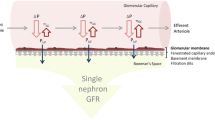
Abstract
Background
Peritubular capillary rarefaction plays an important role in the progression of chronic kidney disease. Little is known about the relation between peritubular capillary density, glomerular volume and filtration rate in the healthy kidney.
Methods
In this single-center study, we included 69 living kidney donors who donated between 2005 and 2008 and had representative renal biopsies available. In all donors, glomerular filtration rate was measured using 125I-Iothalamate before donation and at five years after donation. Before donation, the increase in glomerular filtration rate after dopamine stimulation was measured. Glomerular volume and peritubular capillary density were determined in biopsies taken at the time of transplantation. Pearson’s correlation coefficient and linear regression were used to assess relations between parameters.
Results
Mean donor age was 52 ± 11 years and mean measured glomerular filtration rate was 119 ± 22 mL/min before donation and 82 ± 15 mL/min at five years after donation. While peritubular capillary density (measured by either number of peritubular capillaries/50,000 μm2 or number of peritubular capillaries/tubule) was not associated with measured glomerular filtration rate before or after donation, number of peritubular capillaries/tubule was associated with the increase in measured glomerular filtration rate after dopamine stimulation (St.β = 0.33, p = 0.004), and correlated positively with glomerular volume (R = 0.24, p = 0.047). Glomerular volume was associated with unstimulated measured glomerular filtration rate before donation (St.β = 0.31, p = 0.01) and at five years (St.β = 0.30, p = 0.01) after donation, independent of age.
Conclusions
In summary, peritubular capillary density was not related to unstimulated kidney function before or after kidney donation, in contrast to glomerular volume. However, number of peritubular capillaries/tubule correlated with the increase in glomerular filtration rate after dopamine stimulation in healthy kidneys, and with glomerular volume. These findings suggest that peritubular capillary density and glomerular volume differentially affect kidney function in healthy living kidney donors.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microstructural changes such as glomerular hypertrophy, interstitial fibrosis and tubular atrophy can be present to various degrees in kidneys of healthy individuals without clinical signs of kidney damage [1]. Glomerular volume is positively associated with single-nephron glomerular filtration rate (GFR) in healthy individuals, probably as a compensation mechanism to maintain a normal total GFR in the case of loss of nephrons or increased renal demand [2]. Moreover, a higher glomerular volume is associated with hypertension, overweight, height and family history of end-stage kidney disease [2, 3]. Glomerular enlargement has been explained as the result of either increased intraglomerular pressure or an increased glomerular ultrafiltration coefficient, accompanied by prolongation of glomerular capillaries and subsequent enlargement of the glomerular tuft [4, 5]. Indeed, hypertrophic glomeruli have more capillaries, and a greater total capillary area [6, 7]. It is unknown whether these glomerular capillary changes also affect the peritubular capillaries (PTCs), and if so, whether PTC density is also related to kidney function in the healthy kidney.
The peritubular capillary bed predominantly evolves from the efferent glomerular arteriole [8, 9], while the glomerular capillary bed is situated behind the afferent arteriole. A single nephron unit consists of a glomerulus with accompanying tubular system, in which distal tubuli “return” to their own glomerulus, but the PTC microcirculation forms a coalescing plexus surrounding tubuli from different nephrons. Both cortical capillary beds are highly permeable to water and solutes which are filtered in the glomerulus and almost totally reabsorbed via tubuli in peritubular capillaries. They differ in blood pressure as well as in oxygen tension: blood pressure and oxygen levels are high in the glomerulus, while blood pressure is lower and there is a steep decrease in oxygen gradient in the interstitium [9, 10]. In patients with insulin-dependent diabetes mellitus, an independent relationship of glomerular and interstitial biopsy parameters with renal function was found [11]. Based on these differences between the glomerular and peritubular capillary beds we hypothesize that an increase in glomerular volume is not accompanied by an increase in peritubular capillaries in healthy kidneys. We expect that in early stages of kidney damage, a phase of glomerular capillary hypertrophy occurs followed by peritubular capillary loss and fibrosis in later stages of chronic kidney disease (CKD).
