Abstract
Introduction
Acute kidney injury is a frequent complication in critically ill patients with and without COVID-19. The aim of this study was to evaluate the incidence of, and risk factors for, acute kidney injury and its effect on clinical outcomes of critically ill COVID-19 patients in Tyrol, Austria.
Methods
This multicenter prospective registry study included adult patients with a SARS-CoV-2 infection confirmed by polymerase chain reaction, who were treated in one of the 12 dedicated intensive care units during the COVID-19 pandemic from February 2020 until May 2022.
Results
In total, 1042 patients were included during the study period. The median age of the overall cohort was 66 years. Of the included patients, 267 (26%) developed acute kidney injury during their intensive care unit stay. In total, 12.3% (n = 126) required renal replacement therapy with a median duration of 9 (IQR 3–18) days. In patients with acute kidney injury the rate of invasive mechanical ventilation was significantly higher with 85% (n = 227) compared to 41% (n = 312) in the no acute kidney injury group (p < 0.001). The most important risk factors for acute kidney injury were invasive mechanical ventilation (OR = 4.19, p < 0.001), vasopressor use (OR = 3.17, p < 0.001) and chronic kidney disease (OR = 2.30, p < 0.001) in a multivariable logistic regression analysis. Hospital and intensive care unit mortality were significantly higher in patients with acute kidney injury compared to patients without acute kidney injury (Hospital mortality: 52.1% vs. 17.2%, p < 0.001, ICU-mortality: 47.2% vs. 14.7%, p < 0.001).
Conclusion
As in non-COVID-19 patients, acute kidney injury is clearly associated with increased mortality in critically ill COVID-19 patients. Among known risk factors, invasive mechanical ventilation has been identified as an independent and strong predictor of acute kidney injury.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affected intensive care units (ICUs) around the world for more than 2 years [1]. In addition to the lungs, numerous other organs including the kidneys were involved, particularly in critically ill patients [2, 3].
The incidence of acute kidney injury (AKI) in patients admitted to an ICU before the COVID-19 pandemic was up to 57.3% in the multinational AKI-EPI study [4]. Early reports of the incidence of AKI in COVID-19 patients varied widely from very low rates in China [5] to relatively high rates in Europe and the United States [6, 7]. The same was seen for the use of renal replacement therapy (RRT) [8,9,10,11]. Recent meta-analyses show rates between 29 and 33% for the occurrence of AKI [12, 13].
As in non-COVID-19 patients, AKI is associated with increased mortality in critically ill COVID-19 patients [4, 14,15,16].
While renal tropism of SARS-CoV-2 on the kidney was presumed to be the primary cause at the beginning of the pandemic [17], histological findings suggest a multifactorial etiology of AKI in COVID-19 patients [18]. Since COVID-19 in critically ill patients affects mostly the lungs, proposed lung-kidney interactions may be of particular importance [19]. Invasive mechanical ventilation (IMV) [20], as well as the acute respiratory distress syndrome (ARDS) [21] are known risk factors for the development of AKI in critically ill patients. During the first wave, IMV was used predominantly in critically ill COVID-19 patients due to reports from China of rapid deterioration [15, 22]. Over the course of the pandemic, the rates of IMV declined and non-invasive ventilation (NIV) strategies became more widespread. Associated with that, several studies reported a significant drop in AKI rates [23, 24].
At the beginning of the SARS-CoV-2 pandemic a registry study was established in Tyrol, Austria, which included all ICUs treating critically ill COVID-19 patients [25]. This covers a region with about 750,000 inhabitants.
The aim of this study was to investigate the incidence of, and the risk factors for, AKI in critically ill COVID-19 patients and its association with mortality in this cross-regional registry in Tyrol, Austria.
Methods
Patients
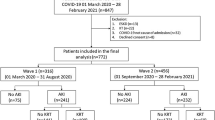
This prospective, multicenter registry study includes all critically ill COVID-19 patients admitted to an ICU in Tyrol (i.e., 13 departments allocated in 8 hospitals; a detailed list of all sites is available in the electronic supplemental material, [ESM] Supplemental Table 1) in the period from 1st February, 2020 until 1st May, 2022 [24, 25]. Patients required at least one positive SARS-CoV-2 by polymerase chain reaction (PCR) test for COVID-19 diagnosis. This registry was approved by the local ethics committee (Nr. 1099/2020).
