Abstract
Background and purpose
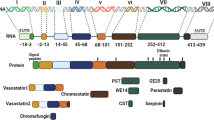
Asprosin was discovered as a new endocrine hormone originating from fibrillin-1 cleavage that plays a crucial role in various metabolic-related diseases, such as obesity, nonalcoholic fatty liver disease (NAFLD), diabetes, polycystic ovary syndrome (PCOS), and cardiovascular diseases. The purpose of this review is to describe the recent advancements of asprosin.
Method
Narrative review.
Result
This comprehensive review explores its tissue-specific functions, focusing on white adipose tissue, liver, hypothalamus, testis, ovary, heart, pancreas, skeletal muscle, and kidney.
Conclusion
Asprosin is a multifaceted protein with tissue-specific roles in various physiological and pathological processes. Further research is needed to fully understand the mechanisms and potential of asprosin as a therapeutic target. These insights could provide new directions for treatments targeting metabolic-related diseases.








Similar content being viewed by others
References
Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F et al (2016) Asprosin, a fasting-induced glucogenic protein hormone. Cell 165(3):566–579. https://doi.org/10.1016/j.cell.2016.02.063
Mazur-Bialy AI (2021) Asprosin—a fasting-induced, glucogenic, and orexigenic adipokine as a new promising player will it be a new factor in the treatment of obesity, diabetes, or infertility? A review of the literature. Nutrients 13(2):620. https://doi.org/10.3390/nu13020620
Farrag M, Ait Eldjoudi D, Gonzalez-Rodriguez M, Cordero-Barreal A, Ruiz-Fernandez C, Capuozzo M et al (2022) Asprosin in health and disease, a new glucose sensor with central and peripheral metabolic effects. Front Endocrinol (Lausanne) 13:1101091. https://doi.org/10.3389/fendo.2022.1101091
Naiemian S, Naeemipour M, Zarei M, Lari Najafi M, Gohari A, Behroozikhah MR et al (2020) Serum concentration of asprosin in new-onset type 2 diabetes. Diabetol Metab Syndr. https://doi.org/10.1186/s13098-020-00564-w
Zhang L, Chen C, Zhou N, Fu Y, Cheng X (2019) Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin Chim Acta 489:183–188. https://doi.org/10.1016/j.cca.2017.10.034
Wang R, Lin P, Sun H, Hu W (2021) Increased serum asprosin is correlated with diabetic nephropathy. Diabetol Metab Syndr 13(1):51. https://doi.org/10.1186/s13098-021-00668-x
Diao H, Li X, Xu Y, Xing X, Pang S (2023) Asprosin, a novel glucogenic adipokine implicated in type 2 diabetes mellitus. J Diabet Complicat 37(11):108614. https://doi.org/10.1016/j.jdiacomp.2023.108614
Duerrschmid C, He Y, Wang C, Li C, Bournat JC, Romere C et al (2017) Asprosin is a centrally acting orexigenic hormone. Nat Med 23(12):1444–1453. https://doi.org/10.1038/nm.4432
Hu Y, Xu Y, Zheng Y, Kang Q, Lou Z, Liu Q et al (2021) Increased plasma asprosin levels in patients with drug-naive anorexia nervosa. Eat Weight Disord 26(1):313–321. https://doi.org/10.1007/s40519-020-00845-3
Cantay H, Binnetoglu K, Gul HF, Bingol SA (2022) Investigation of serum and adipose tissue levels of asprosin in patients with severe obesity undergoing sleeve gastrectomy. Obesity 30(8):1639–1646. https://doi.org/10.1002/oby.23471
Wang CY, Lin TA, Liu KH, Liao CH, Liu YY, Wu VC et al (2019) Serum asprosin levels and bariatric surgery outcomes in obese adults. Int J Obes 43(5):1019–1025. https://doi.org/10.1038/s41366-018-0248-1