An ideal setting to study microstructural parameters as glomerular volume and PTC density in healthy kidneys is in living kidney donors, for whom pre-implantation biopsies are often available. Previous kidney biopsy studies in living kidney donors showed that glomerular hypertrophy is associated with higher pre-donation GFR [2], but with lower short- and long-term post-donation GFR [12, 13]. It also has been shown that a higher body mass index (BMI) was associated with glomerular hypertrophy [14], and a reduced increase in GFR in response to a dopamine stress test [15]. Thus, in this study, we investigated the relation between PTC density and glomerular volume, pre- and post-donation-measured-GFR in a cohort of living kidney donors.
Methods
Study population
For this retrospective cohort study, we identified 73 living kidney donors with representative kidney biopsies. Biopsies were taken right after donor nephrectomy (T1), right before implantation (T2) and/or after reperfusion (T3) and were considered representative if T1, T2 and/or T3 had a total cortical surface of minimally 0.6 mm2 with at least 5 glomeruli. All donors donated between August 11, 2005 and June 17, 2008 at the University Medical Center Groningen, The Netherlands. Four donors were excluded because they were part of the Dutch “cross-over” program and only came to our center for the actual nephrectomy procedure, rendering 69 living kidney donors eligible for inclusion in this study. All donors underwent pre- and three-month post-donation clinical and laboratory measurements as part of the regular living kidney donor screening program. In 52 donors, five-year post-donation follow-up was available. In 2014, these data were added to the TransplantLines Biobank and Cohort study (ClinicalTrials.gov identifier: NCT03272841). This is an observational cohort study on short- and long-term outcomes after organ transplantation/donation, as described previously [16]. The study was approved by the institutional ethical review board (METc 2014/077). All procedures were conducted in accordance with the declaration of Helsinki and declaration of Istanbul.
Biopsy analysis
All available T1, T2 and T3 biopsies were stained with periodic-acid-shiff (PAS) and, on a separate section, an immunohistochemical staining for CD34 (Monosan, Uden, the Netherlands) was performed. In brief, parafin-embedded tissue sections were incubated with primary antibody after blocking of endogeneous perioxidase and antigen retrieval by boiling in TRIS EDTA buffer. After washing, the biopsies were incubated with bright vision anti-mouse HRP (Immunologic; Duiven, The Netherlands) followed by washing and thereafter 3,3-diaminobenzidine (DAB) (DAKO cytomation, Glosturp, Denmark) was used as the chromogen. Thereafter the protocol slides were counterstained with hematoxylin (Klinipath, Duiven, The Netherlands). Periodic-acid-shiff and immunohistochemically stained slides were digitalized using a Ventana scanner (Ventana iScan HT (Roche, Basel, Switzerland), and imported in Panoramic Image Viewer (3DHistotech, Budapest, Hungary); examples are shown in Fig. 1. Microstructural parameters were measured on PAS-stained sections by one observer (ML), according to Elsherbiny et al. [14], with the exception that partial glomeruli were counted as 1 and not as 0.5. Briefly, total cortical biopsy area was annotated manually, as well as glomerular tuft surface area of all non-sclerotic glomeruli. Then the profile area of non sclerotic glomeruli was calculated by dividing the number of non sclerotic glomeruli by cortical area. The Weibel Gomez stereological model was used to calculate the non sclerotic glomeruli density. Furthermore, non sclerotic glomeruli volume (glomerular volume) was calculated as described by Elsherbiny et al. [14]. Of all CD34 stained sections a maximum of 10 pictures of 120,000 μm2 were taken in a serpentine manner [17], with Panoramic Viewer 1.15.4 and exported as jpeg into Paint (Microsoft, Seattle, WA, USA). There were no glomeruli present in these pictures. In all pictures, PTCs and tubules were manually traced by one observer (ML), with the exclusion of interlobular arteries. Peritubular capillaries and Tubuli per picture were quantified by Image J. Peritubular capillary density was assessed as number of PTCs per tubule (PTC/tubule) and number of PTCs per surface area (PTC/50,000 μm2).The tubular area was determined by dividing the area of the pictures with the number of tubuli counted per biopsy.
