, and 49% filled three or more prescriptions.
Duration of continuous therapy after initiation of potassium binders up to 365 days after treatment initiation by binder type. Legend: Persistence on therapy was estimated based on repeated dispensations for each agent (see methods). P value denotes statistically significant difference in the persistence of SPS vs novel potassium binders, using the log-rank test
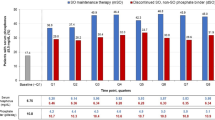
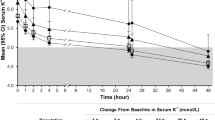
Within the first 15 days after dispensation, mean potassium decreased to 4.6 (SD 0.6) and 4.8 (SD 0.6) mmol/L in new users of sodium polystyrene sulfonate and novel binders, respectively, a level that was maintained during the following 60 days (Table 2 and Fig. 2, panels A and B). Figure 2, panels C and D depict the change in potassium concentration from baseline levels, observing similar average potassium reduction between treatments. There were no statistically significant differences in achieved potassium levels between treatments in multivariable logistic regression that adjusted for differences in patient characteristics between groups. The OR for novel potassium binders vs. sodium polystyrene sulfonate was 0.65 (95% CI 0.38–1.10) during the first 15 days of therapy and 0.89 (95% CI 0.45–1.76) after 60 days (Table 2). In unadjusted analysis, 76% (95% CI 73- 79) of new users of sodium polystyrene sulfonate and 67% (95% CI 56–77) of new users of novel binders reached a potassium value ≤ 5.0 mmol/L after 15 days of therapy (Fig. 3). At 60 days, 73% of patients maintained potassium ≤ 5.0 mmol/L in both groups. Similar results were obtained in a sensitivity analysis defining achieved potassium concentration with the median value of all tests during each interval (Supplementary Figs. 1 and 2). SPS: sodium polystyrene sulfonate, SZC: sodium zirconium cyclosilicate.
Mean potassium concentration during different intervals after treatment start (panels A and B) and mean potassium change from baseline potassium (panels C and D). Legend: Shown is the mean potassium value closest to the end of each time interval. Panels A and C show each agent separately (SPS, patiromer and SZC), and panels B and D combine both novel potassium binders together. There were no statistically significant differences between treatment strategies (P > 0.05) as estimated by t-test or one-way ANOVA. SPS; sodium polystyrene sulfonate, SZC; sodium zirconium cyclosilicate, Novel K + ; Binder (SZC + patiromer)
Proportion of patients with plasma potassium concentration ≤ 5.0 mmol/L (Panels A and C) and ≤ 5.5 mmol/L (Panels B and D) before and after initiation of treatment with potassium binders. Legend: Bars (standard error) represent the percentage of patients with potassium ≤ 5.0 mmol/L and ≤ 5.5 mmol/L, modeling the potassium value closest to the end of each time interval. N () shows the number of patients represented in each of the bars. *There were no statistically significant differences between the binders, except for one observation in panel B during the first 15 days of therapy, where Chi2 test < 0.05. SPS; sodium polystyrene sulfonate, SZC; sodium zirconium cyclosilicate, Novel K + ; Binder (SZC + patiromer)
Within 365 days of follow-up, 3 patients had a major gastrointestinal event (gastrointestinal ulcer and/or perforation in two patients who started sodium polystyrene sulfonate and one patient who started a novel binder). The characteristics of these 3 patients are described in Supplementary Table 4. Minor adverse events are described in Table 3. The most common identified adverse event was hypocalcemia, with a cumulative incidence of 27% of patients who started sodium polystyrene sulfonate by day 90, and 19% of patients who started novel binders. In multivariable analyses, adjusted for age, gender, eGFR, diabetes, hypertension, myocardial infarction, heart failure, and arrhythmia, but not for baseline plasma calcium, the odds for hypocalcemia were numerically lower in the initiators of novel binders than sodium polystyrene sulfonate initiators, however, did not reach statistical significance [OR 0.71 (95% CI 0.44–1.15) p = 0.14]. The second most common adverse event detected was initiation of constipation or diarrhea medications, with a cumulative incidence of 20% in patients who started sodium polystyrene sulfonate by day 90, and 14% in patients who started novel binders (p = 0.17). Hypokalemia, hypophosphatemia, and hypernatremia followed the same pattern, with non-statistically significant lower multivariate odds for novel binders vs. sodium polystyrene sulfonate (Table 3). A description of death events during follow up is reported in Supplementary Table 5.
Discussion
In this study, we observed longer treatment duration for novel binders and more frequent chronic use than for sodium polystyrene sulfonate. However, all three agents showed similar short-term effectiveness in reducing plasma potassium, and similar safety with regard to minor adverse events.