Since SARS-CoV-2 spread episodically in Austria, four waves were defined as follows: first wave: 1st February, 2020 to 16th July, 2020; second wave: 17th July, 2020 to 21st February, 2021; third wave: 22nd February, 2021 to 19th July, 2021; fourth wave: 20th July 2021, to 1st May, 2022 (ESM: Supplemental Figure 1).
The detailed methods of this registry have been published previously [25].
Definitions and data collection
Only adult patients (≥ 18 years) were included in this analysis. Endotracheal intubation and ventilation via tracheostomy were classified as IMV, while nasal high flow (NHF) and continuous positive airway pressure (CPAP) conducted by mask or helmet were categorized as non-invasive ventilation.
The occurrence of AKI during the ICU stay was defined by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines as increased serum creatinine or decreased urine output [26]. In all participating ICUs only continuous forms of RRT were provided. Depending on the ICU, these were continuous venovenous hemofiltration, continuous venovenous hemodialysis or continuous venovenous hemodiafiltration. When the modality was used for at least 2 h in a day it was counted as a day.
In case of ICU-to-ICU transfer, both the initial ICU stay and the subsequent ICU stay were combined and considered as one admission. At the time of ICU admission, the Sequential Organ Failure Assessment Score (SOFA) [27] and Simplified Acute Physiology Score 3 (SAPS 3) [28, 29] were calculated.
Baseline characteristics were extracted from the patient information system and recorded in the Tyrol-CoV-ICU-Reg. A predefined set of comorbidities was checked for every patient as detailed in the supplementary material. Intensive care interventions like IMV, NIV, prone positioning, pharmacological hemodynamic support, RRT, ECMO, duration of interventions and medication, as well as complications like AKI were recorded.
Data were collected until death or hospital discharge, whichever occurred earlier.
All data were collected with an electronic case report form and REDCap electronic data capture, a web platform for managing databases and surveys created by Vanderbilt University and hosted by the Department of Medical Statistics, Information and Health Economics, Medical University Innsbruck [30, 31].
Statistical analysis
Median with interquartile range (IQR) is presented for continuous variables, while categorical variables are described as numbers with corresponding percentages. Adjusted odds ratio (OR) is reported with 95% confidence interval (95% CI).
For statistical analyses we used the software SPSS (version 27; IBM Corp., Armonk, New York) and R software (version 3.4.0, R Foundation for Statistical Computing, Vienna, Austria). To test for normal distribution, we performed the Shapiro–Wilk test. T-test was applied in case of normal distribution, otherwise the Mann–Whitney-U-test or Kruskal–Wallis test was performed. Categorical variables were analyzed by calculating the χ2-test.
We compared the number of observed deaths to the number of expected deaths for each KDIGO stage as predicted by the SAPS 3 and calculated the ratio of observed-to-expected deaths for each stage. The 95% CIs for observed deaths were calculated by bootstrapping. The Hosmer and Lemeshow test was used to test the goodness of fit of the SAPS 3 at each KDIGO stage [32].
Potential predictors for AKI and hospital mortality were evaluated using univariable and multivariable logistic regression analysis. To further identify predictors of hospital mortality a Cox regression was performed. The variables were selected based on univariate analysis. For the Cox regression analysis, we assumed that most AKI episodes occurred during the first 48 h after ICU admission.
All variables with a p-value < 0.05 in the univariable analysis were included in the multivariable analysis.
Statistical significance was defined as a p-value < 0.05 for all tests. All tests were 2-sided.