Representative examples of the microstructural measurements on biopsies. In Periodic acid-Schiff (PAS) stained sections (a and b) the area of cortex was delineated (a), and the area of the tuft of all individual non-sclerosed glomeruli (a and b). On CD34 stained sections (c–e) peritubular capillaries (PTCs) are accentuated. Tubuli (d) peritubular capillaries (e), were annotated manually. Scale bars: a 200 um; b–e 50 um
In cases that met our inclusion criteria of at least 5 glomeruli and 600,000 μm2 of cortex, the PAS stained digital section was scored histologically according to Banff by a pathologist (CPK) [18]. Grade of interstitial fibrosis and tubular atrophy (IF/TA) was determined as highest of tubular atrophy (ct) or interstitial fibrosis (ci). Also, IF/TA was assessed by the pathologist as more or less than 5% of the cortical area.
Assessment of kidney function and other clinical measurements
During screening, clinical parameters as weight, height, hip circumference, waist circumference and blood pressure were measured, medication use was asked as well as smoking history. Kidney function before and at three months and five years after donation was indirectly determined by measuring the clearance of the exogenous filtration marker 125I-iothalamate (measured GFR (mGFR), described in more detail previously) [19]. In short, 125I-Iothalamate and 131I-hippurate infusions were started and after a stabilization period, baseline measurements were performed in a steady state of plasma tracer levels. Clearances were calculated as (U*V)/P and (I*V)/P, where U*V represents the urinary excretion, I*V represents the infusion rate of the tracer and P represents the plasma tracer concentration per clearance period. We calculated mGFR from clearance levels of these tracers using (U*V)/P and corrected the renal clearance of 125I-iothalamate for urine collection errors by multiplying the urinary 125I-Iothalamate clearances with the ratio of plasma and urinary 131I-hippurate clearance by using the following formula:
The mGFR after stimulation with dopamine was also assessed before donation (mGFRdopa). The mGFRdopa was used to calculate the dopamine-induced increase in GFR (ΔmGFRdopa, previously referred to as the renal functional reserve (RFR) [19, 20]) by subtracting the unstimulated mGFR form the mGFRdopa. Dopamine-stimulated mGFR was missing in 4 cases. Serum creatinine was measured routinely in our central chemistry laboratory by an isotope dilution mass spectrometry (IDMS) traceable enzymatic assay on the Roche Modular (Roche Ltd., Mannheim, Germany). In addition, serum HbA1c concentration was recorded.
Statistical analyses and sample size estimation
Data are reported as mean (standard deviation (SD)) for normally distributed variables and median [interquartile range, IQR] for skewed data. Binary variables are shown as “number (%)”. Correlations between glomerular volume, IF/TA, PTC/tubule, tubular area and PTC/50,000 µm2 were assessed by scatter plots and Pearson’s correlation coefficients. In cross-sectional analyses, we investigated which pre-donation characteristics were associated with the microstructural parameters using univariable linear regression analyses. Subsequently, we used linear regression analyses to assess the association between the morphometrical parameters and pre- and post-donation kidney function outcomes. Outcomes were pre- and three months and five year post-donation mGFR. All univariable associations of the microstructural parameters with pre- and post-donation outcomes were adjusted for age using multivariable linear regression analyses, because age is a known determinant of GFR, as well as microstructural features in the kidney [2, 21]. To detect a correlation of 0.3 with an α of 0.05 and a power of 80%, 67 donors are needed. Statistical analyses were performed in SPSS version 28 for Windows (IBM, Armonk, NY), and Graphpad Prism 8 for Windows (Graphpad, San Diego, CA). p values of < 0.05 were considered statistically significant.
Results
Pre- and post-donation characteristics
A total of 69 living kidney donors were included in this study. Mean age was 52 ± 11 years, 46% were female and all donors were white (Table 1). The donors had a mean BMI of 26 ± 4 kg/m2 and a mean systolic blood pressure (SBP) of 130 ± 15 mmHg. Three donors had a pre-donation serum HbA1c level ≥ 6.5%, of which two donors had a BMI of 34 and 35 kg/m2, respectively. Pre-donation mGFR was 119 ± 22 mL/min and decreased to 75 ± 14 at three months post-donation (Table 2). Five years after donation, mGFR was 82 ± 15 mL/min. Before donation, mean glomerular volume was 0.0024 ± 0.0007 mm3, mean number of PTC/tub was 1.97 ± 0.3, mean number of PTC/50,000 μm2 was 25.9 ± 4.4, mean tubular area was 3679.2 ± 835.7 µm2, and 19 donors had > 5% IF/TA (Table 3).
