Abstract
Introduction
Although vascular calcification is a recognised complication for haemodialysis patients, peritoneal dialysis (PD) patients are also at risk. As such we wished to review peritoneal and urinary calcium balance and the effect of calcium containing phosphate binders (CCPBs).
Methods
Twenty-four-hour peritoneal calcium balance and urinary calcium were reviewed in PD patients undergoing their first assessment of peritoneal membrane function.
Results
Results from 183 patients, 56.3% male, 30.1% diabetic, mean age 59.4 ± 16.4 years, median 2.0 (2–6) months of PD, 29% treated by automated PD (APD), 26.8% continuous ambulatory (CAPD) and 44.2% APD with a day-time exchange (CCPD) were reviewed. Peritoneal calcium balance was positive in 42.6%, and remained positive in 21.3% after including urinary calcium losses. PD calcium balance was negatively associated with ultrafiltration (odds ratio 0.99 (95% confidence limits 0.98–0.99), p = 0.005. PD calcium balance was lowest with APD (APD − 0.45 (− 0.78 to 0.05) vs CAPD − 0.14 (− 1.18 to 0.59) vs CCPD − 0.03) − 0.48 to 0.5) mmol/day), p < 0.05, with 82.1% of patients with a positive balance prescribed icodextrin, when combining peritoneal and urinary losses. When considering CCPB prescription, then 97.8% of subjects prescribed CCPD had an over-all positive calcium balance.
Discussion
Over 40% of PD patients had a positive peritoneal calcium balance. Elemental calcium intake from CCPB had a major effect on calcium balance, as median combined peritoneal and urinary calcium losses were < 0.7 mmol/day (26 mg), so caution is required to prevent excessive CCPB prescribing, increasing the exchangeable calcium pool and thus potentially increasing vascular calcification, particularly for anuric patients.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with chronic kidney disease (CKD) are at increased risk of vascular and cardiac valvular calcification. Although initial reports suggested that this risk was greatest for end-stage CKD patients treated by haemodialysis, more recent studies have also shown that peritoneal dialysis (PD) patients are also at increased risk [1]. Historically, PD fluids were formulated with a high dialysate calcium concentration (1.75 mmol/L), as these were developed prior to the introduction of activated forms of vitamin D3 into clinical practice. Due to concerns about a positive calcium gain from these dialysates, lower calcium containing dialysates (1.25 mmol/L) were introduced in the 1990s. However, use of these dialysates was reported to induce a negative calcium balance resulting in increasing parathyroid hormone concentrations [2], and leading to some centres opting to use combinations of both higher and lower calcium dialysates [3].
Although higher calcium dialysates were reported to induce a positive peritoneal calcium balance, and lower calcium dialysates a negative balance, this was not a universal finding, and other factors including serum calcium, PD dwell time, ultrafiltration volumes, and PD modality were also reported to affect peritoneal calcium balance, along with the use of calcium and non-calcium containing phosphate binders [4,5,6].
The demographics of CKD patients treated by PD has changed over recent times in Europe and North America, with increasing numbers of elderly patients now being treated by PD, with a corresponding increase in the prevalence of both sarcopenia and osteoporosis [7, 8]. As such we wished to revisit calcium balance in a contemporary cohort of PD patients and to determine the potential effect of additional calcium containing phosphate binders.
Methods
We reviewed the PD calcium balance in a cohort of adult PD patients attending a United Kingdom (UK) university hospital for their first assessment of peritoneal membrane function. All patients had started PD electively, and in addition had undergone dual-energy X-ray absorptiometry (DXA) to assess bone mineral density, as recommended by the Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD group [10]. The DXA scans were performed after drainage of PD dialysate, post voiding and with patients weighed wearing only a thin gown and height measured using a stadiometer (Hologic Discovery A (S/N87402.1), software version 13.5.2.1, Hologic, USA) [9]. Bone mineral density was measured at the lumbar spine (L1–L5) and femoral neck, and additionally reported as T-scores, the bone density comparison to that of a 30-year-old healthy gender-matched person, and Z scores comparing bone density to the average values for a person of the same age and gender. According to WHO criteria, patients were categorized into three groups: normal bone mineral density with a T-score no less than − 1.0, osteopenia with a T-score between − 1.0 and − 2.5, and osteoporosis for patients with a T-score less than − 2.5, or a Z score of < − 2.0 [10,11,12]. Appendicular lean mass and body fat were also measured by DXA.
