Abstract
Purpose
Cushing’s disease is associated with substantial morbidity and impaired quality of life (QoL) resulting from excess cortisol exposure. The current study explored improvements in clinical signs and additional specific manifestations of hypercortisolism during osilodrostat (potent oral 11β-hydroxylase inhibitor) therapy by degree of control of mean urinary free cortisol (mUFC).
Methods
LINC 3 (NCT02180217) was a prospective, open-label, 48-week study of osilodrostat (starting dose: 2 mg bid; maximum: 30 mg bid) that enrolled 137 adults with Cushing’s disease and mUFC > 1.5 times the upper limit of normal (ULN). mUFC (normal range 11‒138 nmol/24 h), cardiometabolic parameters (blood pressure, weight, waist circumference, body mass index, total cholesterol, fasting plasma glucose, glycated haemoglobin), physical manifestations of hypercortisolism (facial rubor, striae, fat distribution, bruising, hirsutism [females], muscle atrophy) and QoL were evaluated. mUFC was defined as controlled if ≤ ULN, partially controlled if > ULN but ≥ 50% reduction from baseline, and uncontrolled if > ULN and < 50% reduction from baseline. Concomitant medications were permitted throughout the study.
Results
At weeks 24 and 48, respectively, mUFC was controlled in 93 (67.9%) and 91 (66.4%) patients, partially controlled in 20 (14.6%) and 13 (9.5%), and uncontrolled in 24 (17.5%) and 33 (24.1%). Overall, mean improvements from baseline in cardiometabolic at week 24 were greater in patients with controlled or partially controlled versus uncontrolled mUFC; at week 48, improvements occurred irrespective of mUFC control. Generally, physical manifestations and QoL progressively improved from baseline irrespective of mUFC control.
Conclusions
Improvements in clinical signs and additional specific manifestations of hypercortisolism associated with Cushing’s disease occurred alongside decreases in mUFC.
Trial registration NCT02180217 (first posted July 2014).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cushing’s disease, the most common form of endogenous Cushing’s syndrome, is a rare and debilitating condition caused by an adrenocorticotropic hormone (ACTH)-secreting pituitary tumour, resulting in excessive secretion of cortisol from the adrenal glands [1]. The subsequent exposure to elevated cortisol levels is associated with a wide range of comorbidities, impaired health-related quality of life (HRQoL) and, especially in the case of untreated disease, increased mortality [2]. Many clinical features and physical manifestations are associated with Cushing’s disease. Clinical complications include visceral obesity, hypertension, impaired glucose metabolism (including diabetes) and dyslipidaemia [3, 4]; these complications result in an increased risk of cardiovascular disease, one of the main causes of death in patients with Cushing’s disease [2, 5]. Dermatol-ogical manifestations of hypercortisolism, most commonly including skin thinning with purple striae, ecchymoses and hirsutism (in females), together with fatigue and proximal myopathy, are also commonly associated with Cushing’s disease [2, 3]. Multiple comorbidities are a defining feature of patients with Cushing’s disease [6] and contribute to impaired patient HRQoL [7], which may persist even following remission [8]. Osilodrostat is an oral adrenal steroidogenesis inhibitor that targets 11β-hydroxylase (the enzyme that catalyses the final step of cortisol synthesis) and is currently approved for the treatment of patients with Cushing’s disease (USA) or endogenous Cushing’s syndrome (EU and Japan) who are eligible for medical therapy [9,10,11].
The current report explores changes in clinical signs and additional specific manifestations of hypercortisolism in patients with Cushing’s disease following medical treatment with osilodrostat. Osilodrostat treatment led to rapid and sustained normalisation of mean urinary free cortisol (mUFC) in many patients during the 48 week core phase of the Phase III LINC 3 study [12], which continued into the long-term extension [13]. The majority of enrolled patients (n = 132/137, 96.4%) achieved normal mUFC (below the upper limit of normal [ULN]) at least once during the study, and more patients maintained a complete response with osilodrostat versus with placebo following the 8 week randomised-withdrawal period (n = 31 [86.1%] vs n = 10 [28.6%]; odds ratio 13.7 [95% confidence interval (CI) 3.7–53.4]; P < 0.0001) [12]. Late-night salivary cortisol (LNSC) was also measured; mean levels rapidly decreased within the first 12 weeks, then remained below baseline values [12]. However, LNSC data were not recorded for all patients at all time points, precluding meaningful analyses of outcomes in patients with control of mUFC and/or LNSC in the LINC 3 study alone. mUFC is regularly used to evaluate the efficacy of medical therapies in patients with Cushing’s disease, and achieving mUFC normalisation is a key treatment goal [5]. Furthermore, if mUFC improves but does not normalise, alternative medical therapy options may be considered [5]. Changes in clinical features of hypercortisolism according to the degree of mUFC control achieved by each patient are examined in this report.