Correlations between microstructural parameters
Scatterplots of correlations between microstructural parameters are shown in Fig. 2. The strongest correlation was observed for tubular area with PTC/50,000 μm2 (R = − 0.63, p < 0.001), with fewer PTCs per 50,000 μm2 in cases with larger tubular area. However, when the number of PTCs was adjusted for the number of tubules on the biopsy (PTC/tubule), we observed an increase in PTC/tubule in cases with increased tubular area (R = 0.31, p = 0.01), which is as expected because cases with larger tubules display a smaller number of tubules per surface area on the biopsy. Glomerular volume correlated positively with tubular area (R = 0.26, p = 0.03) and with PTC/tub (R = 0.24, p = 0.047), and negatively with a trend towards significance with PTC/50,000 μm2 (R = − 0.21, p = 0.08). There was no correlation between PTC/tubule and PTC/50,000 μm2 (R = 0.05, p = 0.70).
Scatter plots of the morphometrical parameters with each other. A glomerular volume (x-axis) with PTC/tubule (y-axis), Pearson = 0.24, p = 0.047; B glomerular volume (x-axis) with tubular area (y-axis), Pearson = 0.26, p = 0.03; C glomerular volume (x-axis) with PTC/50,000 µm2 (y-axis), Pearson = − 0.21, p = 0.08; D tubular area (y-axis) with PTC/tubule (x-axis), Pearson = 0.31, p = 0.01; E tubular area (y-axis) with PTC/50,000 µm2 (x-axis), Pearson = 0.63, P < 0.001; F PTC/50,000 µm2 (y-axis) with PTC/tubule (x-axis), Pearson = 0.05, p = 0.70. PTC/tubule: peritubular capillary per tubule; PTC/50,000 µm2: peritubular capillary per 50,000 µm2
Clinical determinants of microstructural parameters in living donor kidney biopsies
Univariable linear regression analyses did not reveal associations of clinical variables (e.g. age, sex, weight, blood pressure) with PTC/tubule or PTC/50,000 μm2 (Table 4). Body surface area (BSA) (St.β = 0.30, p = 0.01), waist/hip-ratio (St.β = 0.25, p = 0.05), systolic blood pressure (SBP, St.β = 0.35, p = 0.004) and diastolic blood pressure (DBP, St.β = 0.30, p = 0.01) were all positively associated with glomerular volume (Table 4). A trend towards significance was shown for the association of BMI with glomerular volume (St.β = 0.23, p = 0.06). Smoking correlated negatively and significantly with tubular area (St.β = − 0.38, p = 0.004). Living kidney donors with IF/TA > 5% in their biopsy were older than donors without IF/TA (t-test p = 0.002, Table 5). Also, individuals with IF/TA > 5% had a larger tubular area (Table 5). None of the clinical parameters were associated with PTC/tubule or PTC/50,000 μm2.
Associations of microstructural parameters with pre-donation GFR
Peritubular capillary/tubule was significantly and independent of age associated with the ΔmGFRdopa (= dopamine induced increase in mGFR, St.β = 0.25, p = 0.04, Table 6), but not with unstimulated mGFR (St.β = 0.17, p = 0.14). Peritubular capillary/50,000 µm2 was not associated with mGFR or ΔmGFRdopa (St.β = 0.01, p = 0.97 and St.β = 0.04, p = 0.74, respectively). Glomerular volume was significantly and positively associated with pre-donation mGFR (St.β = 0.31, p = 0.01, Table 6), but not with the ΔmGFRdopa (St.β = − 0.13, p = 0.31). Tubular area and IF/TA were not associated with pre-donation kidney function (Table 6). In a multivariable linear regression model including glomerular volume and PTC/tubule, both were independently associated with pre-donation mGFRdopa (R2 = 0.29), with glomerular volume being associated with pre-donation mGFR, and PTC/tub with pre-donation ΔmGFRdopa (Table 7). The association of PTC/tubule with mGFRdopa and ΔmGFRdopa remained significant after adjustment for tubular area (PTC/tubule with mGFRdopa: St.β = 0.29, p = 0.01; PTC/tubule with ΔmGFRdopa: St.β = 0.26, p = 0.045, Table 8).
