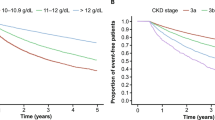
Abstract
Background
Limited data are available on the epidemiology and clinical management of anaemia in patients with non-dialysis-dependent chronic kidney disease (NDD-CKD).
Methods
This retrospective observational study was based on records from databases of five Local Health Units across Italy. Adults with reported NDD-CKD stage 3a–5 between 1 January 2014 and 31 December 2016 were identified. Annual prevalence and incidence of anaemia (age- and sex-standardised) and clinical management (erythropoiesis-stimulating agents [ESAs], intravenous [IV] iron, and blood transfusions) were evaluated. Eligibility for ESAs was defined by ≥ 2 records of Hb < 10 g/dL, or < 11 g/dL over 6 months.
Results
Overall, 101,143 individuals with NDD-CKD (3a–5) recorded between 2014 and 2016 were identified, of whom 40,020 (39.6%) were anaemic. Prevalence of anaemia was 33.8% in 2016 and incidence of anaemia was stable (11.4–12.4%) from 2014 to 2016. Prevalence and incidence of anaemia increased with CKD stage. Among eligible patients, 12.8% with Hb < 11 g/dL and 15.5% with Hb < 10 g/dL received ESAs, and the proportion treated increased with CKD stage. Among ESA-treated patients with at least 2 years of follow up, 18.4% and 19.3% received IV iron in the Hb < 11 and < 10 g/dL groups, respectively, and 16.5% and 19.4% received blood transfusions. Corresponding proportions for the overall anaemic cohort were 9.0% and 11.3%, respectively.
Conclusions
Anaemia is a significant issue in patients with NDD-CKD. Low rates of ESA treatment indicate a potential treatment gap and suggest that anaemia may not be adequately controlled in many patients.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) has an estimated global prevalence of 11–13% and is a growing public health issue worldwide [1]. Data from the 2008–2012 Cardiovascular risk profile in Renal patients of the Italian Health Examination Survey (CARHES), which examined Italian residents aged 35–79 years, showed a CKD prevalence of about 7%, with early stages (1 and 2) accounting for nearly 60% of cases [2].
Anaemia is a common complication in patients with CKD, and its prevalence increases with advancing CKD stage [3, 4]. The main cause of anaemia associated with CKD is reduced production of erythropoietin by the failing kidneys as a result of impaired oxygen-sensing mechanisms in CKD [5]. Anaemia is associated with CKD progression and other adverse outcomes, including major adverse cardiovascular events, hospitalisation, and all-cause mortality [3].
Iron supplementation and erythropoiesis-stimulating agents (ESAs) are the mainstays of treatment for anaemia in CKD [6,7,8]. Recent data from the prospective, multinational Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps) on the management of anaemia in patients with non-dialysis-dependent CKD (NDD-CKD) under nephrologist care in Brazil, France, Germany, and the USA, highlighted that anaemia monitoring and treatment are suboptimal, with many patients untreated despite guideline-based indications to treat [9, 10]. With respect to Italy, data on the prevalence and management of anaemia of NDD-CKD are limited. In a prospective cohort study of adults with NDD-CKD stage 3–5 attending 25 renal clinics in Italy in 2003, the prevalence of mild anaemia was 41.3% at the first visit [11]. Another prospective study in 19 outpatient renal clinics across Italy reported an unexpectedly high prevalence of anaemia (severe 18.0–19.3%, mild 43.2–44.0%) among patients with NDD-CKD over a 6-month period, which was linked to clinical inertia in initiating anaemia treatment [12].
Therefore, this observational study was designed to evaluate the prevalence of NDD-CKD (stage 3a–5), in addition to the prevalence and incidence of anaemia of NDD-CKD (stage 3a–5), and the therapeutic management of this complication in Italian clinical practice.
Materials and methods
This was a retrospective cohort study involving adult patients (aged ≥ 18 years) with reported NDD-CKD stage 3a–5 between 1 January 2014 and 31 December 2016 (the inclusion period). Records were extracted from administrative (demographic, hospitalisation, pharmaceutical, and outpatient specialist services) and laboratory databases of five Local Health Units (LHUs) across Italy (full details provided in Online Resource 1). The Ethics Committee of each participating LHU was notified of this study and provided approval (reference numbers: Prot. N AslVC.Farm.19.02, Prot. 96/SegCE/2019, Prot. N. 0,219,118, Prot. N. 03, and Prot. CEUR-2020-Os-029 [Online Resource 1]).