Methods
Patients and study design
Full details of the study design for the 48 week core phase of the LINC 3 study have been described previously [12]. Briefly, adult patients with confirmed Cushing’s disease and mUFC (calculated from three 24 h urine samples) > 1.5 × ULN were enrolled. All patients received osilodrostat (starting dose: 2 mg twice daily [bid]; maximum dose: 30 mg bid) throughout the study, except those randomised to placebo during an 8-week randomised-withdrawal phase after receiving osilodrostat for 26 weeks (weeks 26–34). Dose titration was permitted based on efficacy (to achieve the goal of mUFC ≤ ULN) and tolerability. Concomitant medications were permitted throughout the study, including medications for the treatment of hypertension, diabetes and dyslipidaemia. Patients were classified as having hypertension if they had one or more of the following: history of antihypertensive medication; medical history of hypertension; baseline systolic blood pressure (SBP) > 130 mmHg; baseline diastolic blood pressure (DBP) > 90 mmHg. Glycaemic status was defined as follows based on fasting plasma glucose (FPG): normoglycaemia, FPG < 100 mg/dL; impaired fasting glucose, FPG 100– < 126 mg/dL; diabetic, FPG ≥ 126 mg/dL. Patients could also be classified as having diabetes at baseline if they had one or more of the following: history of antidiabetic medication; medical history of diabetes mellitus; glycated haemoglobin (HbA1c) ≥ 6.5%; FPG ≥ 126 mg/dL. Patients were classified as having dyslipidaemia if they had one or more of the following: history of lipid-lowering medication; medical history of dyslipidaemia; baseline total cholesterol ≥ 200 mg/dL (≥ 5.2 mmol/L); triglycerides > 150 mg/dL (> 1.7 mmol/L); high-density lipoprotein cholesterol (HDL-c) < 40 mg/dL (< 1.0 mmol/L) in male patients or < 50 mg/dL (< 1.3 mmol/L) in female patients; low-density lipoprotein cholesterol (LDL-c) > 100 mg/dL (> 2.6 mmol/L). The study was conducted in accordance with the Declaration of Helsinki, with an independent ethics committee/institutional review board at each site approving the study protocol. Patients provided written informed consent to participate. The LINC 3 study is registered at ClinicalTrials.gov (NCT02180217).
Study objectives and assessments
In the present post hoc analysis from the LINC 3 study, changes from baseline in clinical features of hypercortisolism and HRQoL were assessed by degree of mUFC control. Mean 24 h UFC concentration (three 24 h urine samples) was determined using liquid chromatography-tandem mass spectrometry (normal range: 11–138 nmol/24 h [4–50 μg/24 h]) at baseline, then every 2–4 weeks (depending on the study period), as used in the assessment of the primary and secondary endpoints of the study [12]. Degree of mUFC control was defined as controlled (mUFC ≤ ULN), partially controlled (> ULN but ≥ 50% reduction from baseline) or uncontrolled (mUFC > ULN and < 50% reduction from baseline).
Physical examination, including measurement of body weight (with calculation of body mass index [BMI]), waist circumference, SBP and DBP, was conducted at baseline, then every 2–4 weeks (depending on the study period). SBP and DBP were recorded as the mean of three values at 1–2 min intervals after the patient had been sitting for 5 min (with back supported and both feet placed on the floor).
Physical features of hypercortisolism (facial rubor, hirsutism [in females], striae, bruising, proximal muscle wasting, central obesity, supraclavicular and dorsal fat pads) were assessed at baseline, then at the end of each study period (weeks 12, 24, 34 and 48). Two photographs were taken, one frontal and one lateral, from the shoulders up to assess facial rubor and supraclavicular and dorsal fat pads. Two additional photographs were taken, one frontal and one dorsal, of the trunk with the patient in a standing position to assess hirsutism, striae, proximal muscle atrophy, central obesity and bruising. Photographs were rated subjectively by local investigators (0 = absent; 1 = mild; 2 = moderate; 3 = severe).
Assessment of FPG, HbA1c and cholesterol was performed at a central laboratory. FPG and HbA1c were measured every 12 weeks, except during the randomised-withdrawal period, when measurements were taken every 2 weeks. Cholesterol was measured every 4 weeks throughout the study.