Results
Study population and patient characteristics
In total, 1059 patients were included during the study period in the Tyrol-CoV-ICU-Reg study. After exclusion of 17 patients < 18 years, 1042 patients were included in the final analysis. Baseline characteristics and scores at admission are reported in Table 1. The median age of the overall cohort was 66 years (IQR, 56–75) and the most common comorbidities were hypertension (57.6%), cardiovascular diseases (37.1%), obesity (Body Mass Index [BMI] > 30; 32.1%), diabetes mellitus type 2 (23.3%) and chronic kidney disease (CKD, 18.9%). At ICU admission the median SAPS 3 and SOFA score was 53 (IQR, 46–61), and 5 (IQR, 4–7), respectively. The median time from symptom onset to hospital and ICU admission was 6 days (IQR, 4–9) and 8 days (IQR, 5–11), respectively.
Acute kidney injury
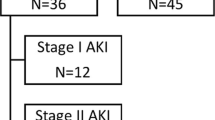
Of the included patients, 267 (25.6%) developed AKI during the ICU stay. According to the KDIGO classification 8.1% had AKI stage 1, 4.9% had AKI stage 2 and 12.7% had AKI stage 3.
Patients with AKI were older and more often had a pre-existing diagnosis of hypertension, cardiovascular disease, or a known CKD. Distribution of sex and the time from symptom onset to hospital and ICU admission was similar in both (AKI and no AKI) groups (hospitalization: 6 days vs. 6 days, p = 0.704; ICU admission: 8 days vs. 9 days, p = 0.055). Compared to patients without AKI, patients with AKI had higher SOFA and SAPS 3 scores on admission (SAPS 3: 58 vs. 51, p < 0.001; SOFA: 7 vs. 4, p < 0.001) and stayed significantly longer in the ICU (19 days vs. 9 days, p < 0.001) and in the hospital (28 days vs. 20 days, p < 0.001). Interventions and outcome of patients classified according to their KDIGO stage are reported in the ESM (Supplemental Table 2). In total, 126 patients (12.3%) required RRT with a median duration of 9 days (IQR, 3–18).
Outcome
Overall hospital and ICU mortality rates in our cohort were 26.4% and 23.1%, respectively (Table 2). In patients with AKI these rates were significantly higher (hospital mortality: 52.1% vs. 17.2%, p < 0.001, ICU-mortality: 47.2% vs. 14.7%, p < 0.001) and increased for each KDIGO stage (Fig. 1).
After adjustment for confounding variables such as age, IMV, vasopressor use, SAPS 3 and comorbidities (cardiovascular, hypertension and CKD), AKI KDIGO stage 2 and stage 3 were both independent predictors of hospital mortality in a logistic regression analysis (Table 3).
Similar results were obtained when predictors of hospital mortality were analyzed by Cox-regression analysis (ESM Supplemental Table 3). After adjustment for confounding variables KDIGO stage 2 and stage 3 remained significant.
To account for differences regarding location we created another model, including the site of the first presentation (reference: tertiary hospital). After adjustment for confounding variables, the site of presentation was not a significant predictor for hospital mortality (ESM Supplemental Tables 4a and b). However, KDIGO stage 2 and stage 3 both remained significant after adjustment.
The frequency of AKI and the hospital mortality for each participating center is shown in ESM Supplemental Tables 5 and 6.
Figure 2 shows the standardized mortality ratios ([SMR], i.e., observed-to-expected mortality ratios). In patients without AKI the SMR was 0.68 and increased with each KDIGO stage up to a maximum of nearly 2 in AKI KDIGO stage 3. The goodness of fit of the SAPS 3 score was 0.173 in AKI KDIGO stage 3.
Variations over time
The study period can be divided into 4 waves according to the dynamics of the pandemic (ESM Supplemental Figure 1). In the first wave the rate of AKI was high, at 49.1% and it decreased in the subsequent waves (ESM Supplemental Figure 2). The main difference between the four waves was the rate of IMV, which was higher in the first compared to the other three waves (67.9% vs. 50.7% vs. 50.3% vs. 50.5%).