The observed longer treatment duration of novel binders than for sodium polystyrene sulfonate agrees with their marketed indication for chronic use. It also agrees with observations from a German study of patients who received prescriptions for patiromer, where the majority were maintained on therapy for up to 1 year [13]. A strength of our analysis is that it is based on filled dispensations, a better method of ascertainment of exposure than prescriptions. The observed absolute and relative potassium reductions in our study are also in line with trials [6, 8, 9, 14, 32] and observational studies [13, 16,17,18,19,20], A summary table of all identified trials and observational evidence to date is shown in Supplementary Table 6 and 7.
A novelty in our study is the observation that all agents were associated with similar reductions in potassium levels. With one exception [10], available trial evidence to date compared single potassium binders against the non-pharmacological standard-of-care. This lack of formal comparisons precludes the ability to conclude that one agent is more or less potent than another. Although we did not find statistically significant differences in potassium levels between agents, the odds ratio for reaching normokalemia (potassium < 5.0 mmol/L) in the first 15 days of follow-up was numerically lower for novel potassium binders vs. sodium polystyrene sulfonate, and this tendency continued throughout the follow-up. A recent randomized cross-over trial [10], where 48 patients on hemodialysis were given 2-weeks of sodium polystyrene sulfonate 15 g tid or patiromer 16.8 g daily with a 2-week washout period, showed that mean weekly potassium was 4.6 mmol/L during sodium polystyrene sulfonate periods compared to 5.2 mmol/L during washout periods (difference of 0.6 mmol/L, p < 0.01), and compared to 5.0 mmol/L during patiromer periods (difference of 0.2 mmol/L, p < 0.01), suggesting higher efficacy for sodium polystyrene sulfonate than for patiromer. We note that a controlled head-to-head trial has just been initiated in the setting of acute hyperkalemia [33]. In a retrospective observational study, Huda et al. compared effectiveness in 138 patients with hyperkalemia receiving sodium zirconium cyclosilicate or calcium polystyrene sulphonate in the United Kingdom, and observed similar efficacy in promptly reducing elevated potassium levels [18]. A similar study in Japan by Nakayama et al. [33] that included 132 patients, observed however a greater reduction in potassium levels by sodium zirconium cyclosilicate compared to calcium polystyrene sulfonate. Calcium polystyrene sulfonate is not commercialized in Sweden and we cannot confirm or refute these observations.
Another novelty in this study is the observation that all agents showed similar short-term incidence of minor adverse effects. The rates of adverse events for sodium polystyrene sulfonate in our study are comparable to the two existing small trials: hypocalcemia 18.8%, hypokalemia 18.8%, nausea 25–43%, constipation 8–37.5% and anorexia 34% [9, 34]. Our observed rates are also compatible with the original Food and Drug Administration (FDA) filings for sodium zirconium cyclosilicate approval, the adverse events reported were edema (13.7%), hypertension (11%) and heart failure (4.6%) [35], with higher incidence of edema in high-risk individuals (CKD, heart failure). In the original FDA filings for patiromer approval, hypokalemia and hypomagnesemia were noted to occur in 3–10% and 5–17% based on dose, respectively [11].
We did not find any statistically significant differences in the rate of adverse effects between agents. However, absolute risks were numerically higher for sodium polystyrene sulfonate users, and the relative risks showed consistently lower odds ratios for novel binders compared to sodium polystyrene sulfonate. The broad confidence intervals are likely due to our small sample size, but results may also be impacted by perception of risk by clinicians, resulting in different monitoring rates and laboratory testing between treatment groups, thereby increasing the chance of finding abnormalities. This is different from the scenario of trials, where all patients are monitored with the same rate and frequency as per protocol [36, 37]. Finally, very few serious gastrointestinal events identified through administrative data have been reported to date for sodium polystyrene sulfonate alone, finding that small absolute risks that compared to non-pharmacological standard of care were statistically significant [23, 38] or no different [39]. Large administrative data studies on rare adverse events with novel binders are currently not available, and our study was not powered to evaluate them.