Bone mineral density (BMD; L1–L4 lumbar spine and total hip) was assessed by dual-energy X-ray absorptiometry and standardised against BMD T-score at baseline and week 48. Each patient was scanned with the same instrument throughout the study, and images were reviewed centrally.
HRQoL was measured using the CushingQoL questionnaire (scored from 12 [worst] to 60 [best]) [14] and the Beck Depression Inventory II (BDI-II; scored from 0 [best] to 63 [worst]) [15] prior to any clinical assessments, drug dosing or diagnostic testing at baseline, then at weeks 4, 8, 12, 24, 26, (28 for randomised patients only,) 30, (32 for randomised patients only,) 34 and 48.
Statistical methods
All data were analysed descriptively. Mean change from baseline and corresponding 95% CIs were provided for clinical signs of hypercortisolism by degree of mUFC control at weeks 24 and 48. Correlations were evaluated using the Pearson’s correlation coefficient.
Results
Patient characteristics
Overall, 77.4% of patients enrolled in the study were female. Mean baseline mUFC was 7.3 × ULN (Table 1). All patients had at least one relevant medical history/current medical condition, which included hypertension, diabetes and dyslipidaemia (Table 1). At baseline, the most frequently reported physical manifestation rated as severe was central obesity (16.1% mild, 29.9% moderate, 25.5% severe). Additional physical manifestations were rated mostly as mild or moderate: supraclavicular fat pad (24.8% mild, 35.0% moderate, 8.8% severe); dorsal fat pad (32.1% mild, 34.3% moderate, 7.3% severe); facial rubor (34.3% mild, 23.4% moderate, 5.8% severe); hirsutism (females; 37.7% mild, 17.0% moderate, 3.8% severe); proximal muscle atrophy (30.7% mild, 15.3% moderate, 5.8% severe); striae (24.1% mild, 18.2% moderate, 6.6% severe); ecchymoses (22.6% mild, 11.7% moderate, 4.4% severe).
Effect of osilodrostat on mUFC
Median duration of osilodrostat exposure from baseline to core study data cut-off was 75 weeks (range 1–165, IQR 48–117), and average median daily osilodrostat dose was 7.1 mg/day (range 1.1–53.9, IQR 3.8–14.0). The majority (71.5%) of patients achieved control of mUFC (≤ ULN) by week 12, with a further 13.9% achieving partial control. The proportion of patients with mUFC ≤ ULN remained high throughout the core study (Fig. 1). Overall, 132 (96.4%) patients achieved control of mUFC at least once during the 48 weeks. Descriptive analyses of the proportion of patients with mUFC control by age, sex, race and time since diagnosis suggest that these factors do not affect response to treatment at weeks 24 and 48 (Fig. 2; Supplementary Table 1).
Proportion of patients with controlled, partially controlled and uncontrolled mUFC over time. Based on patients in the full analysis set (N = 137). Patients who had discontinued or otherwise had missing mUFC values at a given visit were counted as uncontrolled. Controlled, mUFC ≤ ULN; partially controlled, mUFC > ULN but ≥ 50% reduction from baseline; uncontrolled, mUFC > ULN and < 50% reduction from baseline
Changes in blood pressure
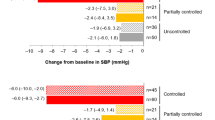
In total, 86.9% of patients were classified as hypertensive at baseline (Table 1). Of patients with baseline SBP > 130 mmHg (n = 79), 58.2, 50.6 and 49.4% had SBP ≤ 130 mmHg after 12, 24 and 48 weeks of osilodrostat treatment, respectively; of patients with baseline DBP > 90 mmHg (n = 50), 72.0, 62.0 and 66.0% had DBP ≤ 90 mmHg at weeks 12, 24 and 48, respectively. In patients without hypertension at baseline, both SBP and DBP generally remained stable during osilodrostat treatment. At week 48, five patients with baseline SBP ≤ 130 mmHg had SBP > 130 mmHg, and four patients with baseline DBP ≤ 90 mmHg had DBP > 90 mmHg. Of all patients classified as hypertensive at baseline, at week 48, mean (95% CI) SBP and DBP improved both in patients who did and in those who did not receive antihypertensive medications during the study (SBP − 8.7 [− 12.6, − 4.8] and − 14.5 [− 20.1, − 9.0] mmHg, respectively; DBP − 5.1 [− 7.7, − 2.5] and − 10.2 [− 14.5, − 6.0] mmHg, respectively). Overall, at week 48, 40% (n = 34/85) of patients taking antihypertensive medication at baseline had either stopped or reduced the dose; 40% (n = 34/85) had an increase in dose of antihypertensive medication or number of