Associations of microstructural parameters with post-donation GFR
There was no association of PTC/tubule with unstimulated mGFR at three months or five years post-donation. Glomerular volume was significantly and positively associated with both three-month, and five-year post-donation mGFR (St.β = 0.27, p = 0.02 and St.β = 0.30, p = 0.01, respectively, Table 9). Tubular area, PTC/50,000μm2 and IF/TA were not associated with post-donation mGFR (Table 6).
Discussion
The present study aimed to investigate the relationship between peritubular capillary density and other microstructural parameters including glomerular volume, tubular area and IF/TA in healthy kidneys. Furthermore, we investigated whether PTC density and other microstructural parameters were associated with clinical characteristics and pre- and post-donation-measured GFR. In this study we confirm associations of glomerular volume with mGFR, systolic blood pressure and body size measurements at donation. We found no association of PTC density (measured by either PTC/50,000 μm2 or PTC/tubule) with clinical characteristics or pre- or post-donation mGFR. However, we did find a positive association between PTC/tubule and ΔmGFRdopa. Our results indicate that glomerular volume and peritubular capillary density have a differential relationship with kidney function. In addition, our findings suggest that an increase in glomerular capillaries (i.e. glomerular volume) is not associated with an increase in number of peritubular capillaries in healthy individuals. Peritubular capillary density may therefore not provide prognostic information in potential living kidney donors.
It has been broadly recognized that peritubular capillary rarefaction plays an important role in the development of interstitial fibrosis and tubular atrophy and the progression of CKD [22,23,24,25]. In recipients of a kidney from a deceased donor, an average decrease in the PTC/tubule ratio of nearly 25% in the first three months after transplantation is associated with lower graft function [7]. Gaining knowledge on how PTCs react to early compensatory/pathological microstructural changes in the kidney can contribute to better understanding their role in the development of CKD. We observed a negative correlation (with trend towards significance) between PTC/50,000 μm2 and glomerular volume, i.e. larger glomerular volume is associated with fewer peritubular capillaries in the pre-implantation biopsy. In a case report of two cases with low birth weight (known to be associated with low nephron number and CKD), proteinuria and polycythemia, a decreased PTC per surface area was also found together with glomerular hypertrophy [26]. The association between glomerular volume and tubular area that we observed was in line with previous findings [14]. The positive relationship of glomerular volume with PTC/tubule that we found is likely due to a combination of a decrease in PTC density and an increase in tubular area (i.e., fewer tubules per picture) in individuals with larger glomeruli. Experimental studies show that even subtle alterations in tubular cells [27] or pericytes [28, 29] can induce PTC loss and IF/TA, indicating that the tubulovascular ratio (measured by PTC/tubule) provides additional information besides counting PTC numbers per surface area.
Even though PTC density is clearly decreased in advanced CKD [22,23,24,25], we found no association of PTC density with kidney function in our cohort, possibly because only healthy kidneys with normal GFR were included in this study. Total GFR is the result of single nephron GFR and number of nephrons [2], so it would be interesting for future studies to investigate whether PTC density is in fact related to single-nephron GFR, and whether this explains the lack of an association with total GFR in healthy kidneys. Our finding that IF/TA in the pre-donation biopsy is not related to measured GFR post-donation confirms results from Buus et al. [30]. We observed that individuals with more than 5% IF/TA had an increased tubular area and (a trend towards) a larger glomerular volume. In biopsies of patients with IgA nephropathy and various forms of chronic tubulointerstitial disease, hypertrophic tubuli expressed vascular endothelial growth factor (VEGF), which did not protect from PTC loss with concomitant loss of renal function [31, 32]. Further studies are needed to investigate whether tubular hypertrophy may be a first response to glomerular enlargement in healthy individuals, that, if not compensated for by an increase in PTC density, might lead to decreased tubular oxygen supply resulting in tubular atrophy, PTC loss, interstitial fibrosis, and renal function decline.