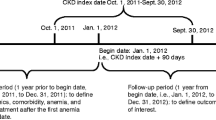
NDD-CKD (3a–5) and respective stages were defined as detailed in Online Resource 2. Anaemia was defined as a haemoglobin (Hb) value below the cut-off specified by Kidney Disease Improving Global Outcomes (KDIGO) guidelines (< 13 g/dL in males and < 12 g/dL in females) [7]. Patients were grouped into three cohorts: NDD-CKD (3a–5) cohort, anaemia of NDD-CKD (3a–5) cohort, and anaemia of NDD-CKD (3a–5) ESA-treated cohort (referred to hereafter as NDD-CKD, anaemic, and ESA-treated cohorts, respectively). The three patient cohorts had distinct index dates during the study period (Fig. 1). Patients were excluded if 1) they were on dialysis at or before the CKD index date; 2) they had a cancer diagnosis ≤ 5 years before the CKD index date; 3) the characterisation (‘look-back’) period was < 1 year; 4) the anaemia index date was ≥ 1 month before the CKD index date (incident anaemic patients); or 5) the ESA treatment index date was ≥ 1 month prior to the anaemia index date (incident ESA-treated patients). For each cohort, the index date marked the beginning of the follow-up period (Fig. 1). An identification period of ≥ 1 year before the respective index dates was used to characterise patients in terms of comorbidities (see Online Resource 2 for details) and previous treatment for the management of anaemia. The follow-up period was 2 years after the respective index date, or from index date until death, dialysis, transplant, or exiting the database, whichever occurred first.
Study design. aFor the NDD−CKD cohort (CKD index date), date of first diagnosis of CKD stage 3a–5 or earliest record of eGFR <60 mL/min/1.73 m2 within the inclusion period; for the anaemic cohort (anaemia of CKD index date): date of earliest second of two consecutive tests (within 3 months of each other) reporting Hb <13 g/dL (males) or <12 g/dL (females) within the inclusion period; for the ESA−treated cohort (ESA treatment index date), date of first ESA prescription within the inclusion period. bOr until death, dialysis, transplant, or exiting the database (whichever occurred first). CKD chronic kidney disease; eGFR estimated glomerular filtration rate; ESA erythropoiesis−stimulating agent; Hb haemoglobin; NDD−CKD non−dialysis dependent chronic kidney disease
The objectives of this study were to: 1) estimate the annual prevalence of NDD-CKD; 2) estimate the annual prevalence and incidence of anaemia among patients with NDD-CKD; and 3) evaluate the management of anaemia in patients with NDD-CKD, including treatment with ESAs (frequency and types of ESAs used), intravenous iron, and blood transfusions.
Incident and prevalent patients within the three cohorts are defined in Online Resource 3. Results were age- and sex-standardised according to Italian census data from 1 January of the year following the inclusion date. Prevalence was defined as the presence of NDD-CKD or anaemia of NDD-CKD at 31 December of the year. Incident cases were defined as newly diagnosed NDD-CKD or anaemia of NDD-CKD during the year of interest. Eligibility for ESA treatment was defined using two Hb thresholds: ≥ 2 records of Hb < 10 g/dL over 6 months; and ≥ 2 records of Hb < 11 g/dL over 6 months. The Hb < 10 g/dL threshold is recommended by international guidelines [7], while the Hb < 11 g/dL threshold is supported by the Italian Society of Nephrology [13] and defined in the Italian Therapeutic Plan for ESA prescription [14, 15]. Patients with a record of ESA prescription and ≥ 2 records of Hb < 10 or < 11 g/dL over 6 months were categorised as the ESA-treated cohort (referred to hereafter as Hb < 10 g/dL ESA-treated cohort and Hb < 11 g/dL ESA-treated cohort). ESAs were sub-categorised as short-acting (epoetin alfa/beta) or long-acting (darbepoetin alfa, methoxy polyethylene glycol-epoetin beta). Autoimmune diseases were defined by ICD-9-CM codes 696, 714, 720, 555, and 556 (see Online Resource 2 for further details). ICD-9-CM 250.0x (diabetes mellitus without mention of complication) and ATC A10 (drugs used in diabetes) codes were used as a proxy for diabetes.