Risk factors for AKI
Overall, 52.4% (n = 545) of the study population received IMV during the ICU stay for a median of 13 days (IQR, 7–23). In patients with AKI the IMV rate was significantly higher with 85.0% compared to 41.0% in the no AKI group. Patients with AKI required mechanical ventilation for a longer period compared to patients without AKI (16 days vs. 12 days, p = 0.003).
After adjusting for confounding variables, IMV and vasopressor use were significant predictors of AKI in a multivariable logistic regression analysis (Table 4).
Discussion
In this prospective observational multicenter cohort study of critically ill adult COVID-19 patients we found an incidence of AKI of 25.6% and a rate of RRT of 12.3%. Patients with AKI showed significantly higher hospital mortality compared to patients without AKI. After adjustment for other important risk factors, AKI KDIGO stage 2 and stage 3 remained independent predictors of hospital mortality. The most important risk factors for AKI were IMV, vasopressor use and CKD in a multivariable logistic regression analysis. Among known risk factors, IMV seems to be of particular importance as a predictor of AKI in critically ill COVID-19 patients.
The rate of AKI in patients with confirmed SARS-CoV-2 varied in single reports at the onset of the pandemic. Meanwhile, systematic reviews and meta-analyses found a range from 29% to 37% [12, 33]. In our cohort we found a relatively low overall rate of AKI of 23%, compared to other studies [34, 35]. This may be due to differences in case-mix and baseline characteristics or treatment standards, as shown in our previous studies [24, 25].
It is well known that AKI is associated with increased mortality in critically ill non-COVID-19 patients [4]. Acute kidney injury is also an important risk factor for mortality in COVID-19 ICU patients as shown by previous investigations [36]. We observed a fourfold increased risk of death in patients with AKI KDIGO Stage 3. Even after adjustment for the most common risk factors for COVID-19, AKI KDIGO Stages 2 & 3 remained significant predictors of mortality in critically ill COVID-19 patients. A recent study indicated that AKI may contribute independently to mortality only in less severe critically ill COVID-19 patients [37]. When we corrected for disease severity by including the SAPS 3 in our model, AKI remained an independent predictor of mortality (ESM Supplemental Tables 3 and 4b).
Correspondingly, we found an increased SMR (based on the SAPS 3) with each AKI KDIGO stage. In patients without AKI the SMR mortality ratio was below 1, reflecting good performance of the participating ICUs. However, AKI appeared to be an independent factor contributing to significantly impaired outcome. KDIGO 1 was already associated with a significantly increased O/E mortality ratio of 1.17. This was further increased in KDIGO stage 2 and stage 3, contributing to a nearly twofold increased excess SMR. Similar findings have been reported for AKI in critically ill patients not suffering from COVID-19 [38].
Rates of AKI in the first wave were higher compared to the consecutive waves in our study population. Interestingly in the first wave, IMV rates were also much higher (ESM Supplemental Figure 2). One reason for the high rates of IMV observed at the beginning of the pandemic might be the tendency to resort to early intubation due to uncertainty in the management of rapid respiratory deterioration in COVID-19-related ARDS [39]. Invasive mechanical ventilation rates decreased in the subsequent waves and more patients were primarily treated with non-invasive ventilation. Accordingly, AKI rates decreased from the first to the following waves. This occurred even though patients in the second wave were significantly older than in the first wave, which even resulted in increased mortality in that period, as previously reported [24]. Since age is an established risk factor for AKI [40], this enhances the importance of these findings.
In a (pre-pandemic) meta-analysis, van den Akker et al. showed that IMV is an independent risk factor for AKI in critically ill patients [20]. The mechanisms are complex and multi-factorial. One mechanism of kidney damage from IMV is high ventilation pressures (especially positive end-expiratory pressure [PEEP]) and the resulting intrathoracic pressures. This reduces the venous return to the heart, which leads to decreased cardiac output, venous congestion and increased abdominal pressure. Consequently, these altered pressure conditions accompanied by activated neurohormonal responses may lead to a reduction in renal blood flow and glomerular filtration [19]. A small pilot study suggested that these effects may be particularly pronounced in COVID-19 compared to mechanically ventilated patients with ARDS of different etiology [41]. Exceptionally high ventilatory pressures were often required in COVID-19 ARDS patients [42], contributing to barotrauma and biotrauma of the lung associated with increased release of pro-inflammatory cytokines [43]. However, COVID-19-specific lung-kidney interactions may become effective even before mechanical ventilation is required. Pulmonary microthrombi formation, increased pulmonary resistance and central venous pressure may contribute to renal congestion and impair renal blood flow as well as renal function, as shown for other patient populations [44].