PD adequacy was calculated by standard methods from 24-h urinary collections and corresponding spent PD dialysate samples [13], along with estimated protein nitrogen appearance (PNA) calculated from standard equations [14, 15]. Peritoneal membrane transport was calculated from 4-h peritoneal dialysate dwell and plasma creatine concentrations using a standard 2.0 L 22.7 g/L peritoneal dialysate [13, 14]. Calcium was measured photometrically in serum, peritoneal dialysate and urine (5-nitro-5′-methyl-BAPTA method) (Roche Modular P® analyser, Roche Diagnostics Limited, Burgess Hill, UK), in a UK accredited laboratory. Peritoneal calcium removal was calculated by the difference between the daily amount of calcium instilled in fresh dialysate and the calcium measured in the 24-h effluent dialysate. Patients and staff were instructed to allow 15 s for the flush before fill, continuous ambulatory peritoneal dialysis (CAPD) technique, and the median volume measured was 90 mL, as such calcium balance in CAPD patients was then adjusted from an initial volume of 2.15 L in a fresh dialysate bag [16]. Volumetric measurements were obtained for patients dialysing with automated peritoneal dialysis (APD) cyclers without and with an additional day fill (CCPD). Peritoneal dialysis prescriptions used standard glucose dialysates (calcium 1.25 mmol/L) and icodextrin (1.75 mmol/L) (Baxter Health Care, Deerfield, Illinois, USA). No patient had been treated for PD peritonitis or had an acute hospital admission within the preceding 2 months.
Hospital computerised records were reviewed to retrieve patient demographics, relevant laboratory investigations and medications. Daily ingestion from medications was estimated from the elemental calcium content of prescribed calcium containing phosphate binders (calcium carbonate, calcium acetate and combination of calcium and magnesium carbonate). Dietary absorption of elemental calcium has been estimated between 20 and 40%, but is decreased in patients with CKD due to Vitamin D deficiency and to prevent calcium overload [17]. However, studies have shown that although absorption of normal amounts of dietary calcium are reduced, when large doses of calcium are administered, as with calcium containing phosphate binders, then absorption is similar to healthy individuals [18]. As such we have estimated elemental calcium absorption at 20%. Patient co-morbidity was assessed by Stoke-Davies and patient functionality by the Clinical Frailty Scale scores [19, 20].
Statistical analysis
Normally distributed continuous variables were expressed by mean values ± standard deviation (SD), and non-parametric continuous variables reported as median (25th and 75th percentile). Categorical variables were expressed by frequencies and percentages. Standard analyses included t-test, and ANOVA for parametric continuous variables, Mann–Whitney U test and Kruskal–Wallis for nonparametric continuous variables, and the chi-square (X2) test was performed for categorical variables. Tukey and Games-Howell adjustments were made in cases of multiple testing. Univariate analysis was carried out by Spearman correlation. Determinants of a positive peritoneal and urinary calcium balance, and then overall calcium balance considering the elemental calcium content of calcium containing phosphate binders were analysed using a step backward multivariable logistic regression using variables associated with p < 0.1 on univariate analysis. Variables were then excluded if not statistically significant, unless they improved model fit. If required, nonparametric variables were log transformed. Analyses were performed using Statistical Package for Social Sciences (SPSS Version 28.0 software, IBM Corp., Armonk, New York, USA), Prism 9.4 (Graph Pad, San Diego, USA) and Microsoft Excel Version 2107 (Build 14,228.20226). A two-tailed p value < 0.05 was considered statistically significant.
Ethics
This retrospective audit was conducted according to United Kingdom (UK) National Research Ethics guidelines and did not require additional local ethical approvals or individual patient consent. The audit was registered with the University hospital, and all patient data was anonymised in keeping with UK regulations for audit and service development.