Wiecek M, Szymura J, Sproull J, Szygula Z (2019) Decreased blood asprosin in hyperglycemic menopausal women as a result of whole-body cryotherapy regardless of metabolic syndrome. J Clin Med. https://doi.org/10.3390/jcm8091428
Gozel N, Kilinc F (2021) Investigation of plasma asprosin and saliva levels in newly diagnosed type 2 diabetes mellitus patients treated with metformin. Endokrynol Pol 72(1):37–43. https://doi.org/10.5603/EP.a2020.0059
Long W, Xie X, Du C, Zhao Y, Zhang C, Zhan D et al (2019) Decreased circulating levels of asprosin in obese children. Horm Res Paediatr 91(4):271–277. https://doi.org/10.1159/000500523
Corica D, Aversa T, Currò M, Tropeano A, Pepe G, Alibrandi A et al (2021) Asprosin serum levels and glucose homeostasis in children with obesity. Cytokine 142:155477. https://doi.org/10.1016/j.cyto.2021.155477
Sunnetci Silistre E, Hatipogl HU (2020) Increased serum circulating asprosin levels in children with obesity. Pediatr Int 62(4):467–476. https://doi.org/10.1111/ped.14176
Wang M, Yin C, Wang L, Liu Y, Li H, Li M et al (2019) Serum asprosin concentrations are increased and associated with insulin resistance in children with obesity. Ann Nutr Metab 75(4):205–212. https://doi.org/10.1159/000503808
Shabir K, Brown JE, Afzal I, Gharanei S, Weickert MO, Barber TM et al (2021) Asprosin, a novel pleiotropic adipokine implicated in fasting and obesity-related cardio-metabolic disease: comprehensive review of preclinical and clinical evidence. Cytokine Growth Factor Rev 60:120–132. https://doi.org/10.1016/j.cytogfr.2021.05.002
Hekim MG, Kelestemur MM, Bulmus FG, Bilgin B, Bulut F, Gokdere E et al (2021) Asprosin, a novel glucogenic adipokine: a potential therapeutic implication in diabetes mellitus. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2021.1894178
Ke F, Xue G, Jiang X, Li F, Lai X, Zhang M et al (2020) Combination of asprosin and adiponectin as a novel marker for diagnosing non-alcoholic fatty liver disease. Cytokine 134:155184. https://doi.org/10.1016/j.cyto.2020.155184
Summers KM, Bush SJ, Davis MR, Hume DA, Keshvari S, West JA (2023) Fibrillin-1 and asprosin, novel players in metabolic syndrome. Mol Genet Metab 138(1):106979. https://doi.org/10.1016/j.ymgme.2022.106979
Zhang Z, Zhu L, Wang Z, Hua N, Hu S, Chen Y (2023) Can the new adipokine asprosin be a metabolic troublemaker for cardiovascular diseases? State Art Rev Prog Lipid Res 91:101240. https://doi.org/10.1016/j.plipres.2023.101240
Mishra I, Duerrschmid C, Ku Z, He Y, Xie W, Silva ES et al (2021) Asprosin-neutralizing antibodies as a treatment for metabolic syndrome. Elife. https://doi.org/10.7554/eLife.63784
Sakai LY, Keene DR, Renard M, De Backer J (2016) FBN1: the disease-causing gene for marfan syndrome and other genetic disorders. Gene 591(1):279–291. https://doi.org/10.1016/j.gene.2016.07.033
Milewicz DM, Grossfield J, Cao SN, Kielty C, Covitz W, Jewett T (1995) A mutation in FBN1 disrupts profibrillin processing and results in isolated skeletal features of the Marfan syndrome. J Clin Invest 95(5):2373–2378. https://doi.org/10.1172/JCI117930
Reinhardt DP, Gambee JE, Ono RN, Bachinger HP, Sakai LY (2000) Initial steps in assembly of microfibrils formation of disulfide-cross-linked multimers containing fibrillin-1. J Biol Chem 275(3):2205–2210. https://doi.org/10.1074/jbc.275.3.2205