While PTC density was not associated with pre- or post-donation mGFR, we did find an association between PTC/tubule and the GFR increase after dopamine infusion (ΔmGFRdopa). Dopamine infusion induces dilatation of the afferent and efferent arterioles, and the GFR increase after dopamine infusion has been referred to as “renal stress testing” [19]. As hypothesized by Van Londen et al., the ΔmGFRdopa may be a measure of the hemodynamic response range of the kidney [19]. It could be that loss of PTC/tubule goes hand-in-hand with overall decreased tubulovascular health in the kidney, resulting in a diminished hemodynamic response to dopamine infusion, but more detailed data on renal hemodynamics are needed to further substantiate this. This would be in line with the hypothesis by Johnson et al. that subtle tubulointerstitial injury with PTC rarefaction makes individuals (and experimental animals) prone to develop salt-sensitive hypertension [33, 34]. Contrary to PTC/tubule, glomerular volume was not associated with pre-donation ΔmGFRdopa. It is known that glomerular enlargement is accompanied by an increase in single-nephron GFR [1, 2], which is also demonstrated in our study by a positive association between glomerular volume and pre-donation mGFR. We expected that an increase in glomerular volume would result in smaller ΔmGFRdopa, but this was not seen in our cohort. Power could be an issue here or maybe this association does not exist in a healthy population. In multivariable analysis, glomerular volume and PTC/tubule had an additive effect on ΔmGFRdopa, suggesting that their effects are partially independent. It might be that in individuals with larger glomerular volume, PTC/tubule provides information on the efficacy of the tubuloglomerular feedback mechanism after “renal stress”.
It has been thought that glomerular enlargement, i.e. hypertrophy, is a compensatory mechanism in response to an increased metabolic or hemodynamic demand and that over time it could lead to glomerulosclerosis, proteinuria and kidney function decline [35,36,37]. Consistent with this theory and in line with previous literature, the current study shows a positive and significant association of glomerular volume with blood pressure, waist/hip-ratio and BSA and borderline significant with BMI, all established risk factors of CKD (i.e., nephron loss) [38,39,40]. In a large U.S. cohort, glomerular volume is associated with a post-donation mGFR < 60 mL/min/1.73m2 [20], and with a ten-year post-donation mGFR < 45 mL/min/1.73m2 (but not < 60 mL/min/1.73m2) [12]. However, our study showed that larger glomerular volume was positively associated with three-month- and five-year post-donation mGFR. When comparing the characteristics of our donors to the aforementioned studies, the contrary results could possibly (partly) be explained by the seemingly higher BMI and lower pre-donation eGFR in the U.S. cohort (Mayo Clinic) compared to our cohort, which are both risk factors for lower post-donation kidney function [12]. In addition, glomerular density seemed higher in our cohort, compared to the U.S [14, 41]. Possibly, there was a lower number of nephrons in individuals in the U.S. cohort, whereas in our cohort glomerular enlargement may have remained within physiological ranges. Physiological enlargement of glomeruli is supported by Lenihan et al. who postulated that glomerular hypertrophy post-donation is probably attributable to an increase in the glomerular ultrafiltration coefficient (Kf) and not to glomerular hypertension [5]. Moreover, recent findings in our cohort showed that a stronger short-term increase in post-donation single-kidney GFR, possibly accompanied by glomerular enlargement, predicted better five- and ten-year post-donation GFR [42]. Another reason for the contradictory results could be that kidney function impairment resulting from glomerular hypertrophy was not captured by the follow-up time in our cohort. More studies with greater sample size and follow-up beyond five years are warranted to clarify these discrepancies.
Strengths of this study include the precise kidney function measurements, and the presence of dopamine-related renal function. Furthermore, our study is the first to study PTC density in relation to glomerular morphology and kidney function in healthy individuals. Although we did a power calculation, our study consisted of a small sample size, increasing the risk of missing effects due to limited power. Secondly, we used biopsies taken from living donors before surgery, during surgery and/or after surgery (respectively T1, T2 and/or T3 biopsies). We cannot exclude that the surgical procedure affects PTC density, although in living donors with only little ischemic damage this effect is deemed small [3]. Furthermore, biopsies of different regions of the kidney may have been taken; however, Denic et al. found that clinical characteristics show similar associations with glomerulosclerosis and glomerular volume at different cortical depths [36]. In addition, we found similar associations of glomerular morphology with clinical characteristics as previous studies, supporting the validity of our biopsies. Finally, the majority of our donors were Caucasian, making conclusions not generalizable to other ethnicities.