Our study has additional limitations: The study population is representative of Stockholm, which may not necessarily reflect experiences in other regions or demographic groups. In our study, we assumed standard doses and lacked information on prescribed doses. Results may thus be in part explained by the prescription of a higher effective dose rather than differences between agents at equipotent doses. No direct information on dose equivalence is available in the literature, except for the observational study by Kovesdy et al. [16], which found sodium polystyrene sulfonate 15 g tid more effective than patiromer 16.8 g daily. We have no information on co-interventions during the same period [such as changes in diet, stopping renin-angiotensin system inhibitors or angiotensin receptor neprilysin inhibitor, or prescribing potassium-lowering diuretics]. Our study focuses on the reduction of plasma potassium as a surrogate endpoint for preventing severe hyperkalemia and related adverse events. However, it does not directly investigate clinical outcomes such as arrhythmia or mortality, which are the ultimate endpoints of interest. We believe that a potentially slow adoption of novel binders in our region, as seen in this study, could reflect a combination of cultural and historical practices, a need for more familiarity and confidence among clinicians, and financial considerations. There is also a need for better data on relative effectiveness and for data on cost effectiveness.
To conclude, this study of new users of potassium-lowering agents from Stockholm shows similar effectiveness and adverse event rates for patients treated with sodium polystyrene sulfonate and those treated with novel binders. However, treatment duration was longer for the novel agents, which may raise questions on cost-effectiveness [40]. Moving forward, it would be important to confirm these findings through a clinical trial and evaluation of cost-effectiveness [18, 41]. In addition to preventing hyperkalemia-related arrhythmic death, it has been suggested that prolonged use of binders may prevent clinically important events by permitting continuation of the evidence-based cardiovascular medications [42], thereby reducing cardiovascular outcomes. However, these cardiovascular events are rare and numbers needed to treat, as well as duration of treatment with potassium binders to prevent one event, will likely be very high compared to other cardiovascular prophylactic strategies [43].
Data availability
The data contain patient-related information and cannot be shared publicly as per European General Data Protection Regulation. The data can be accessed through collaborative research applications address to the principal investigator JJC (juan.jesus.carrero@ki.se), and subjected to data sharing agreements that fulfill institutional and national regulations.
References
Palmer BF et al (2021) Clinical Management of Hyperkalemia. Mayo Clin Proc 96(3):744–762
Clase CM et al (2020) Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 97(1):42–61
Agarwal R et al (2019) Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 394(10208):1540–1550
Butler J et al. (2022) Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J
Bushinsky DA et al (2015) Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia. Kidney Int 88(6):1427–1433
Weir MR et al (2015) Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 372(3):211–221
Bakris GL et al (2015) Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 314(2):151–161
Zannad F et al (2020) Efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: the randomized, placebo-controlled HARMONIZE-Global study. ESC Heart Fail 7(1):54–64
Lepage L et al (2015) Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol 10(12):2136–2142
Jaques DA et al (2022) Comparative efficacy of patiromer and sodium polystyrene sulfonate on potassium levels in chronic haemodialysis patients: a randomized crossover trial. Clin Kidney J 15(10):1908–1914
Spinowitz BS et al (2019) Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol 14(6):798–809
Dong L et al (2022) Efficacy and safety of potassium binders in the treatment of patients with chronic kidney disease and hyperkalemia. Eur J Pharmacol 931:175174
Pecoits-Filho R et al (2023) Patiromer utilization in patients with advanced chronic kidney disease under nephrology care in Germany. Clin Kidney J 16(1):176–183
Pinnell D et al (2022) Real-world evaluation of patiromer utilization and its effects on serum potassium in veterans with end stage kidney disease. Medicine (Baltimore) 101(50):e32367
Di Palo KE, Sinnett MJ, Goriacko P (2022) Assessment of patiromer monotherapy for hyperkalemia in an acute care setting. JAMA Netw Open 5(1):e2145236
Kovesdy CP et al (2020) Real-world management of hyperkalemia with patiromer among United States Veterans. Postgrad Med 132(2):176–183
Nakayama T et al (2023) Compared effectiveness of sodium zirconium cyclosilicate and calcium polystyrene sulfonate on hyperkalemia in patients with chronic kidney disease. Front Med 10:1137981
Huda AB et al (2022) Hyperkalaemia and potassium binders: retrospective observational analysis looking at the efficacy and cost effectiveness of calcium polystyrene sulfonate and sodium zirconium cyclosilicate. J Clin Pharm Ther 47(12):2170–2175
Patel S et al (2023) Assessing patiromer utilization and associated serum potassium changes in US veterans with prior sodium polystyrene sulfonate exposure. Medicine (Baltimore) 102(9):e33134
Kovesdy CP et al (2019) Real-world evaluation of patiromer for the treatment of hyperkalemia in hemodialysis patients. Kidney Int Rep 4(2):301–309
Zhuo M et al (2022) Risk of hospitalization for heart failure in patients with hyperkalemia treated with sodium zirconium cyclosilicate versus patiromer. J Card Fail 28(9):1414–1423
Carrero JJ, Elinder CG (2022) The Stockholm CREAtinine measurements (SCREAM) project: fostering improvements in chronic kidney disease care. J Intern Med 291(3):254–268
Laureati P et al (2020) Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant 35(9):1518–1526
https://www.fass.se/LIF/atcregister?userType=0&atcCode 08 march 2023].