medications. Of patients with controlled, partially controlled and uncontrolled mUFC at week 48, 43.1% (n = 25/58), 33.3% (n = 3/9) and 33.3% (n = 6/18), respectively, had either stopped or reduced the dose of antihypertensive medication; 39.7% (n = 23/58), 44.5% (n = 4/9) and 38.9% (n = 7/18), respectively, had an increase in dose or number of medications, and 17.2% (n = 10/58), 22.2% (n = 2/9) and 27.8% (n = 5/18), respectively, had no change. Clinically relevant improvements from baseline in mean (95% CI) SBP and DBP were noted by week 24 in patients with controlled (− 7.1 [− 10.3, − 3.9] and − 4.9 [− 7.1, − 2.7] mmHg, respectively) and partially controlled mUFC (− 7.2 [− 14.0, − 0.4] and − 5.2 [− 9.8, − 0.6] mmHg), but not in patients with uncontrolled mUFC (2.0 [− 9.7, 13.7] and 5.3 [− 3.3, 13.9] mmHg). By week 48, improvements in both SBP and DBP were seen irrespective of mUFC control (Fig. 3A). There was no correlation between change from baseline in mUFC and change from baseline in SBP or DBP at week 24 (r = − 0.02, P = 0.8326 and r = 0.02, P = 0.8160, respectively) and a weak correlation at week 48 (r = 0.20, P = 0.0433 and r = 0.18, P = 0.0715, respectively).
Changes in body weight, waist circumference and BMI
Most patients had physical evidence of central obesity at baseline; mean weight was 80.8 kg and mean BMI was 30.3 kg/m2 (Table 1). Clinically relevant improvements in mean (95% CI) weight, waist circumference and BMI were noted by week 24 in patients with controlled mUFC (− 2.7 [− 3.6, − 1.8] kg, − 3.4 [− 4.9, − 1.8] cm and − 1.1 [− 1.4, − 0.7] kg/m2, respectively); improvements were not clinically relevant in patients with partially controlled mUFC (− 1.7 [− 3.8, 0.3] kg, − 2.4 [− 5.0, 0.3] cm and − 0.6 [− 1.4, 0.2] kg/m2) or uncontrolled mUFC (− 1.6 [− 3.5, 0.3] kg, 0.1 [− 2.4, 2.6] cm and − 0.6 [− 1.4, 0.1] kg/m2). By week 48, improvements from baseline were seen irrespective of mUFC control (Fig. 3A). No correlation was observed between change in mUFC and change in mean body weight, waist circumference or BMI at either week 24 or week 48 (Supplementary Table 2).
Changes in lipid profile
In total, 83.9% of patients had dyslipidaemia at baseline (Table 1). Mean changes in cholesterol (including LDL-c and HDL-c) and triglycerides were similar across mUFC response subgroups at both week 24 and week 48 (Fig. 3B). There were no strong correlations between change in mUFC and change in total cholesterol, LDL-c, HDL-c or triglycerides at either week 24 or week 48 (Supplementary Table 2).
Changes in glycaemic status
At baseline, 61 (44.5%) patients were classified as diabetic. Based on FPG levels only, most (67.9%) patients had normoglycaemia (FPG < 100 mg/dL) at baseline; 26.3% of patients had either impaired fasting glucose or diabetes (FPG ≥ 100 mg/dL). Of patients with baseline FPG ≥ 100 mg/dL (n = 36), 58.3, 63.8 and 44.4% had FPG < 100 mg/dL by weeks 12, 24 and 48, respectively. In total, at week 48, 48.8% (n = 21/43) of patients taking antidiabetic medication at baseline had stopped or reduced the dose, and 23.3% (n = 10/43) increased the dose or number of medications. Of patients with controlled, partially controlled and uncontrolled mUFC at week 48, 55.6% (n = 15/27), 75% (n = 3/4) and 25% (n = 3/12), respectively, had either stopped or reduced the dose of antidiabetic medication; 18.5% (n = 5/27), 25% (n = 1/4) and 33.3% (n = 4/12), respectively, had an increase in dose or number of medications, and 25.9% (n = 7/27), 0% (n = 0/4) and 41.7% (n = 5/12), respectively, had no change. Improvements in mean (95% CI) FPG and HbA1c at week 24 occurred irrespective of mUFC control, although they were more evident in patients with controlled mUFC (− 13.3 [− 18.9, − 7.7] mg/dL and − 0.3% [− 0.5, − 0.2], respectively) or partially controlled mUFC (− 21.2 [− 37.0, − 5.4] mg/dL and − 0.4% [− 0.9, 0.0]) than in those with uncontrolled mUFC (− 7.4 [− 17.1, 2.4] mg/dL and − 0.0% [− 0.3, 0.3]). A similar pattern of mean changes in FPG and HbA1c was observed at week 48 (Fig. 3C). There was a weak correlation between change from baseline in mUFC and change from baseline in FPG (r = 0.25, P = 0.008) and HbA1c (r = 0.23, P = 0.012) at week 24. At week 48, the correlation was stronger for FPG (r = 0.33, P = 0.001) but absent for HbA1c (r = 0.14, P = 0.161).