Data analysis was primarily descriptive. Statistical significance was determined for binary variables using the Cochran–Armitage test, continuous variables using linear regression, and categorical variables using a Cochran–Mantel–Haenszel test. Analyses were reported by CKD stage as well as for the overall sample. All analyses were performed using STATA SE, version 12.0 (StataCorp LLC, College Station, TX, USA). End-stage renal disease (ESRD) risk assessment was determined using the Fine and Gray proportional sub-distribution hazards model, with death as a competing risk factor. Risk of death was determined using the Cox proportional hazards model. Covariates for each included: CKD stage, age, sex, cardiovascular disease, hypertension, diabetes mellitus, and autoimmune diseases.
Results
Disposition of patients
The five LHU databases included records for just over 1.5 million inhabitants, which corresponds to about 3% of the entire adult population of Italy [16]. A total of 101,143 individuals with NDD-CKD were included in the analysis, of whom 40,020 (39.6%) were anaemic (Fig. 2).
Patient flowchart. aAnaemia of NDD-CKD (3a–5) cohort. bAnaemia of NDD-CKD (3a–5) ESA-treated cohort (eligibility criterion for ESA treatment: ≥ 2 records of Hb < 11 g/dL over a 6-month period). cAnaemia of NDD-CKD (3a–5) ESA-treated cohort (eligibility criterion for ESA treatment: ≥ 2 records of Hb < 10 g/dL over a 6-month period). ESA erythropoiesis-stimulating agent; Hb haemoglobin; LHU local health unit; NDD-CKD non-dialysis dependent chronic kidney disease
Patient demographics and characteristics at index date
Over 70% of NDD-CKD patients had CKD stage 3a (Table 1). Mean age was 76.1 years and the majority of patients (> 90%) were aged ≥ 60 years. Approximately half of male patients were 60–79 years of age (50.7%), and approximately half of female patients were ≥ 80 years of age (50.2%) (Online Resource 4). No clear trends were observed for mean age across the CKD stages in the anaemic and ESA-treated cohorts (Online Resource 5 and 6). The proportion of males ranged between 39 and 48% across the CKD stages in the NDD-CKD cohort (Table 1). Of those patients with NDD-CKD, 43.3% of males and 37.0% of females were included in the anaemic cohort (Online Resource 4).
In general, rates of comorbidities tended to be slightly higher in the anaemic cohort than in the NDD-CKD cohort (Table 2 and Online Resource 7 and 8). Cardiovascular disease, hypertension, diabetes mellitus, autoimmune diseases, and myelodysplastic syndrome were detected in 23.5%, 89.9%, 32.4%, 2.1%, and 0.8% of patients with anaemia, respectively, without any apparent trends across the CKD stages (Table 2). The proportions of patients in the anaemic cohort who had received previous treatments for anaemia (ESAs, iron, blood transfusion) showed an upward trend with CKD progression (Table 2). Clinical characteristics of patients with anaemia of NDD-CKD stratified by sex can be found in Online Resource 9.
Prevalence of NDD-CKD
In 2016, the standardised prevalence of NDD-CKD was 5.6% (Table 3). CKD stage 3a was the most common (4.2%), while the prevalence of stages 3b–5 ranged from 0.1–1.0%. Overall, there was a numerically higher proportion of female (6.3%) than male (4.7%) patients with NDD-CKD in 2016.
Prevalence and incidence of anaemia of NDD-CKD
Standardised prevalence of anaemia among patients with NDD-CKD in 2016 was 33.8% (Table 3) and increased with CKD stage, rising from 28.2% among patients with stage 3a to 78.9% among those with stage 5. There was a numerically higher proportion of male (37.0%) than female (31.7%) patients with anaemia of NDD-CKD. Overall, the standardised incidence of anaemia of NDD-CKD was 12.4%, 12.3%, and 11.4% in 2014, 2015, and 2016, respectively, and increased with CKD stage within each year (Table 4). For all years, overall standardised incidence of anaemia of CKD was numerically higher for males than females.