Jensen SA, Aspinall G, Handford PA (2014) C-terminal propeptide is required for fibrillin-1 secretion and blocks premature assembly through linkage to domains cbEGF41-43. Proc Natl Acad Sci U S A 111(28):10155–10160. https://doi.org/10.1073/pnas.1401697111
Lonnqvist L, Reinhardt D, Sakai L, Peltonen L (1998) Evidence for furin-type activity-mediated C-terminal processing of profibrillin-1 and interference in the processing by certain mutations. Hum Mol Genet 7(13):2039–2044. https://doi.org/10.1093/hmg/7.13.2039
Lee SS, Knott V, Jovanovic J, Harlos K, Grimes JM, Choulier L et al (2004) Structure of the integrin binding fragment from fibrillin-1 gives new insights into microfibril organization. Structure 12(4):717–729. https://doi.org/10.1016/j.str.2004.02.023
Loeys B, Nuytinck L, Delvaux I, De Bie S, De Paepe A (2001) Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med 161(20):2447–2454. https://doi.org/10.1001/archinte.161.20.2447
Loeys BL, Gerber EE, Riegert-Johnson D, Iqbal S, Whiteman P, McConnell V et al (2010) Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3000488
Garg A, Xing C (2014) De novo heterozygous FBN1 mutations in the extreme C-terminal region cause progeroid fibrillinopathy. Am J Med Genet A 164A(5):1341–1345. https://doi.org/10.1002/ajmg.a.36449
Passarge E, Robinson PN, Graul-Neumann LM (2016) Marfanoid-progeroid-lipodystrophy syndrome: a newly recognized fibrillinopathy. Eur J Hum Genet 24(9):1244–1247. https://doi.org/10.1038/ejhg.2016.6
Zhang Y, Zhu Z, Zhai W, Bi Y, Yin Y, Zhang W (2021) Expression and purification of asprosin in Pichia pastoris and investigation of its increase glucose uptake activity in skeletal muscle through activation of AMPK. Enzyme Microb Technol 144:109737. https://doi.org/10.1016/j.enzmictec.2020.109737
Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J et al (2014) Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteom 13(2):397–406. https://doi.org/10.1074/mcp.M113.035600
Kerslake R, Hall M, Vagnarelli P, Jeyaneethi J, Randeva HS, Pados G et al (2021) A pancancer overview of FBN1, asprosin and its cognate receptor OR4M1 with detailed expression profiling in ovarian cancer. Oncol Lett 22(3):650. https://doi.org/10.3892/ol.2021.12911
Lee T, Yun S, Jeong JH, Jung TW (2019) Asprosin impairs insulin secretion in response to glucose and viability through TLR4/JNK-mediated inflammation. Mol Cell Endocrinol 486:96–104. https://doi.org/10.1016/j.mce.2019.03.001
Li E, Shan H, Chen L, Long A, Zhang Y, Liu Y et al (2019) OLFR734 mediates glucose metabolism as a receptor of asprosin. Cell Metab 30(2):319–28 e8. https://doi.org/10.1016/j.cmet.2019.05.022
Tatematsu M, Yoshida R, Morioka Y, Ishii N, Funami K, Watanabe A et al (2016) Raftlin controls lipopolysaccharide-induced TLR4 internalization and TICAM-1 signaling in a cell type-specific manner. J Immunol 196(9):3865–3876. https://doi.org/10.4049/jimmunol.1501734
da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ (2001) Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex transfer from CD14 to TLR4 and MD-2. J Biol Chem 276(24):21129–21135. https://doi.org/10.1074/jbc.M009164200
Ugur K, Aydin S (2019) Saliva and blood asprosin hormone concentration associated with obesity. Int J Endocrinol 2019:2521096. https://doi.org/10.1155/2019/2521096
Alan M, Gurlek B, Yilmaz A, Aksit M, Aslanipour B, Gulhan I et al (2019) Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol 35(3):220–223. https://doi.org/10.1080/09513590.2018.1512967