Levey AS et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney inter., Suppl. 2013; 3: 1–150.
Visseren FLJ et al (2022) 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol 29(1):5–115
Howlett JG et al (2016) The canadian cardiovascular society heart failure companion: bridging guidelines to your practice. Can J Cardiol 32(3):296–310
Schnelle K et al (2020) Largest experience of safety and efficacy of patiromer in solid organ transplant. Transplant Direct 6(9):e595
Haller H et al. (2022) Safety and efficacy of patiromer in hyperkalemic patients with CKD: a pooled analysis of three randomized trials. kidney 360, 3(12): 2019–2026.
Scicchitano P et al. Optimizing Therapies in Heart Failure: The Role of Potassium Binders. Biomedicines, 2022. 10(7).
Pitt B et al (2011) Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 32(7):820–828
Cañas AE et al (2023) A randomized study to compare oral potassium binders in the treatment of acute hyperkalemia. BMC Nephrol 24(1):89
Nasir K, Ahmad A (2014) Treatment of hyperkalemia in patients with chronic kidney disease: a comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. J Ayub Med Coll Abbottabad 26(4):455–458
http://fda.com/. 2023–02–20].
Trevisan M et al (2021) Pharmacoepidemiology for nephrologists (part 1): concept, applications and considerations for study design. Clin Kidney J 14(5):1307–1316
Fu EL et al (2021) Pharmacoepidemiology for nephrologists (part 2): potential biases and how to overcome them. Clin Kidney J 14(5):1317–1326
Noel JA et al (2019) Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med 179(8):1025–1033
Ferreira JP et al (2021) Adverse gastrointestinal events with sodium polystyrene sulphonate and calcium polystyrene sulphonate use in dialysis patients: a nationwide registry study. Nephrol Dial Transplant 36(2):339–345
Carrero JJ et al. Pharmacological strategies to manage hyperkalemia: out with the old, in with the new? Not so fast…. Clinical Kidney Journal, 2023.
Kim K et al (2022) Cost effectiveness of sodium zirconium cyclosilicate for the treatment of hyperkalaemia in patients with CKD in Norway and Sweden. BMC Nephrol 23(1):281
Butler J et al (2022) Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J 43(41):4362–4373
Packer M (2022) Potassium binders for patients with heart failure? The real enlightenment of the DIAMOND. trial Euro Heart J 43(41):4374–4377
Acknowledgements
JJC is supported by the Swedish Research Council, the National Institute of Health (NIH R01DK115534) and the Swedish Heart and lung Foundation. AGO receives research support from The National Council of Science and Technology (CONACYT), CVU 373297. ELF is supported by a Rubicon grant from the Netherlands Organization for Scientific Research.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
AGO participated in study conception and design of the study, analysis of the data and writing the paper. JJC and CMC participated in study conception and design, analysis of the data, writing the paper and approval of the final version of the manuscript. AB, ELF, BEPG, ALF, ME, and CZ, participated in interpretation of the data and/or critical revision of the manuscript to its final form. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
JJC has conducted studies for which Karolinska Institutet has received support from AstraZeneca (manufacturer of sodium zirconium cyclosilicate), ViforPharma (manufacturer of patiromer), Novonordisk, Astellas, MSD, GSK, Boehringer Ingelheim and Amgen; He also reports receiving lecture fees from Baxter, Fresenius Kabi, AstraZeneca, Astellas, GSK and Abbott; and participation in advisory boards for Astrazeneca, Nestle and Bayer; ME reports payment for lectures (Astellas pharma, Vifor pharma, Astra Zeneca, Baxter healthcare and Fresenius Medical care) and attendance in advisory boards (Astellas pharma, Vifor pharma, Astra Zeneca). The rest of the authors have no conflict of interest to report.
Ethical approval
The regional ethical review board in Stockholm approved the study.
Human and animal rights
The present study complies with the guidelines for human studies.
Informed consent
Informed consent was not deemed necessary because all data were deidentified at the Swedish Board of Health and Welfare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonzalez-Ortiz, A., Clase, C.M., Bosi, A. et al. Evaluation of the introduction of novel potassium binders in routine care; the Stockholm CREAtinine measurements (SCREAM) project. J Nephrol 37, 961–972 (2024). https://doi.org/10.1007/s40620-023-01860-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-023-01860-0