Eligibility for ESAs and ESA treatment
Using the Hb < 11 g/dL criterion to determine ESA eligibility, 25,360 patients were considered eligible for ESA treatment, of whom 3238 were prescribed ESAs. Using the Hb < 10 g/dL criterion, 17,703 patients were considered eligible for ESA treatment, and 2742 were prescribed ESAs (Fig. 2). Over the whole study period (2014–2016), the overall proportion of patients with anaemia of NDD-CKD who were eligible for ESA treatment increased with CKD stage, regardless of the eligibility criterion used, or sex (Table 5; Online Resource 10). The proportion of ESA-eligible patients was 63.4% using the Hb < 11 g/dL threshold (57.6% for male and 68.0% for female patients), and 44.2% using the Hb < 10 g/dL threshold (40.7% for male and 47.1% for female patients). There was an upward trend across the CKD stages for the proportion of eligible patients who were treated with ESAs. The proportion of eligible patients treated with ESAs was 12.8% when the Hb threshold was < 11 g/dL, ranging from 6.7% for patients in stage 3a to 39.8% for those in stage 5. Using the stricter eligibility criterion of Hb < 10 g/dL, a slightly higher proportion of eligible patients were treated with ESAs overall (15.5%) and for each CKD stage.
Overall, 59.5% (1927/3238) of patients in the Hb < 11 g/dL ESA-treated cohort received short-acting ESAs and 49.3% (1597/3238) received long-acting ESAs. The corresponding proportions for the Hb < 10 g/dL ESA-treated cohort were 62.0% (1699/2742) and 47.4% (1300/2742), respectively. Some patients switched between the two ESA types, receiving both long- and short-acting ESAs during the study period. In the Hb < 11 g/dL ESA-treated cohort, the proportion of patients receiving short-acting ESAs decreased with CKD stage from 69.6% to 54.0%, in favour of long-acting ESAs (the proportion of patients increased from 36.1% for stage 3a to 63.7% for stage 5). A similar pattern was observed for the Hb < 10 g/dL ESA-treated cohort.
Iron supplementation and blood transfusions (patients with at least 2 years of follow up)
Intravenous (IV) iron infusions were received by 9.0% of patients in the anaemic cohort, and 18.4% and 19.3% of patients in the Hb < 11 g/dL ESA-treated and Hb < 10 g/dL ESA-treated cohorts, respectively (Table 6). Data for patients who received oral iron during the follow-up period were not consistently available in the study databases, and thus were not analysed here. Blood transfusions were received by 11.3%, 16.5%, and 19.4% of patients in the anaemic cohort, Hb < 11 g/dL ESA-treated cohort, and Hb < 10 g/dL ESA-treated cohort, respectively.
Risk of end stage renal disease and death
The sub-distribution model showed that CKD stage, age, sex, hypertension, diabetes mellitus, and anaemia had a significant effect on the development of ESRD in patients with CKD (p < 0.001 for all; Online Resource 11). In particular, anaemia was associated with a fourfold higher risk of ESRD (sub-distribution hazard ratio 4.00, confidence interval [CI]: 2.75–5.81; p < 0.001). Similarly, we found an increased risk of death in CKD patients with anaemia (hazard ratio 2.24, CI: 2.17–2.31; p < 0.001) compared to patients without anaemia.
Discussion
To date, this is the largest real-world study on anaemia of NDD-CKD in Italy. Starting with records for approximately 1.5 million inhabitants from a pool of five areas geographically distributed across Italy, 101,143 patients with NDD-CKD were identified. Anaemia of NDD-CKD was characterised in terms of epidemiology, demographics, clinical characteristics, and management.
The overall standardised prevalence of NDD-CKD in 2016 was 5.6%. Previous Italian studies describing prevalence rates for CKD have not reported data on anaemia, or focused on NDD-CKD, and used different patient inclusion criteria; therefore, those findings are not directly comparable with the results from this research [2, 17]. In this study, the standardised prevalence rate in 2016 for anaemia among patients with NDD-CKD was 33.8%, which differs from rates (41–62%) reported by other Italian studies enrolling patients regularly followed in renal clinics [11, 12]. Different prevalence rates reported by studies are expected given the variations in study populations and methodology. Patients with NDD-CKD were predominantly female in the present study. This is consistent with previous studies, which report that the prevalence of CKD was higher in female patients, despite increased severity and risk of progression of CKD in male patients [18]. In the present study, the prevalence of anaemia among patients with NDD-CKD was numerically higher in males than females, which conflicts with findings from previous studies that females were more likely to develop anaemia of CKD than males [3, 19]. This may be a consequence of the higher Hb cut-off value in male patients compared to females (< 13 g/dL versus < 12 g/dL) for the definition of anaemia in the present study. As a result, the Hb cut-off value might be attained at an earlier stage of disease in male than female patients [20]. Furthermore, comorbidities with the potential to induce anaemia (e.g., CV disease and diabetes) were reported in higher proportions of male patients than female patients in this study. Standardised prevalence and incidence of anaemia among patients with NDD-CKD increased through CKD stages, suggesting a greater need for anaemia management in patients with more advanced CKD. Trends in the standardized incidence of anaemia among patients with NDD-CKD from 2014 to 2016, overall and by CKD stage, were variable; particularly for stage 3a where anaemia was less frequent.