Results
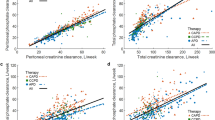
We reviewed the results from 183 adult PD patients who underwent their first assessment of peritoneal membrane function between July 2016 and April 2021, median 3 (2–7) months after starting PD, who had a DXA scan 2 (2–4) months after starting PD (Table 1). Seventy-eight (42.6%) patients had a daily positive peritoneal calcium balance, 19 of 53 (35.8%) APD, 20 of 48 (41.7%) CAPD and 39 of 82 (47.6%) CCPD (Fig. 1). When urinary calcium losses were considered, then 39 (21.3%) had a daily combined peritoneal and urinary positive calcium balance (Table 1). Patients with a positive calcium balance had lower urine volumes and urinary calcium and peritoneal ultrafiltration and calcium removal. Fewer patients were treated by APD, and more used icodextrin, a higher calcium dialysate. These patients had lower serum calcium levels and lower LS and FN DXA Z scores, and more patients had been prescribed calcium containing phosphate binders. Body weight was not significantly lower, and although PNA was lower, when adjusted for body weight (nPNA) it did not differ (0.90 ± 0.24 vs 0.94 ± 0.24 g/kg/day).
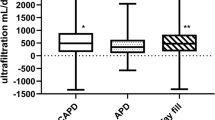
Net peritoneal calcium balance according to peritoneal dialysis (PD) modality; Net balance is the difference in the amount of calcium instilled in the fresh dialysate and that drained in 24 h. Automated PD with a dry day (APD), continuous ambulatory PD (CAPD), automated PD with a daytime exchange (CCPD). APD patients all used lower calcium dialysates (1.25 mmol/L) and the majority had a negative balance, whereas nearly all CAPD and CCPD patients used a long icodextrin exchange (1.75 mmol/L), and fewer patients treated by CAPD and CCPD had a negative daily peritoneal calcium balance. Median, interquartile and 95% confidence limits. *p < 0.05 vs APD
Elemental calcium from calcium-based phosphate binders was calculated, and assuming that only 20% of this calcium could be absorbed, the number of patients with a positive calcium balance considering peritoneal and urinary calcium fluxes and elemental calcium intake increased to 105 (57.4%) (Table 2). Only 2 (2.2%) patients who were prescribed calcium containing phosphate binders had a net calcium loss, when considering calcium absorption from the phosphate binders. Patients with a positive calcium balance had lower peritoneal and urinary calcium losses, lower serum calcium and urinary urea and total urea clearance, lower LS Z scores, body fat, and fewer were diabetic (Table 2). Besides greater prescription of calcium-based phosphate binders and alfacalcidol, and elemental calcium intake, patients had greater peritoneal urea clearance, higher haemoglobin and serum creatinine.
On univariate analysis, daily combined peritoneal and urinary calcium balance was associated with a positive peritoneal calcium balance and icodextrin usage, and negatively with body composition, particularly lean mass, urinary volume and calcium, PNA, FN T and Z scores, serum calcium and phosphate and age (Table 3). When the estimated amount of elemental calcium absorbed from calcium containing phosphate binders was added to the overall daily calcium balance, this had a major effect (Table 3).
These variables were then entered into logistic multivariable models to determine which factors were independently associated with a positive calcium balance. Considering urinary and calcium balances, a positive peritoneal and urinary calcium balance was associated with CCPD, and negatively with peritoneal ultrafiltration, serum calcium and PNA (Table 4). When considering a positive calcium balance after including elemental calcium from phosphate binders, the prescription of calcium containing phosphate binders was very strongly associated with a positive balance, whereas 24-h peritoneal calcium loss and urinary volume were associated with a negative balance.
Discussion
Medial vascular calcification was first described in diabetic patients and associated with peripheral vascular disease. Patients with CKD, and those treated by haemodialysis, and PD are also at increased risk of vascular calcification [1]. Longitudinal studies in PD patients have demonstrated an increased prevalence of cardiac valve and arterial calcification with time [21]. Interestingly, the study by Gallieni M et al., did not demonstrate any association between progressive vascular calcification and serum calcium phosphate or PTH concentrations [21]. As peritoneal dialysates contain calcium concentrations ranging between 1.25 AND 1.75 mmol/L, this may be above the normal serum ionised calcium range of 1.2–1.4 mmol/L, thereby potentially allowing calcium to diffuse from the peritoneal dialysate. Historically, glucose-containing PD dialysates had a calcium concentration of 1.75 mmol/L. Studies involving CAPD patients who were switched from 1.75 to 1.25 mmol/L dialysates [22], or comparing patients using both dialysates [23], showed that although PTH values increased, this could be compensated by increasing alfacalcidol prescription, and no changes in bone biopsy histology were reported [23].