Luis C, Fernandes R, Soares R, von Hafe P (2020) A state of the art review on the novel mediator asprosin in the metabolic syndrome. Porto Biomed J. https://doi.org/10.1097/j.pbj.0000000000000108
Scheja L, Heeren J (2019) The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol 15(9):507–524. https://doi.org/10.1038/s41574-019-0230-6
Hong T, Li J-Y, Wang Y-D, Qi X-Y, Liao Z-Z, Bhadel P et al (2021) High serum asprosin levels are associated with presence of metabolic syndrome. Int J Endocrinol 2021:1–7. https://doi.org/10.1155/2021/6622129
Ceylan HI, Saygin O, Ozel TU (2020) Assessment of acute aerobic exercise in the morning versus evening on asprosin, spexin, lipocalin-2, and insulin level in overweight/obese versus normal weight adult men. Chronobiol Int 37(8):1252–1268. https://doi.org/10.1080/07420528.2020.1792482
Mirr M, Braszak-Cymerman A, Ludziejewska A, Kregielska-Narozna M, Bogdanski P, Bryl W et al (2023) Serum asprosin correlates with indirect insulin resistance indices. Biomedicines. https://doi.org/10.3390/biomedicines11061568
O’Neill B, Simha V, Kotha V, Garg A (2007) Body fat distribution and metabolic variables in patients with neonatal progeroid syndrome. Am J Med Genet A 143A(13):1421–1430. https://doi.org/10.1002/ajmg.a.31840
Miao Y, Qin H, Zhong Y, Huang K, Rao C (2021) Novel adipokine asprosin modulates browning and adipogenesis in white adipose tissue. J Endocrinol. https://doi.org/10.1530/joe-20-0503
Yin T, Chen S, Zeng G, Yuan W, Lu Y, Zhang Y et al (2022) Angiogenesis-browning interplay mediated by asprosin-knockout contributes to weight loss in mice with obesity. Int J Mol Sci. https://doi.org/10.3390/ijms232416166
Kawai T, Autieri MV, Scalia R (2021) Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol 320(3):C375–C391. https://doi.org/10.1152/ajpcell.00379.2020
Mazur-Bialy AI (2023) Asprosin enhances cytokine production by a Co-culture of fully differentiated mature adipocytes and macrophages leading to the exacerbation of the condition typical of obesity-related inflammation. Int J Mol Sci. https://doi.org/10.3390/ijms24065745
Trefts E, Gannon M, Wasserman DH (2017) The liver. Curr Biol 27(21):R1147–R1151. https://doi.org/10.1016/j.cub.2017.09.019
Liu LJ, Kang YR, Xiao YF (2021) Increased asprosin is associated with non-alcoholic fatty liver disease in children with obesity. World J Pediatr 17(4):394–399. https://doi.org/10.1007/s12519-021-00444-x
Ertuna GN, Sahiner ES, Yilmaz FM, Ates I (2023) The role of irisin and asprosin level in the pathophysiology of prediabetes. Diabetes Res Clin Pract 199:110642. https://doi.org/10.1016/j.diabres.2023.110642
Nedeva IS, Assyov Y, Karamfilova V, Vodenicharov V, Gerganova A, Hristova J et al (2023) Circulating asprosin concentrations in patients with obesity and carbohydrate disturbances. Horm Metab Res 55(4):284–289. https://doi.org/10.1055/a-2033-6109
Wang Y, Qu H, Xiong X, Qiu Y, Liao Y, Chen Y et al (2018) Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediators Inflamm 2018:9471583. https://doi.org/10.1155/2018/9471583
Wang R, Lin P, Sun H, Hu W (2021) Increased serum asprosin is correlated with diabetic nephropathy. Diabetol Metab Syndr. https://doi.org/10.1186/s13098-021-00668-x