Anaemia is known to become more common as CKD progresses, and may be associated with increased risks of cardiovascular disease, hospitalisation, cognitive impairment, and death [6]. In the current study, hypertension was the most frequent comorbidity, with rates of 83.8% to 96.4% across the three cohorts, and without a clear relationship with CKD stage. These observations are in line with other studies in patients with NDD-CKD, in which about 80–87% of patients had hypertension [21, 22]. Similarly, no trend across CKD stages was observed for the prevalence of diabetes mellitus, a clinical condition more frequently associated with new-onset anaemia [23]. The present study also showed that a diagnosis of anaemia in patients with CKD was associated with a higher risk of ESRD, and of death, independently of risk factors related to adverse outcomes in this patient population [11, 22, 24, 25].
Among anaemic patients, we applied two different cut-offs of Hb concentration (≥ 2 records of either Hb < 10 or < 11 g/dL over 6 months) in estimating ESA eligibility to reflect international guidelines and clinical practice in Italy, respectively. This ensured that the data would be relevant worldwide and not just in Italy. Requiring ≥ 2 records over 6 months rather than a single record reduced the chance that a reduction in Hb may have had a temporary cause such as inflammation, infection, bleeding, or surgery [26]. Based on the two eligibility criteria, 44.2% (Hb < 10 g/dL) and 63.4% (Hb < 11 g/dL) of anaemic patients were eligible for ESA treatment. Regardless of the Hb cut-off, the proportion of eligible patients actually treated with ESAs was low (≤ 15.5%), although the proportion treated increased across the CKD stages (from < 10% in stage 3a to approximately 40% in stage 5). These results suggest that patients who required treatment for anaemia were inadequately treated when ESAs could have been prescribed. Therapeutic inertia has been reported previously in the context of nephrology care, with 34% of NDD-CKD patients not receiving ESAs despite having anaemia over a 6-month observation period [12]. This phenomenon has been confirmed recently by the CKDopps analysis, which showed that a large proportion of patients with anaemia of NDD-CKD did not receive anaemia medication within 1 year [10]. However, it would be an oversimplification to conclude from the present study that anaemia was undertreated. Indeed, international guidelines recommend that the decision to treat with ESAs is not based on Hb level alone, but should also consider symptoms related to anaemia, prior cardiovascular history (e.g., stroke), active or past history of malignancy, rate of decrease of Hb concentration, prior response to iron therapy, the risk for transfusion, and risks related to ESA therapy [7, 26]. Therefore, the large proportion of untreated patients may reflect the need for healthcare professionals to understand the underlying causes of anaemia before prescribing ESAs, while also reducing the burden of cost of inappropriate prescriptions on the National Health Service. In addition, this large untreated patient population may rely on a sub-optimal healthcare service, which may not refer all patients to the appropriate specialist, such as a nephrologist, to receive treatment. Of note, the present analysis was based on ESAs prescribed to patients in the outpatient setting, and ESA use during hospitalisation was not captured. However, ESAs prescribed during hospitalisation continue to be given after discharge, and therefore the absence of hospital records of ESA use is unlikely to have affected the study findings.
The proportion of anaemic patients who received IV iron infusions in the present study (9.0%) was generally consistent with data reported in other studies. A study using data from the CKDopps found that IV iron was received by 12%, 9%, 33%, and 8% of patients with anaemia of CKD stages 3a–5 in Brazil, France, Germany, and the USA, respectively [9]. Another study conducted in Italy found that IV iron was prescribed to approximately 3% of NDD-CKD patients receiving iron supplements [12]. A challenge remains in the estimation of the proportion of patients receiving oral iron therapy. Oral iron supplements are likely to be the predominant form of iron therapy in patients with NDD-CKD, but their over-the-counter availability makes their use more difficult to track. As oral iron data were not readily available in the databases used for the current study, these data were not analysed for the follow-up period.