More patients treated by CCPD had a positive peritoneal calcium balance when compared to APD, with all but 4 (5.1%) CCPD patients receiving icodextrin with a concentration of 1.75 mmol/L as the day exchange. Although we found no difference between APD and CAPD, a much smaller study reported greater peritoneal calcium removal with CAPD, however these APD and CAPD patients used the same glucose dialysates [4], whereas the great majority of our CAPD patients (93.8%) used one or more icodextrin exchanges. However, by not taking into account the additional volume in CAPD dialysate bags to allow for the flush before fill technique, this would have led to an overestimation of calcium removal reported by CAPD in this earlier study. In keeping with previous reports, calcium removal by PD was associated with both peritoneal ultrafiltration [4, 5, 19, 20] and higher serum calcium [4, 5]. Previous studies have reported that higher glucose dialysates increase calcium removal, but this was related to greater peritoneal ultrafiltration, particularly with 38.6 g/L dextrose [24, 25].
Although ultrafiltration is an important factor for peritoneal clearance of calcium, we also found that serum calcium concentration was also independently associated with calcium balance. This supports other studies which have reported that peritoneal calcium loss is greater in patients with higher serum calcium concentrations, as a higher serum calcium level would potentially influence diffusive calcium clearance, limiting or even reversing calcium influx from the peritoneal dialysate [4, 5].
Just over half of our patients had a negative peritoneal calcium balance, and this increased to just below 80% when urinary calcium losses were included. So, although urinary losses increased the proportion of patients achieving a net calcium loss with PD, PD losses made a greater contribution to the combination, due to the reduced amount of calcium in the urine of CKD patients [26]. However, the amount of elemental calcium in calcium containing phosphate binders dwarfed peritoneal and urinary losses, with only 2% of patients prescribed calcium containing phosphate binders having a negative calcium balance. Studies in CKD patients have demonstrated the role of 1,25 (OH)2 vitamin D3 in increasing intestinal calcium absorption [27]. Although there was no difference in the prescription of alfacalcidol (activated Vitamin D3) in determining peritoneal or urinary calcium losses, when including elemental calcium in prescribed phosphate binders then those patients with a positive balance were prescribed more alfacalcidol. Balance studies in CKD patients have demonstrated that administration of calcium containing phosphate binders leads to a substantially increased positive balance when compared to healthy controls [28, 29]. Our data also show that almost all patients were in a positive calcium balance when prescribed calcium containing phosphate binders. A positive calcium balance will increase the exchangeable calcium pool, thus potentially leading to increased vascular calcification. So, although patients with more osteoporosis, defined by more negative DXA lumbar spine and femoral neck Z scores, had positive peritoneal and urinary calcium balance, an increase in the exchangeable calcium pool does necessarily result in improved bone mineralisation [29, 30]. As this was a cross-sectional study, we cannot comment on whether a positive balance led to an improvement in DXA Z scores. Indeed, many studies have highlighted the relationship between osteoporosis and increased vascular calcification, so simply aiming for a positive calcium balance in PD patients may not reduce the risk of osteoporosis, but potentially increase vascular calcification [31, 32].
This was a cross-sectional study including patients recently starting PD, so most patients had some residual renal function, and as such many patients had some urinary calcium losses to mitigate against a positive peritoneal calcium balance. Although PD ultrafiltration was an important factor in determining peritoneal calcium losses, higher serum calcium also had an effect on increasing peritoneal calcium losses. Peritoneal calcium losses were greater with APD compared to CAPD and CCPD, although whether this was due to differences in dwell times between PD modalities or to the use of icodextrin with a higher dialysate calcium concentration used by CAPD- and CCPD-treated patients remains to be determined. We were unable to collect accurate dietary data to estimate dietary calcium intake and overall calcium balance. The European Best Practice Guideline group recommended that the total intake of elemental calcium should not exceed 2000 mg/day, including calcium obtained from calcium-based phosphate binders [33]. Other studies in dialysis patients have reported that dietary calcium intake was usually below 1000 mg/day, [34], in keeping with UK recommendations of 700 mg/day for adults aged 19–64 years [35]. We were unable to collect detailed information on the dietary calcium intake from our multi-ethnic patient population. However, not all dietary calcium is absorbed, and this may vary not only with age, but can also be lower with higher intakes, (45% with 200 mg/day to 15% with 2000 mg/day). In addition, some food stuffs reduce absorption, such as those containing phytates, and oxalate.