Zhang B, Lu J, Jiang Y, Feng Y (2023) Asprosin contributes to nonalcoholic fatty liver disease through regulating lipid accumulation and inflammatory response via AMPK signaling. Immun Inflamm Dis 11(8):e947. https://doi.org/10.1002/iid3.947
Kocaman N, Kuloglu T (2020) Expression of asprosin in rat hepatic, renal, heart, gastric, testicular and brain tissues and its changes in a streptozotocin-induced diabetes mellitus model. Tissue Cell 66:101397. https://doi.org/10.1016/j.tice.2020.101397
Liu Y, Long A, Chen L, Jia L, Wang Y (2020) The asprosin-OLFR734 module regulates appetitive behaviors. Cell Discov 6:19. https://doi.org/10.1038/s41421-020-0152-4
Mishra I, Xie WR, Bournat JC, He Y, Wang C, Silva ES et al (2022) Protein tyrosine phosphatase receptor delta serves as the orexigenic asprosin receptor. Cell Metab 34:549-63e8. https://doi.org/10.1016/j.cmet.2022.02.012
Dampney RA, Michelini LC, Li DP, Pan HL (2018) Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol 315(5):H1200–H1214. https://doi.org/10.1152/ajpheart.00216.2018
Wang XL, Wang JX, Chen JL, Hao WY, Xu WZ, Xu ZQ et al (2022) Asprosin in the paraventricular nucleus induces sympathetic activation and pressor responses via cAMP-dependent ROS production. Int J Mol Sci. https://doi.org/10.3390/ijms232012595
Wei F, Long A, Wang Y (2019) The asprosin-OLFR734 hormonal signaling axis modulates male fertility. Cell Discov 5:55. https://doi.org/10.1038/s41421-019-0122-x
Maurya S, Krishna A, Lal B, Singh A (2022) Asprosin promotes steroidogenesis and spermatogenesis with improved glucose metabolism in adult mice testis. Andrologia 54(11):e14579. https://doi.org/10.1111/and.14579
Sathoria P, Chuphal B, Rai U, Roy B (2023) Molecular cloning, characterization and 3D modelling of spotted snakehead fbn1 C-terminal region encoding asprosin and expression analysis of fbn1. Sci Rep 13(1):4470. https://doi.org/10.1038/s41598-023-31271-x
Deniz R, Yavuzkir S, Ugur K, Ustebay DU, Baykus Y, Ustebay S et al (2021) Subfatin and asprosin, two new metabolic players of polycystic ovary syndrome. J Obstet Gynaecol 41(2):279–284. https://doi.org/10.1080/01443615.2020.1758926
Li X, Liao M, Shen R, Zhang L, Hu H, Wu J et al (2018) Plasma asprosin levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic-related diseases. Mediators Inflamm 2018:7375294. https://doi.org/10.1155/2018/7375294
Perez-Lopez FR, Lopez-Baena MT, Perez-Roncero GR, Chedraui P, Varikasuvu SR, Garcia-Alfaro P (2023) Asprosin levels in women with and without the polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol 39(1):2152790. https://doi.org/10.1080/09513590.2022.2152790
Chang CL, Huang SY, Hsu YC, Chin TH, Soong YK (2019) The serum level of irisin, but not asprosin, is abnormal in polycystic ovary syndrome patients. Sci Rep 9(1):6447. https://doi.org/10.1038/s41598-019-42061-9
Maurya S, Singh A (2022) Asprosin modulates testicular functions during ageing in mice. Gen Comp Endocrinol 323–324:114036. https://doi.org/10.1016/j.ygcen.2022.114036
Keskin T, Erden Y, Tekin S (2021) Intracerebroventricular asprosin administration strongly stimulates hypothalamic-pituitary-testicular axis in rats. Mol Cell Endocrinol 538:111451. https://doi.org/10.1016/j.mce.2021.111451
Maylem ERS, Spicer LJ, Batalha I, Schutz LF (2021) Discovery of a possible role of asprosin in ovarian follicular function. J Mol Endocrinol 66(1):35–44. https://doi.org/10.1530/JME-20-0218