In conclusion, we found no association of PTC density with clinical characteristics or pre- and post-donation-measured GFR, while glomerular volume is associated with pre-donation blood pressure, body size measurements and GFR. Measurement of PTC density may not provide prognostic information on kidney function after living kidney donation. Our findings support that glomerular and tubular enlargement in healthy kidneys may not be accompanied by an increase in peritubular capillaries. The association of the ratio between peritubular capillaries and tubules with kidney function after dopamine infusion may provide information on hemodynamic response mechanisms and warrants further investigation. Lastly, the relationship between peritubular capillaries and glomerular and tubular parameters in the preservation of renal function merits further study in health and disease.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy of the research participants but are available from the corresponding author on reasonable request.
References
Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD et al (2016) Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis 68(1):58–67
Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP et al (2017) Single-nephron glomerular filtration rate in healthy adults. N Engl J Med 376(24):2349–2357
Denic A, Ricaurte L, Lopez CL, Narasimhan R, Lerman LO, Lieske JC et al (2019) Glomerular volume and glomerulosclerosis at different depths within the human kidney. J Am Soc Nephrol 30(8):1471–1480
Taal MW (2020) Mechanisms of progression in chronic kidney disease. In Lu ASL, Cherktow GM, Luyckx VA et al (eds) Brenner and Rector’s The Kidney, 11th edn. Elsevier, Philadelphia, pp 1742–1789
Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC (2015) Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest 125(3):1311–1318
Hill GS, Heudes D, Bariéty J (2003) Morphometric study of arterioles and glomeruli in the aging kidney suggests focal loss of autoregulation. Kidney Int 63(3):1027–1036
Hill GS, Heudes D, Jacquot C, Gauthier É, Bariéty J (2006) Morphometric evidence for impairment of renal autoregulation in advanced essential hypertension. Kidney Int 69(5):823–831
Kida Y (2020) Peritubular capillary rarefaction: an underappreciated regulator of CKD progression. Int J Mol Sci 21(21):1–24
Navar LG, Maddox DA, Munger KA (2000) The renal circulations and glomerular filtration. In: Lu ASL, Chertow GM, Luyckx VA et al (eds) Brenner and Rector’s The Kidney, 11 edn. Elsevier, Philadelphia, pp 80–114
Evans RG, Ince C, Joles JA, Smith DW, May CN, O’Connor PM et al (2013) Haemodynamic influences on kidney oxygenation: clinical implications of integrative physiology. Clin Exp Pharmacol Physiol 40(2):106–122
Lane PH, Steffes MW, Fioretto P, Mauer SM (1993) Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int 43(3):661–667
Merzkani M, Denic A, Narasimhan R, Lopez CL, Larson JJ, Kremers WK et al (2021) Kidney microstructural features at the time of donation predicts long-term risk of chronic kidney disease in living kidney donors. Mayo Clin Proc 96(1):40–51
Issa N, Vaughan LE, Denic A, Kremers WK, Chakkera HA, Park W et al (2019) Larger nephron size, low nephron number, and nephrosclerosis on biopsy as predictors of kidney function after donating a kidney. Am J Transplant 19(7):1989–1998
Elsherbiny HE, Alexander MP, Kremers WK, Park WD, Poggio ED, Prieto M et al (2014) Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol 9(16):1892–1902
Rook M, Bosma RJ, Van Son WJ, Hofker HS, Van Der Heide JJH, Ter Wee PM et al (2008) Nephrectomy elicits impact of age and BMI on renal hemodynamics: Lower postdonation reserve capacity in older or overweight kidney donors. Am J Transplant 8(10):2077–2085
Mariat C, Mjøen G, Watschinger B, Sukru Sever M, Crespo M, Peruzzi L et al (2021) Assessment of pre-donation glomerular filtration rate: going back to basics. Nephrol Dial Transplant 37(3):430–437
Steegh FMEG, Gelens MACJ, Nieman FHM, Van Hooff JP, Cleutjens JPM, Van Suylen RJ et al (2011) Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol 22(6):1024–1029
Roufosse C, Simmonds N, Clahsen-Van Groningen M, Haas M, Henriksen KJ, Horsfield C et al (2018) A 2018 reference guide to the banff classification of renal allograft pathology. Transplantation 102(11):1795–1814
van Londen M, Kasper N, Hessels NR, Navis G, de Borst MH, Bakker SJL et al (2018) Renal functional reserve capacity before and after living kidney donation. Am J Physiol Physiol 315(6):F1550–F1554