Although vascular calcification is a well recognised complication for haemodialysis patients, PD patients are also equally at risk of progressive vascular and cardiac valvular calcification [21]. Just over 40% of our patients had a positive peritoneal calcium balance. More patients treated by APD, only using 1.25 mmol/L calcium glucose dialysates, had a negative peritoneal calcium balance, whereas fewer patients treated by CCPD using a long day dwell with a 1.75 mmol/L icodextrin dialysate had a negative balance. More than 80% of patients with a positive balance were prescribed icodextrin. Thus, the calcium content of PD dialysates has a major influence on calcium balance. Unless patients have a high daily peritoneal ultrafiltration volume or residual renal function, then the prescription of higher calcium dialysates will result in a positive calcium balance. As the median combined peritoneal and urinary calcium loss was less than 30 mg/day, the prescription of calcium containing phosphate binders, with an elemental calcium content ranging between 110 and 500 mg/tablet, would have risked a positive calcium balance (Fig. 2). As such, more thought is required when prescribing calcium containing phosphate binders to PD patients to prevent excessive calcium loading and increasing the exchangeable calcium pool thereby potentially increasing the risk of progressive vascular calcification.
Availability of data and material
Data held UCL Department of Nephrology V drive, data availability upon reasonable request and within NHS guidelines.
Code availability
Not applicable.
References
Wang AY (2014) Calcium balance and negative impact of calcium load in peritoneal dialysis patients. Perit Dial Int 34(4):345–352
Armstrong A, Beer J, Noonan K, Cunningham J (1997) Reduced calcium dialysate in CAPD patients: efficacy and limitations. Nephrol Dial Transplant 12(6):1223–1228
Zhao HP, Wu B, Lu LX, Qiao J, Wu XL, Wang M (2012) Effect of combining different calcium concentration dialysate on calcium balance in peritoneal dialysis patients. Chin Med J (Engl) 125(22):4009–4013
Montenegro J, Saracho R, Aguirre R, Martinez I (1993) Calcium mass transfer in CAPD: the role of convective transport. Nephrol Dial Transplant 8(11):1234–1236
Hamada C, Tomino Y (2013) Transperitoneal calcium balance in anuric continuous ambulatory peritoneal dialysis and automated peritoneal dialysis patients. Int J Nephrol 2013:863791
Davenport A, Goel S, MacKenzie JC (1992) Audit of the use of calcium carbonate as a phosphate binder in 100 patients treated with continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 7(7):632–635
Davenport A (2022) Comparison of frailty, sarcopenia and protein energy wasting in a contemporary peritoneal dialysis cohort. Perit Dial Int. https://doi.org/10.1177/08968608221077462
Davenport A (2022) Frailty, appendicular lean mass, osteoporosis and osteosarcopenia in peritoneal dialysis patients. J Nephrol. https://doi.org/10.1007/s40620-022-01390-1
Fürstenberg A, Davenport A (2011) Assessment of body composition in peritoneal dialysis patients using bioelectrical impedance and dual-energy x-ray absorptiometry. Am J Nephrol 33(2):150–156
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO (2017) clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017(7):1–59
Kanis J, Kanis J (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporosis Int 4(6):368–381
Dimai HP (2017) Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T- and Z-score, and reference databases. Bone 104:39–43
Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, Plum J, Rodrigues A, Selgas R, Struijk D, Verger C; EBPG Expert Group on Peritoneal Dialysis. European best practice guidelines for peritoneal dialysis. 7 Adequacy of peritoneal dialysis. Nephrol Dial Transplant. 2005;20 Suppl 9:ix24-ix27
NKF-DOQI CLINICAL PRACTICE GUIDELINES FOR PERITONEAL DIALYSIS ADEQUACY (1997) Assessment of nutritional status. Am J Kidny Dis 30(3 Suppl 2):S125–S129
Vongsanim S, Salame C, Eaton S, Grimble G, Davenport A (2019) Differences between measured total nitrogen losses in spent peritoneal dialysate effluent and estimated nitrogen losses. J Ren Nutr 29(3):243–247
Tangwonglert T, Davenport A (2021) Peritoneal sodium removal compared to glucose absorption in peritoneal dialysis patients treated by continuous ambulatory peritoneal dialysis and automated peritoneal dialysis with and without a daytime exchange. Ther Apher Dial 25(5):654–662