Maylem ERS, Spicer LJ, Atabay EP, Atabay EC, Batalha I, Schutz LF (2022) A potential role of fibrillin-1 (FBN1) mRNA and asprosin in follicular development in water buffalo. Theriogenology 178:67–72. https://doi.org/10.1016/j.theriogenology.2021.11.004
Batalha IM, Maylem ERS, Spicer LJ, Pena Bello CA, Archilia EC, Schutz LF (2023) Effects of asprosin on estradiol and progesterone secretion and proliferation of bovine granulosa cells. Mol Cell Endocrinol 565:111890. https://doi.org/10.1016/j.mce.2023.111890
Acara AC, Bolatkale M, Kiziloglu I, Ibisoglu E, Can C (2018) A novel biochemical marker for predicting the severity of ACS with unstable angina pectoris: asprosin. Am J Emerg Med 36(8):1504–1505. https://doi.org/10.1016/j.ajem.2017.12.032
Moradi N, Fouani FZ, Vatannejad A, Bakhti Arani A, Shahrzad S, Fadaei R (2021) Serum levels of asprosin in patients diagnosed with coronary artery disease (CAD): a case-control study. Lipids Health Dis 20(1):88. https://doi.org/10.1186/s12944-021-01514-9
Wen MS, Wang CY, Yeh JK, Chen CC, Tsai ML, Ho MY et al (2020) The role of asprosin in patients with dilated cardiomyopathy. BMC Cardiovasc Disord 20(1):402. https://doi.org/10.1186/s12872-020-01680-1
You M, Liu Y, Wang B, Li L, Zhang H, He H et al (2022) Asprosin induces vascular endothelial-to-mesenchymal transition in diabetic lower extremity peripheral artery disease. Cardiovasc Diabetol 21(1):25. https://doi.org/10.1186/s12933-022-01457-0
Liang G, Wang S, Shao J, Jin YJ, Xu L, Yan Y et al (2022) Tenascin-X mediates flow-induced suppression of EndMT and atherosclerosis. Circ Res 130(11):1647–1659. https://doi.org/10.1161/CIRCRESAHA.121.320694
Halling JF, Pilegaard H (2020) PGC-1alpha-mediated regulation of mitochondrial function and physiological implications. Appl Physiol Nutr Metab 45(9):927–936. https://doi.org/10.1139/apnm-2020-0005
Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M et al (2006) Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4(11):e369. https://doi.org/10.1371/journal.pbio.0040369
Ko JR, Seo DY, Kim TN, Park SH, Kwak HB, Ko KS et al (2019) Aerobic exercise training decreases hepatic asprosin in diabetic rats. J Clin Med. https://doi.org/10.3390/jcm8050666
Zou J, Xu C, Zhao ZW, Yin SH, Wang G (2022) Asprosin inhibits macrophage lipid accumulation and reduces atherosclerotic burden by up-regulating ABCA1 and ABCG1 expression via the p38/Elk-1 pathway. J Transl Med 20(1):337. https://doi.org/10.1186/s12967-022-03542-0
Ross R (1999) Atherosclerosis–an inflammatory disease. N Engl J Med 340(2):115–126. https://doi.org/10.1056/NEJM199901143400207
Zhang Z, Tan Y, Zhu L, Zhang B, Feng P, Gao E et al (2019) Asprosin improves the survival of mesenchymal stromal cells in myocardial infarction by inhibiting apoptosis via the activated ERK1/2-SOD2 pathway. Life Sci 231:116554. https://doi.org/10.1016/j.lfs.2019.116554
Goodarzi G, Setayesh L, Fadaei R, Khamseh ME, Aliakbari F, Hosseini J et al (2021) Circulating levels of asprosin and its association with insulin resistance and renal function in patients with type 2 diabetes mellitus and diabetic nephropathy. Mol Biol Rep 48(7):5443–5450. https://doi.org/10.1007/s11033-021-06551-2
Zhang X, Jiang H, Ma X, Wu H (2020) Increased serum level and impaired response to glucose fluctuation of asprosin is associated with type 2 diabetes mellitus. J Diabetes Investig 11(2):349–355. https://doi.org/10.1111/jdi.13148