Changes in BMD
Mean baseline L1‒L4 lumbar spine and total hip BMD in all patients was 1.0 and 0.9 g/cm2, respectively. Mean change from baseline to week 48 in BMD at both the lumbar spine and total hip was 0.0 g/cm2 in both male and female patients; as a mean percentage change from baseline (95% CI), the increase in BMD was more pronounced in males (lumbar spine: + 3.9% [− 0.2, 8.0]; total hip: + 1.7% [− 0.5, 4.0]) than females (lumbar spine: + 2.7% [1.3, 4.1]; total hip: − 0.1% [− 1.6, 1.3]). The degree of mUFC response had no effect on outcomes (Supplementary Table 3).
Changes in physical manifestations of hypercortisolaemia
Physical manifestations of hypercortisolaemia were prevalent at baseline. At weeks 24 and 48, improvements in rated severity score from baseline occurred across all physical manifestations, with few patients rated with worsening scores. Improvements in physical manifestation severity scores were seen irrespective of the degree of mUFC response, with the exception of proximal muscle atrophy at week 24, whereby a numerically larger proportion of patients with controlled (26.5%) and partially controlled (43.8%) mUFC had improved severity scores compared with uncontrolled patients (14.3%; Fig. 4).
Proportion of patients with improvements in rated severity score from baseline for physical manifestations of hypercortisolism by degree of mUFC control at week 24 and week 48. An improvement was defined as the symptom score being lower (ie less severe) than at baseline. The denominator for the percentage is the number of patients in the full analysis set (all enrolled patients who received at least one dose of osilodrostat) with data available at both baseline and the given visit. *Females only
Changes in HRQoL
Clinically meaningful improvements in mean CushingQoL scores for the overall study population were reached at weeks 26, 30, 32, 34 and 48, and for mean change in BDI-II scores at weeks 24, 26, 28, 30 and 48 [12, 14, 15]. At week 24, numerical improvements in both mean (95% CI) CushingQoL and BDI-II scores occurred irrespective of whether patients had controlled mUFC (9.2 [6.1, 12.4] and − 3.6 [− 5.4, − 1.9], respectively), partially controlled mUFC (9.4 [0.9, 17.8] and − 6.8 [− 11.2, − 2.4]) or uncontrolled mUFC (10.1 [2.0, 18.2] and − 4.6 [− 11.2, 2.0]). A similar finding was observed at week 48, although the 95% CIs were notably wider for patients with uncontrolled mUFC (Fig. 5). There was a weak correlation between change in mUFC and change in CushingQoL score at week 24 (r = − 0.15, P = 0.0875), which was not evident at week 48 (r = 0.02, P = 0.8028). A weak correlation between change in mUFC and change in BDI-II score was observed at both week 24 and week 48 (r = 0.24, P = 0.0081 and r = 0.30, P = 0.0016, respectively).
Discussion
Cushing’s syndrome, including Cushing’s disease, is associated with many clinical complications that affect multiple organ systems. The patients enrolled in LINC 3 typically presented with multiple underlying conditions and exhibited sometimes severe physical manifestations of hypercortisolism. Treatment with osilodrostat resulted in rapid and sustained normalisation of mUFC in many patients, with almost all patients achieving control of mUFC at least once during the study. Alongside controlling excess cortisol levels, medical therapy with osilodrostat resulted in improvements in multiple clinical features of hypercortisolism, which supports findings from trials of different medical therapies that act by reducing cortisol levels [16,17,18,19,20,21,22,23]. In the present study, to fully explore improvements in specific clinical signs and additional specific manifestations of hypercortisolism during osilodrostat therapy, a large number of clinical features were analysed over several time points. Initial improvements following osilodrostat treatment, as measured at week 24, were generally greater in patients with controlled or partially controlled mUFC than in patients with uncontrolled mUFC, which may be clinically important for some patients. It is important to note that the addition or adjustment of concomitant medications to treat comorbidities was permitted throughout the study and may have contributed to over- or underestimation of the observed improvements, such as in hypertension, dyslipidaemia and diabetes. The results of the present analysis also demonstrated that age, sex, race or time since diagnosis did not affect a patient achieving mUFC control during osilodrostat treatment.