Other observational studies reporting AKI in COVID-19 found a similar association with IMV [35, 45]. In our study IMV was among the most important predictors of AKI, together with vasopressor use. However, it should be noted that these two interventions are closely interrelated, mainly because of the hemodynamic impact of sedation during IMV, and therefore it is difficult to consider their effects separately.
Another important risk factor for AKI is pre-existing kidney damage [46]. Many studies investigating AKI in COVID-19 patients identified CKD as a risk factor for AKI [47]. In our study CKD was a predictor for AKI, which is consistent with many reports independently of the pandemic [47, 48]. Reduced renal reserve leading to higher susceptibility to AKI is among the proposed mechanisms.
Treatment recommendations changed over time, especially after the publication of the RECOVERY trial on dexamethasone [49]. The impact of steroid treatment on kidney outcomes is difficult to determine since nearly all COVID-19 patients who required oxygen therapy were treated with steroids after the first wave. This may also have contributed to the lower IMV rates after the first wave. However, it is likely that a better understanding of SARS-CoV-2 and improvements in treatment also contributed to a decrease in the AKI rates. The same applies to the different emerging variants in the four waves. We cannot exclude that the different viral mutations might also have contributed to different susceptibility for AKI.
In our cohort, we found a rate of RRT in AKI patients of about 40%, which is higher than rates reported by studies performed in the pre-pandemic era [4]. This may be a COVID-19-specific finding due to the multifactorial mechanisms contributing to AKI in this population [19] influencing requirement of RRT. Other studies comparing COVID-19 to non-COVID-19 patients [9] and a recent meta-analysis [50] also found higher rates of RRT in (hospitalized) COVID-19 patients. In a sepsis cohort however, similar high rates of around 30% have been reported by Peters et al. [51].
Limitations of this study are related to its observational design. We cannot fully exclude that the higher disease severity scores in patients with IMV led to a higher rate of AKI, whereby it must be noted that both the SAPS 3 and the SOFA score considering the modality of ventilation for score calculation and therefore, IMV and vasopressor use, actually led to higher scores themselves. As a consequence of the multicenter character of this study, differences in treatment strategies among centers cannot be excluded. However, we included the location of the first presentation in an additional analysis in the supplementary material. Due to the ongoing pandemic, changes in medical care over time may also have influenced the results. Unfortunately, some important data, such as CKD stage, baseline eGFR or the time-point of the occurrence of AKI are not available for our data set.
The main strengths of this study are the inclusion of all critically ill patients treated over the first four COVID-19 waves in the whole region of Tyrol, Austria. Therefore, our study includes patients from central as well as peripheral hospitals.
Conclusions
AKI is common in critically ill COVID-19 patients and is associated with impaired outcome. Patients with IMV have a higher risk of AKI during their ICU stay. Even when adjusting for common risk factors, IMV remains an independent predictor of AKI in critically ill COVID-19 patients. Therefore, preventive actions may be particularly important in COVID-19 patients undergoing intermittent mechanical ventilation.
Data availability
The datasets used and analysed in the current study are available from the corresponding author on reasonable request.