Chittezhath M, Wai CMM, Tay VSY, Chua M, Langley SR, Ali Y (2020) TLR4 signals through islet macrophages to alter cytokine secretion during diabetes. J Endocrinol 247(1):87. https://doi.org/10.1530/JOE-20-0131
Nackiewicz D, Dan M, He W, Kim R, Salmi A, Rutti S et al (2014) TLR2/6 and TLR4-activated macrophages contribute to islet inflammation and impair beta cell insulin gene expression via IL-1 and IL-6. Diabetologia 57(8):1645–1654. https://doi.org/10.1007/s00125-014-3249-1
Yung JHM, Giacca A (2020) Role of c-Jun N-terminal Kinase (JNK) in obesity and type 2 diabetes. Cells. https://doi.org/10.3390/cells9030706
Shabir K, Gharanei S, Orton S, Patel V, Chauhan P, Karteris E et al (2022) Asprosin exerts pro-inflammatory effects in THP-1 macrophages mediated via the toll-like receptor 4 (TLR4) pathway. Int J Mol Sci. https://doi.org/10.3390/ijms24010227
Wang R, Hu W (2021) Asprosin promotes beta-cell apoptosis by inhibiting the autophagy of beta-cell via AMPK-mTOR pathway. J Cell Physiol 236(1):215–221. https://doi.org/10.1002/jcp.29835
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293. https://doi.org/10.1016/j.cell.2012.03.017
Merz KE, Thurmond DC (2020) Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol 10(3):785–809. https://doi.org/10.1002/cphy.c190029
DeFronzo RA, Tripathy D (2009) Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32(S2):S157–S163. https://doi.org/10.2337/dc09-S302
Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A (2019) Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol 234(6):8152–8161. https://doi.org/10.1002/jcp.27603
Jung TW, Kim HC, Kim HU, Park T, Park J, Kim U et al (2019) Asprosin attenuates insulin signaling pathway through PKCdelta-activated ER stress and inflammation in skeletal muscle. J Cell Physiol 234(11):20888–20899. https://doi.org/10.1002/jcp.28694
Wiecek M, Szymura J, Maciejczyk M, Kantorowicz M, Szygula Z (2018) Acute anaerobic exercise affects the secretion of asprosin, irisin, and other cytokines-a comparison between sexes. Front Physiol 9:1782. https://doi.org/10.3389/fphys.2018.01782
Zhang H, Hu W, Zhang G (2020) Circulating asprosin levels are increased in patients with type 2 diabetes and associated with early-stage diabetic kidney disease. Int Urol Nephrol 52(8):1517–1522. https://doi.org/10.1007/s11255-020-02509-8
Deng X, Zhao L, Guo C, Yang L, Wang D, Li Y et al (2020) Higher serum asprosin level is associated with urinary albumin excretion and renal function in type 2 diabetes. Diabetes Metab Syndr Obes 13:4341–4351. https://doi.org/10.2147/DMSO.S283413
Author information
Authors and Affiliations
Contributions
Ying Zhang: conceptualization, writing-original draft preparation, literature search, visualization; Ping Yang: writing—reviewing and editing, literature search, supervision; Xiaojun Zhang: writing—reviewing and editing, literature search, visualization; Shudong Liu: writing—reviewing and editing, literature search; Kai Lou: conceptualization, writing—reviewing and editing, visualization, supervision. All authors revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Yang, P., Zhang, X. et al. Asprosin: its function as a novel endocrine factor in metabolic-related diseases. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-024-02360-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40618-024-02360-z