Improving cardiometabolic parameters, such as blood pressure, weight, BMI and waist circumference, is an important aspect of care for patients with Cushing’s syndrome in order to reduce the risk of cardiovascular disease [24,25,26,27]. During osilodrostat treatment in patients with Cushing’s disease in the LINC 3 study, improvements in both SBP and DBP were observed, with over half of the patients with high blood pressure at baseline achieving normal SBP and DBP within 12 weeks, which remained within normal limits throughout the 48 weeks of the study. Furthermore, 40.0% of patients were able to stop or reduce their medication for hypertension during the study as prescribed at the discretion of the investigator. In addition, dose or number of antihypertensive medications was reduced or stopped in a numerically greater proportion of patients with controlled mUFC than in those with partially or uncontrolled mUFC, whereas a similar proportion of patients had an increase in dose or number of antihypertensive medications irrespective of mUFC control. Notably, improvements in SBP and DBP were numerically greater in patients with hypertension at baseline who did not receive antihypertensive medication during the study. Furthermore, patients who achieved control or partial control of mUFC at week 24 had numerically greater improvements in both SBP and DBP than those with uncontrolled mUFC. By week 48, when most patients had controlled mUFC (or could have achieved control of mUFC at some point during this time), it was not unexpected that improvements were evident irrespective of mUFC control. The correlation between change in mUFC and change in SBP or DBP from baseline was weak and indicated a weak relationship at week 48. Taken together, these findings highlight the benefits of controlling mUFC, which has the concomitant benefit of also improving blood pressure.
Although there was no correlation between change in mUFC and change in body weight, waist circumference or BMI, again, there was a tendency for patients with controlled mUFC at week 24 to have better outcomes. As weight and waist circumference are only gross indicators of fat mass, it is also possible that improvements occurred in visceral body fat that were not detected in this study. Overall, changes in cholesterol and triglycerides occurred irrespective of mUFC control, and there was no indication that improvements were related to the reduction in mUFC. Notably, some clinical features of hypercortisolism may take years to reverse, particularly when they have been present for some time [28].
Impaired glucose tolerance, including diabetes, is one of the main contributors to mortality identified in patients with Cushing’s syndrome [29,30,31]. During treatment with osilodrostat in the LINC 3 study, approximately 50% of patients with Cushing’s disease reduced their FPG levels to within normal limits, with some patients able to reduce or stop antidiabetic medications as prescribed at the discretion of the investigator; this was particularly notable in patients with controlled or partially controlled mUFC compared with those with uncontrolled mUFC. Improvements in FPG and HbA1c were more evident in patients with controlled or partially controlled mUFC at both weeks 24 and 48. Furthermore, the correlation between change in mUFC and change in FPG from baseline was notable at both time points, indicating a possible direct relationship. Again, these data support the notion of aiming to control mUFC given the additional benefits to patients in relation to comorbidities.
Reduced BMD is common in patients with Cushing’s syndrome [2]. Even in patients with normal BMD, there is an increased risk of fractures as a result of cortisol excess [5, 32]. Data suggest that bone damage is reversible with control of hypercortisolism following surgery [2, 33]; however, data are limited on the effects of medical therapies [33]. Data from the 48-week core phase of the LINC 3 study indicated some improvement in BMD, which was more pronounced in male than female patients. It is possible that cortisol had not been at normal levels for long enough to have a clinically significant impact on BMD in all patients [28, 34]. Improvements in BMD may require longer-term medical treatment; patients with low BMD should be closely monitored and treated accordingly [5, 35].
Improvements in physical manifestations of hypercortisolism, including hirsutism in females, and HRQoL indicators (CushingQoL and BDI-II scores) generally occurred irrespective of the degree of mUFC control, suggesting that improvements may occur independently of the extent of cortisol reduction. This is similar to findings in prospective studies of pasireotide and levoketoconazole [16, 17]. For hirsutism in particular, the proportion of patients with improvements in rated severity score progressively increased, despite the potential for increases in testosterone levels following the initiation of osilodrostat [13]. Preliminary data suggest excess 11-oxygenated C19 steroids as the primary cause, the synthesis of which can be blocked by osilodrostat [36]. It is possible that a relationship could become more evident with longer follow-up [13, 37], considering the severity of hypercortisolism in patients at baseline in this study. Furthermore, as physical manifestations of hypercortisolism were assessed subjectively by different investigators, the findings should be considered with some caution. Interestingly, improvements in the rated score for proximal muscle atrophy at week 24 were numerically greater for patients with controlled or partially controlled mUFC than for those with uncontrolled mUFC. Data from the European registry have highlighted the prevalence of muscle weakness in patients with Cushing’s syndrome [29, 38], which was frequently reported in patients who died [29]. Improving muscle strength in these patients remains a challenge even in patients with surgical remission [39]; therefore, the early improvements in proximal muscle atrophy observed in patients with control of mUFC following osilodrostat treatment could be of clinical importance.