An original finding of this study was the rate of blood transfusions in the NDD-CKD population. Studies from the USA have reported a rising prevalence of blood transfusions in the non-dialysis population, which has been linked with a concomitant and marked reduction in the use of ESAs [27]. This greater use of blood transfusions than ESAs or IV iron is believed to have been triggered by policy changes, including drug reimbursement, and additional FDA ‘black box’ warnings related to the potential adverse effects of ESAs [27]. Indeed, in one US study, the proportion of NDD-CKD patients aged 66–85 years with anaemia who received blood transfusions (22.2%) was found to be almost double the proportion observed in anaemic patients (11.3%) in the present study [27]. This finding could be explained by differences between the US and Italian healthcare systems. Of note, in this study the use of blood transfusions decreased with advancing CKD stage in parallel with increasing ESA prescriptions. This trend was independent of the severity of anaemia (either Hb < 10 g/dL or < 11 g/dL).
The ‘real-world’ setting and large sample size are major strengths of this study. Using population-based data, a ‘snapshot’ has been provided of the management of anaemia in more than 100,000 patients with NDD-CKD in Italy, a population for whom these epidemiologic data were lacking. This study, however, has limitations. We cannot exclude that other haemoglobinopathies (in particular thalassaemia) have been classified as renal anaemia in our study, although the advanced age of our study population strongly limits the potential impact of thalassaemia on our estimates. Also, the inclusion of patients with anaemia from non-CKD-related causes in this study may have resulted in a slight overestimation of the number of patients eligible for ESAs, and the occurrence of clinical inertia. Frequently, the use of large administrative databases to investigate a specific clinical question can be associated with limited suitable data, thus reducing cohort size [28]. The databases captured only direct medical healthcare resource use reimbursed by the Italian National Health Service. In patients without ICD-9-CM codes for CKD, only one creatinine value was used to define the presence of CKD, whereas international guidelines require at least two pathological creatinine values or other markers of kidney damage more than 3 months apart [29]. This discrepancy may have resulted in a partial overestimation of CKD cases. A proxy (e.g., specific drugs, hospitalisations) was used for comorbidities, such as diabetes, which may have introduced error in estimating their prevalence.
The length of look-back period can also impact estimates of prevalence and incidence: a shorter look-back period can potentially lead to the overestimation of an incidence rate due to the misclassification of prevalent and recurrent cases as incident cases [30]. The minimum look-back period for inclusion in this study was 1 year but could have been up to 8 years depending on the individual patient’s inclusion date; however, the length of look-back period was not analysed for this study. Other limitations include the evaluation of drug treatments based on prescriptions only, and therefore ESA prescriptions could have been related to conditions other than anaemia of CKD. Patients with a cancer diagnosis were excluded from the study in order to reduce this potential bias, as ESAs could be prescribed to these patients and therefore could have influenced the results for CKD and anaemia. Eligibility for ESA treatment was based on Hb levels alone. As other decision criteria for ESA treatment recommended by the guidelines, such as symptoms of anaemia and prior cardiovascular history, were not recorded in the LHU databases, it is not possible to comment on the extent to which patients with anaemia may have been undertreated [7, 26]. The iron supplementation data were limited to IV iron. Lastly, it is unknown to what extent the data for Italy from the current study may be generalised to other countries given the differences in healthcare systems.
In conclusion, this study demonstrated that anaemia is a significant issue in patients with NDD-CKD, and the incidence increases with CKD stage. The proportion of patients eligible for ESA treatment who actually received ESAs was relatively low, indicating a potential treatment gap, and suggesting that anaemia may not be adequately controlled. There appears to be an unmet need to remedy the apparent clinical inertia and improve the diagnosis and treatment of anaemia of NDD-CKD in Italy. However, this study also highlights the careful choices made by healthcare professionals when prescribing ESAs, accounting for both the underlying conditions of the patients, and the cost of inappropriate ESA prescription to the Italian National Health Service.