Tent H, Rook M, Stevens LA, Van Son WJ, Van Pelt LJ, Hofker HS et al (2010) Renal function equations before and after living kidney donation: a within-individual comparison of performance at different levels of renal function. Clin J Am Soc Nephrol 5(11):1960–1968
Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P et al (2017) The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol 28(1):313–320
Ishii Y, Sawada T, Kubota K, Fuchinoue S, Teraoka S, Shimizu A (2005) Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int 67(1):321–332
Seron D, Alexopoulos E, Raftery MJ, Hartley B, Cameron JS (1990) Number of interstitial capillary cross-sections assessed by monoclonal antibodies: relation to interstitial damage. Nephrol Dial Transplant 5:889–893
Bohle A, Mackensen-Haen S, Wehrmann M (1996) Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res 19(3–4):191–195
Anutrakulchai S, Titipungul T, Pattay T, Mesung P, Puapairoj A, Sirivongs D et al (2016) Relation of peritubular capillary features to class of lupus nephritis. BMC Nephrol 17:169
Asada N, Tsukahara T, Furuhata M, Matsuoka D, Noda S, Naganuma K et al (2017) Polycythemia, capillary rarefaction, and focal glomerulosclerosis in two adolescents born extremely low birth weight and premature. Pediatr Nephrol 32:1275–1278
Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T et al (2012) Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82(2):172–183
Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD (2017) Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28(3):776–784
Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV et al (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176(1):85–97
Buus NH, Nielsen CM, Skov K, Ibsen L, Krag S, Nyengaard JR (2023) Prediction of renal function in living kidney donors and recipients of living donor kidneys using quantitative histology. Transplantation 107(1):264–273
Choi YJ, Chakraborty S, Nguyen V, Nguyen C, Kim K, Suki WN et al (2000) Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol 31(12):1491–1497
Namikoshi T, Satoh M, Horike H, Fujimoto S, Arakawa S, Sasaki T et al (2006) Implication of peritubular capillary loss and altered expression of vascular endothelial growth factor in IgA nephropathy. Nephron Physiol 102:9–16
Johnson RJ, Schreiner GF (1997) Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int 52(5):1169–1179
Johnson RJ, Herrera-Acosta J, Rodriguez-Iturbe B (2002) Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 346(12):913–923
Anderson S, Rennke HG, Brenner BM (1985) Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest 76(2):612–619
Brenner BM, Lawler EV, Mackenzie HS (1996) The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 49(6):1774–1777
Deen W, Robertson C, Brenner B (1972) A model of glomerular ultrafiltration in the rat. Am J Physiol 223(5):1178–1183
Grams M, Sang Y, Levey A, Matsushita K, Ballew S, Chang A (2016) Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med 374(5):411–421
Nelson RG, Grams ME, Ballew SH, Sang Y, Azizi F, Chadban SJ et al (2019) Development of risk prediction equations for incident chronic kidney disease. JAMA 322(21):2104–2114
Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T et al (2007) Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int 71:159–166
Kanzaki G, Puelles VG, Cullen-McEwen LA, Hoy WE, Okabayashi Y, Tsuboi N et al (2017) New insights on glomerular hyperfiltration: a Japanese autopsy study. JCI Insight. https://doi.org/10.1172/jci.insight.94334
van der Weijden J, Mahesh SVK, van Londen M, Bakker SJL, Sanders JS, Navis G et al (2022) Early increase in single-kidney glomerular filtration rate after living kidney donation predicts long-term kidney function. Kidney Int 101(6):1251–1259
Acknowledgements
This study was financed in part by the Dutch kidney foundation (DKF 13A1D303).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was approved by the institutional ethical review board (METc 2014/077). All procedures were conducted in accordance with the declaration of Helsinki and declaration of Istanbul.
Informed consent
Informed consent was obtained from the patient included in the case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Weijden, J., De Hoogt, P.A., Leufkens, M.M.E. et al. The relationship of peritubular capillary density with glomerular volume and kidney function in living kidney donors. J Nephrol 36, 2111–2124 (2023). https://doi.org/10.1007/s40620-023-01734-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-023-01734-5