References
Blumenthal D, Fowler EJ, Abrams M, Collins SR (2020) Covid-19—implications for the health care system. N Engl J Med 383(15):1483–1488
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395(10234):1417–1418
Teixeira JP, Barone S, Zahedi K, Soleimani M (2022) Kidney Injury in COVID-19: epidemiology, molecular mechanisms and potential therapeutic targets. Int J Mol Sci 23(4):2242
Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN et al (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41(8):1411–1423
Cheng Y, Luo R, Wang X, Wang K, Zhang N, Zhang M et al (2020) The incidence, risk factors, and prognosis of acute kidney injury in adult patients with coronavirus disease 2019. Clin J Am Soc Nephrol 15(10):1394–1402
Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL et al (2020) Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98(1):209–218
Silver SA, Beaubien-Souligny W, Shah PS, Harel S, Blum D, Kishibe T et al (2021) The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: a systematic review and meta-analysis. Kidney Med 3(1):83-98.e1
Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML et al (2020) Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 180(11):1436–1447
Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS et al (2020) AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol 31(9):2145–2157
Alessandri F, Pistolesi V, Manganelli C, Ruberto F, Ceccarelli G, Morabito S et al (2021) Acute kidney injury and COVID-19: a picture from an intensive care unit. Blood Purif 50(6):767–771
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720
Fu EL, Janse RJ, de Jong Y, van der Endt VHW, Milders J, van der Willik EM et al (2020) Acute kidney injury and kidney replacement therapy in COVID-19: a systematic review and meta-analysis. Clin Kidney J 13(4):550–563
Passoni R, Lordani TVA, Peres LAB, Carvalho ARDS (2022) Occurrence of acute kidney injury in adult patients hospitalized with COVID-19: a systematic review and meta-analysis. Nefrologia 42(4):404–414
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L et al (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97(5):829–838
Chang R, Elhusseiny KM, Yeh YC, Sun WZ (2021) COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-a systematic review and meta-analysis. PLoS ONE 16(2):e0246318
Hansrivijit P, Qian C, Boonpheng B, Thongprayoon C, Vallabhajosyula S, Cheungpasitporn W et al (2020) Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Investig Med 68(7):1261–1270
Vijayan A, Humphreys BD (2020) SARS-CoV-2 in the kidney: bystander or culprit? Nat Rev Nephrol 16(12):703–704
Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H et al (2020) Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 396(10247):320–332
Joannidis M, Forni LG, Klein SJ, Honore PM, Kashani K, Ostermann M et al (2020) Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 workgroup. Intensive Care Med 46(4):654–672
van den Akker JP, Egal M, Groeneveld AB (2013) Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care 17(3):R98
Darmon M, Clec’h C, Adrie C, Argaud L, Allaouchiche B, Azoulay E et al (2014) Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol 9(8):1347–1353
Marini JJ, Gattinoni L (2020) Management of COVID-19 respiratory distress. JAMA 323(22):2329–2330
Charytan DM, Parnia S, Khatri M, Petrilli CM, Jones S, Benstein J et al (2021) Decreasing incidence of acute kidney injury in patients with COVID-19 critical illness in New York City. Kidney Int Rep 6(4):916–927
Mayerhöfer T, Klein SJ, Peer A, Perschinka F, Lehner GF, Hasslacher J et al (2021) Changes in characteristics and outcomes of critically ill COVID-19 patients in Tyrol (Austria) over 1 year. Wien Klin Wochenschr 133(23–24):1237–1247
Klein SJ, Bellmann R, Dejaco H, Eschertzhuber S, Fries D, Furtwängler W et al (2020) Structured ICU resource management in a pandemic is associated with favorable outcome in critically ill COVID-19 patients. Wien Klin Wochenschr 132(21–22):653–663
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120(4):c179–c184
Jones AE, Trzeciak S, Kline JA (2009) The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med 37(5):1649–1654
Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA et al (2005) SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med 31(10):1336–44
Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA et al (2005) SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 31(10):1345–55
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L et al (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208
Hosmer DW, Lemeshow S (1995) Confidence interval estimates of an index of quality performance based on logistic regression models. Stat Med 14(19):2161–2172