Data availability
Researchers may request access to anonymised participant level data, trial level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
References
Hill NR, Fatoba ST, Oke JL et al (2016) Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS ONE 11:e0158765. https://doi.org/10.1371/journal.pone.0158765
De Nicola L, Donfrancesco C, Minutolo R et al (2015) Prevalence and cardiovascular risk profile of chronic kidney disease in Italy: results of the 2008–12 National Health Examination Survey. Nephrol Dial Transpl 30:806–814. https://doi.org/10.1093/ndt/gfu383
Palaka E, Grandy S, van Haalen H et al (2020) The impact of CKD anaemia on patients: incidence, risk factors, and clinical outcomes: a systematic literature review. Int J Nephrol 2020:7692376. https://doi.org/10.1155/2020/7692376
Fishbane S, Ross DW, Hong S (2019) Anemia in non-dialysis-dependent CKD: to treat or not to treat? Am J Kidney Dis 73:297–299. https://doi.org/10.1053/j.ajkd.2018.11.006
Del Vecchio L, Locatelli F (2018) Investigational hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHI) for the treatment of anemia associated with chronic kidney disease. Expert Opin Investig Drugs 27:613–621. https://doi.org/10.1080/13543784.2018.1493455
Babitt JL, Lin HY (2012) Mechanisms of anemia in CKD. J Am Soc Nephrol 23:1631–1634. https://doi.org/10.1681/ASN.2011111078
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group (2012) KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2:279–335. https://doi.org/10.1038/kisup.2012.40
Mikhail A, Brown C, Williams JA et al (2017) Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol 18:1–29. https://doi.org/10.1186/s12882-017-0688-1
Wong MMY, Tu C, Li Y et al (2020) Anemia and iron deficiency among chronic kidney disease Stages 3–5ND patients in the chronic kidney disease outcomes and practice patterns study: often unmeasured, variably treated. Clin Kidney J 13:613–624. https://doi.org/10.1093/CKJ/SFZ091
Lopes MB, Tu C, Zee J et al (2021) A real-world longitudinal study of anemia management in non-dialysis-dependent chronic kidney disease patients: a multinational analysis of CKDopps. Sci Rep 11:1784. https://doi.org/10.1038/s41598-020-79254-6
De Nicola L, Minutolo R, Chiodini P et al (2010) Prevalence and prognosis of mild anemia in non-dialysis chronic kidney disease: a prospective cohort study in outpatient renal clinics. Am J Nephrol 32:533–540. https://doi.org/10.1159/000321468
Minutolo R, Locatelli F, Gallieni M et al (2013) Anaemia management in non-dialysis chronic kidney disease (CKD) patients: a multicentre prospective study in renal clinics. Nephrol Dial Transpl 28:3035–3045. https://doi.org/10.1093/ndt/gft338
Società Italiana di Nefrologia (2012) Linea Guida: identificazione, prevenzione e gestione della Malattia Renale Cronica nell’adulto. https://documenti.sinitaly.org/wp-content/uploads/sites/7/2017/03/LG_MRC2012.pdf. Accessed 15 Nov 2021
Agenzia Italiana del Farmaco Piano terapeutico aifa per prescrizione ssn di eritropoietine. https://www.aifa.gov.it/documents/20142/516919/piano_terapeutico_eritropoietine__ex_nota_12__allegato_1_0.pdf. Accessed 15 Nov 2021
Gazzetta Ufficiale Della Republica Italiana, Anno 160°, Numero 92; Giovedì, 18 aprile 2019. https://www.gazzettaufficiale.it/eli/gu/2019/04/18/92/sg/pdf. Accessed 15 Nov 2021
Varrella S (2020) Population in Italy 2019, by age group. https://www.statista.com/statistics/789270/population-in-italy-by-age-group/. Accessed 22 Jul 2021
Gambaro G, Yabarek T, Graziani MS et al (2010) Prevalence of CKD in Northeastern Italy: results of the INCIPE study and comparison with NHANES. Clin J Am Soc Nephrol 5:1946–1953. https://doi.org/10.2215/CJN.02400310
Goldberg I, Krause I (2016) The role of gender in chronic kidney disease. Eur Med J 1:58–64
Shiferaw WS, Akalu TY, Aynalem YA (2020) Risk factors for anemia in patients with chronic renal failure: a systematic review and meta-analysis. Ethiop J Health Sci 30:829–842. https://doi.org/10.4314/ejhs.v30i5.23
Minutolo R, Provenzano M, Chiodini P et al (2022) New-onset anemia and associated risk of ESKD and death in non-dialysis CKD patients: a multicohort observational study. Clin Kidney J 15:1120–1128. https://doi.org/10.1093/ckj/sfac004