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A (2005) A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 173(5):489–495
Klemmer PJ (2005) Calcium loading, calcium accumulation, and associated cardiovascular risks in dialysis patients. Blood Purif 23(Suppl 1):12–19
Clarkson EM, Eastwood JB, Koutsaimanis KG, De Wardener HE (1973) Net intestinal absorption of calcium in patients with chronic renal failure. Kidney Int 3:258–263
Davies SJ, Phillips L, Naish PF, Russell GI (2002) Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 17:1085–1092
Gallieni M, Caputo F, Filippini A, Gabella P, Giannattasio M, Stingone A, Farina M, ROCK-PD Study Investigators (2012) Prevalence and progression of cardiovascular calcifications in peritoneal dialysis patients: a prospective study. Bone 51(3):332–337
Piraciaba MCT, Cordeiro L, Guimarães EA, Abensur H, Pereira BJ, Jorgetti V, Moysés RMA, Elias RM (2022) A feasibility study of avoiding positive calcium balance and parathyroid hormone increase in patients on peritoneal dialysis. Bone Rep 17:101625
Sánchez C, López-Barea F, Sánchez-Cabezudo J, Bajo A, Mate A, Martínez E, Selgas R, Multicentre Study Group Collaborators (2004) Low vs standard calcium dialysate in peritoneal dialysis: differences in treatment, biochemistry and bone histomorphometry. A randomized multicentre study. Nephrol Dial Transplant 19(6):1587–1593
Simonsen O, Venturoli D, Wieslander A, Carlsson O, Rippe B (2003) Mass transfer of calcium across the peritoneum at three different peritoneal dialysis fluid Ca2+ and glucose concentrations. Kidney Int 64(1):208–215
Kurz P, Roth P, Werner E, Vlachojannis J, Grützmacher P (1992) Factors influencing transperitoneal calcium balance during CAPD. ASAIO J 38(3):M589–M592
Hill Gallant KM, Spiegel DM (2017) Calcium balance in chronic kidney disease. Curr Osteoporos Rep 15(3):214–221
Spiegel DM, Brady K (2012) Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int 81(11):1116–1122
Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M (2013) Oral calcium carbonate affects calcium but not phosphorus balance in stage 3–4 chronic kidney disease. Kidney Int 83(5):959–966
Reid IR, Bolland MJ (2020) Calcium and/or Vitamin D supplementation for the prevention of fragility fractures: who needs it ? Nutrients 12(4):1011
Chiodini I, Bolland MJ (2018) Calcium supplementation in osteoporosis: useful or harmful? Eur J Endocrinol 178(4):D13–D25
García-Gómez MC, Vilahur G (2020) Osteoporosis and vascular calcification: a shared scenario. Clin Investig Arterioscler 32(1):33–42
Cannata-Andía JB, Martín-Carro B, Martín-Vírgala J, Rodríguez-Carrio J, Bande-Fernández JJ, Alonso-Montes C, Carrillo-López N (2021) Chronic kidney disease-mineral and bone disorders: pathogenesis and management. Calcif Tissue Int 108(4):410–422
Fouque D, Vennegoor M, ter Wee P, Wanner C, Basci A, Canaud B, Haage P, Konner K, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Tordoir J, Vanholder R (2007) EBPG guideline on nutrition. Nephrol Dial Transplant 22(suppl 2):ii45–ii87
Bovio G, Esposito C, Montagna G, Brazzo S, Esposito V, Torreggiani M, Semeraro L, Cena H (2016) Inadequate macronutrient and micronutrient intakes in hemodialysis and peritoneal dialysis patients: data from a seven-day weighed dietary record. Nephron 133(4):253–260
https://www.nhs.uk. Vitamins and minerals - Calcium - NHS › Health A to Z › Vitamins and minerals. Accessed 2/09/2022
Acknowledgements
Jieying Zheng for initial data analysis.
Funding
None.
Author information
Authors and Affiliations
Contributions
AD wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional declarations
Retrospective audit complied with UK National Research Ethics guidelines.
Ethical approval
Retrospective audit complied with UK National Research Ethics Services (NRES) guidelines, formal ethical approval not required by NRES.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Davenport, A. Calcium balance in peritoneal dialysis patients treated by continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal dialysis (APD) cyclers. J Nephrol 36, 1867–1876 (2023). https://doi.org/10.1007/s40620-023-01575-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-023-01575-2