Yang X, Jin Y, Li R, Zhang Z, Sun R, Chen D (2020) Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care 24(1):356
Lumlertgul N, Pirondini L, Cooney E, Kok W, Gregson J, Camporota L et al (2021) Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann Intensive Care 11(1):123
Schaubroeck H, Vandenberghe W, Boer W, Boonen E, Dewulf B, Bourgeois C et al (2022) Acute kidney injury in critical COVID-19: a multicenter cohort analysis in seven large hospitals in Belgium. Crit Care 26(1):225
Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S et al (2021) AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 32(1):151–160
Regolisti G, Maggiore U, Di Mario F, Gentile M, Benigno GD, Gandolfini I et al (2022) The association of new-onset acute kidney injury and mortality in critically Ill patients with COVID-19 with less severe clinical conditions at admission: a moderation analysis. Front Med (Lausanne) 9:799298
Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W et al (2009) Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med 35(10):1692–1702
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA 323(11):1061–1069
Grams ME, Sang Y, Ballew SH, Gansevoort RT, Kimm H, Kovesdy CP et al (2015) A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis 66(4):591–601
Fogagnolo A, Grasso S, Dres M, Gesualdo L, Murgolo F, Morelli E et al (2022) Focus on renal blood flow in mechanically ventilated patients with SARS-CoV-2: a prospective pilot study. J Clin Monit Comput 36(1):161–167
Gattinoni L, Gattarello S, Steinberg I, Busana M, Palermo P, Lazzari S et al (2021) COVID-19 pneumonia: pathophysiology and management. Eur Respir Rev 30(162):210138
Mayerhöfer T, Perschinka F, Joannidis M (2022) Acute kidney injury and COVID-19: lung-kidney crosstalk during severe inflammation. Med Klin Intensivmed Notfmed 117(5):342–348
Khoury S, Steinvil A, Gal-Oz A, Margolis G, Hochstatd A, Topilsky Y et al (2018) Association between central venous pressure as assessed by echocardiography, left ventricular function and acute cardio-renal syndrome in patients with ST segment elevation myocardial infarction. Clin Res Cardiol 107(10):937–944
Casas-Aparicio GA, León-Rodríguez I, Alvarado-de la Barrera C, González-Navarro M, Peralta-Prado AB, Luna-Villalobos Y et al (2021) Acute kidney injury in patients with severe COVID-19 in Mexico. PLoS One 16(2):e0246595
Nisula S, Kaukonen KM, Vaara ST, Korhonen AM, Poukkanen M, Karlsson S et al (2013) Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med 39(3):420–428
Kunutsor SK, Laukkanen JA (2020) Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med 52(7):345–353
Singh P, Rifkin DE, Blantz RC (2010) Chronic kidney disease: an inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol 5(9):1690–1695
Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L et al (2021) Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384(8):693–704
Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J et al (2020) Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep 5(8):1149–1160
Peters E, Antonelli M, Wittebole X, Nanchal R, François B, Sakr Y et al (2018) A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: results from The Intensive Care Over Nations audit. Crit Care 22(1):188
Acknowledgements
We would like to express our gratitude to Lalit Kaltenbach for providing support for the REDCAP platform and to Univ.-Prof. Mag. Dr. Hanno Ulmer for his support with the statistical analysis. Furthermore, would like to thank all health care workers, who helped in the treatment of COVID-19 patients in Tyrol, Austria.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. The Tyrolean COVID-19 intensive care registry was supported by the Tyrolean Government.
Author information
Authors and Affiliations
Contributions
TM, FP and MJ designed the study, collected data, performed data analysis, and wrote the first draft of the manuscript. SK, AP, GL, RB, LG, MM, RB, SE, SM, AF, DF, MS, EF, WH, RH, LK, BS, CK, TH, EL, CT, CPH, AM, MP, BR, JB, SZH, AR collected data, read, and approved the final manuscript. All authors contributed to the study conception and registry design.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest to declare regarding this manuscript.
Ethical approval
The study was approved by the Ethics Committee of the Medical University Innsbruck (Nr. 1099/2020). Informed consent was obtained according to local regulations. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mayerhöfer, T., Perschinka, F., Klein, S.J. et al. Incidence, risk factors and outcome of acute kidney injury in critically ill COVID-19 patients in Tyrol, Austria: a prospective multicenter registry study. J Nephrol 36, 2531–2540 (2023). https://doi.org/10.1007/s40620-023-01760-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-023-01760-3