Cozzolino M, Bolasco P, Ronco C et al (2018) Clinical management of chronic kidney disease patients in Italy: results from the IRIDE study. Nephron 140:39–47. https://doi.org/10.1159/000490769
De Nicola L, Chiodini P, Zoccali C et al (2011) Prognosis of CKD patients receiving outpatient nephrology care in italy. Clin J Am Soc Nephrol 6:2421–2428. https://doi.org/10.2215/CJN.01180211
El-Achkar TM, Ohmit SE, McCullough PA et al (2005) Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: the Kidney Early Evaluation Program. Kidney Int 67:1483–1488. https://doi.org/10.1111/j.1523-1755.2005.00226.x
Choi HS, Han K-D, Jung J-H et al (2019) The risk of end-stage renal disease in systemic lupus erythematosus. Medicine (Baltimore) 98:e16420. https://doi.org/10.1097/MD.0000000000016420
Kato S, Chmielewski M, Honda H et al (2008) Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 3:1526–1533. https://doi.org/10.2215/CJN.00950208
Locatelli F, Bárány P, Covic A et al (2013) Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transpl 28:1346–1359. https://doi.org/10.1093/ndt/gft033
St Peter WL, Guo H, Kabadi S et al (2018) Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non-dialysis-dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol 19:67. https://doi.org/10.1186/s12882-018-0861-1
Adibuzzaman M, DeLaurentis P, Hill J, Benneyworth BD (2018) Big data in healthcare the promises, challenges and opportunities from a research perspective: a case study with a model database. AMIA Annu Symp Proc 2018:384–392
Kidney Disease: Improving Global Outcomes (KDIGO) (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3:4477–4483
Kim M, Chae KH, Chung YJ et al (2020) The effect of the look-back period for estimating incidence using administrative data. BMC Health Serv Res 20:166. https://doi.org/10.1186/s12913-020-5016-y
Acknowledgements
This study was initiated and funded by Astellas Pharma Inc. Medical writing support was provided by Sarah Whitfield, PhD, and Anne-Marie Edwards, MChem, for Lumanity, funded by Astellas Pharma Inc. Francesca Donà had the initial idea for this study and worked as part of the Astellas study team until the first interim results were released.
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement. This study was initiated and funded by Astellas Pharma Inc. Medical writing support was funded by Astellas Pharma Inc.
Author information
Authors and Affiliations
Contributions
All authors reviewed and contributed to this manuscript. RM, GG, and LDE conceived the idea for this study. PDR, LDE, VP, LT, and RS designed and conducted the study. Data was acquired by LDE and VP, and analysed and interpreted by GG, RM, PDR, LDE, VP, LT, and RS.
Corresponding author
Ethics declarations
Conflict of interest
RM has been a member of Advisory Boards for Amgen and Astellas, and invited speaker at meetings supported by Amgen, Astellas, and Vifor Pharma. GG has been an advisory board member for Astellas. PDR is an employee of Astellas Pharma Italia S.P.A. RS is an employee of Astellas Pharma Europe Ltd. LDE and VP are employees of CliCon Srl, an independent health, economics, and outcomes research company. The agreement signed by Clicon Srl and Astellas Pharma Inc. does not create any entityship, joint venture, or any similar relationship between parties. LT is an employee of Astellas Pharma Europe Ltd.
Ethical approval
Ethics Committees that approved the study: Comitato Etico Interaziendale (Vercelli), Comitato Etico Interprovinciale Area 1, Comitato Etico Lazio 2, Comitato Etico per le province di L'Aquila e Teramo, and Comitato Etico Unico Regionale del Friuli Venezia Giulia.
Previous presentation
These data were presented in part as a mini-oral presentation at the 58th European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) Annual Congress, 5–8 June 2021, Berlin, Germany.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Minutolo, R., Grandaliano, G., Di Rienzo, P. et al. Prevalence, incidence, and treatment of anaemia in patients with non-dialysis-dependent chronic kidney disease: findings from a retrospective real-world study in Italy. J Nephrol 36, 347–357 (2023). https://doi.org/10.1007/s40620-022-01475-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01475-x