The current analysis is limited by the fact that patients were not evenly distributed between subgroups based on degree of mUFC control, as most patients achieved either complete or partial mUFC control from week 12 onwards [12]. As a result, some subgroups (eg partially controlled and uncontrolled mUFC) comprised fewer patients than different groups (eg controlled mUFC). Furthermore, it is important to acknowledge the limitations of measuring and guiding treatment decisions based on mUFC only, which was used to assess the primary endpoint in the current study. Measurements of mUFC can be subject to intra-patient variability [40] and can fluctuate among individuals according to their sensitivity to excess cortisol. As such, some comorbidities can persist despite improvements in hypercortisolism [2, 28]. It is probable that mUFC assessment alone is not sufficiently discriminative to fully reflect control of hypercortisolism, given the general improvements in clinical signs and additional manifestations of hypercortisolism irrespective of mUFC control by week 48, as well as the lack of correlation between mUFC and clinical outcomes. Further research into the relationship between clinical improvements and changes within the hypothalamic–pituitary–adrenal axis during medical therapy is warranted. Use of LNSC as a complementary measure for monitoring treatment response continues to be investigated [41], alongside changes in clinical outcomes, in patients who achieve control of both mUFC and LNSC.
In conclusion, improvements in clinical signs and additional specific manifestations of hypercortisolism associated with Cushing’s disease were observed during 48 weeks of therapy with osilodrostat, alongside the rapid and sustained control of mUFC. Normalisation of cortisol levels remains an important treatment goal for patients with Cushing’s disease, particularly when there are indications that controlled mUFC levels can be associated with the greatest clinical improvements.
Data availability
The datasets generated and analysed during the current study are not publicly available but are available from the corresponding author on reasonable request. Recordati Rare Diseases will share the complete de-identified patient dataset, study protocol, statistical analysis plan, and informed consent form upon request, effective immediately following publication, with no end date.
References
Pivonello R, De Martino MC, De Leo M, Lombardi G, Colao A (2008) Cushing’s syndrome. Endocrinol Metab Clin N Am 37(1):135–149
Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A (2016) Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol 4(7):611–629
Valassi E (2022) Clinical presentation and etiology of Cushing’s syndrome: data from ERCUSYN. J Neuroendocrinol 34(8):e13114
Braun LT, Vogel F, Reincke M (2022) Long-term morbidity and mortality in patients with cushing’s syndrome. J Neuroendocrinol 34(8):e13113
Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR et al (2021) Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol 9(12):847–875
Braun LT, Vogel F, Zopp S, Marchant Seiter T, Rubinstein G, Berr CM et al (2022) Whom should we screen for Cushing syndrome? The Endocrine Society practice guideline recommendations 2008 revisited. J Clin Endocrinol Metab 107(9):e3723–e3730
Bride MM, Crespo I, Webb SM, Valassi E (2021) Quality of life in Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab 35(1):
Valassi E, Chiodini I, Feelders RA, Andela CD, Abou-Hanna M, Idres S et al (2022) Unmet needs in Cushing’s syndrome: the patients’ perspective. Endocr Connect 11(7):e220027
Recordati Rare Diseases (2020) ISTURISA (osilodrostat) tablets, for oral use, prescribing information. https://www.isturisa.com/pdf/isturisa-prescribing-information.pdf. Accessed February 2021.
Recordati Rare Diseases (2020) Osilodrostat summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/isturisa-epar-product-information_en.pdf. Accessed February 2021.
Recordati Rare Diseases (2021) Isturisa® Japan prescribing information. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/24990A5/. Accessed August 2021.
Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, Shimatsu A et al (2020) Efficacy and safety of osilodrostat in patients with cushing’s disease (LINC 3): a multicentre Phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol 8(9):748–761
Fleseriu M, Newell-Price J, Pivonello R, Shimatsu A, Auchus RJ, Scaroni C et al (2022) Long-term outcomes of osilodrostat in Cushing’s disease: LINC 3 study extension. Eur J Endocrinol 187(4):531–541
Webb SM, Badia X, Barahona MJ, Colao A, Strasburger CJ, Tabarin A et al (2008) Evaluation of health-related quality of life in patients with Cushing’s syndrome with a new questionnaire. Eur J Endocrinol 158(5):623–630
Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess 67(3):588–597
Lacroix A, Bronstein MD, Schopohl J, Delibasi T, Salvatori R, Li Y et al (2020) Long-acting pasireotide improves clinical signs and quality of life in Cushing’s disease: results from a Phase III study. J Endocrinol Invest 43(11):1613–1622
Geer EB, Salvatori R, Elenkova A, Fleseriu M, Pivonello R, Witek P et al (2021) Levoketoconazole improves clinical signs and symptoms and patient-reported outcomes in patients with Cushing’s syndrome. Pituitary 24(1):104–115
Pivonello R, Elenkova A, Fleseriu M, Feelders RA, Witek P, Greenman Y et al (2021) Levoketoconazole in the treatment of patients with Cushing’s syndrome and diabetes mellitus: results from the SONICS Phase 3 study. Front Endocrinol (Lausanne) 12:595894
Baudry C, Coste J, Bou Khalil R, Silvera S, Guignat L, Guibourdenche J et al (2012) Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol 167(4):473–481
Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D et al (2014) Ketoconazole in Cushing’s disease: is it worth a try? J Clin Endocrinol Metab 99(5):1623–1630
Pivonello R, Zacharieva S, Elenkova A, Tóth M, Shimon I, Stigliano A et al (2022) Levoketoconazole in the treatment of patients with endogenous Cushing’s syndrome: a double-blind, placebo-controlled, randomized withdrawal study (LOGICS). Pituitary 25(6):911–926
Nieman LK, Boscaro M, Scaroni CM, Deutschbein T, Mezosi E, Driessens N et al (2021) Metyrapone treatment in endogenous Cushing’s syndrome: results at week 12 from PROMPT, a prospective international multicentre, open-label, Phase III/IV study. J Endocr Soc 5(Suppl 1):abst A515
Fleseriu M, Pivonello R, Elenkova A, Salvatori R, Auchus RJ, Feelders RA et al (2019) Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing’s syndrome (SONICS): a Phase 3, multicentre, open-label, single-arm trial. Lancet Diabetes Endocrinol 7(11):855–865
Ferrau F, Korbonits M (2015) Metabolic comorbidities in Cushing’s syndrome. Eur J Endocrinol 173(4):M133–M157
Whitworth JA, Williamson PM, Mangos G, Kelly JJ (2005) Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag 1(4):291–299
Varlamov EV, Langlois F, Vila G, Fleseriu M (2021) Management of endocrine disease: cardiovascular risk assessment, thromboembolism, and infection prevention in Cushing’s syndrome: a practical approach. Eur J Endocrinol 184(5):R207–R224
Fallo F, Di Dalmazi G, Beuschlein F, Biermasz NR, Castinetti F, Elenkova A et al (2022) Diagnosis and management of hypertension in patients with Cushing’s syndrome: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens 40(11):2085–2101
Feelders RA, Pulgar SJ, Kempel A, Pereira AM (2012) The burden of Cushing’s disease: clinical and health-related quality of life aspects. Eur J Endocrinol 167(3):311–326
Valassi E, Tabarin A, Brue T, Feelders RA, Reincke M, Netea-Maier R et al (2019) High mortality within 90 days of diagnosis in patients with Cushing’s syndrome: results from the ERCUSYN registry. Eur J Endocrinol 181(5):461–472
Dekkers OM, Horvath-Puho E, Jorgensen JO, Cannegieter SC, Ehrenstein V, Vandenbroucke JP et al (2013) Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab 98(6):2277–2284
Lambert JK, Goldberg L, Fayngold S, Kostadinov J, Post KD, Geer EB (2013) Predictors of mortality and long-term outcomes in treated Cushing’s disease: a study of 346 patients. J Clin Endocrinol Metab 98(3):1022–1030
Scillitani A, Mazziotti G, Di Somma C, Moretti S, Stigliano A, Pivonello R et al (2014) Treatment of skeletal impairment in patients with endogenous hypercortisolism: when and how? Osteoporos Int 25(2):441–446
Luisetto G, Zangari M, Camozzi V, Boscaro M, Sonino N, Fallo F (2001) Recovery of bone mineral density after surgical cure, but not by ketoconazole treatment, in Cushing’s syndrome. Osteoporos Int 12(11):956–960
Braun LT, Fazel J, Zopp S, Benedix S, Osswald-Kopp A, Riester A et al (2020) The effect of biochemical remission on bone metabolism in Cushing’s syndrome: a 2-year follow-up study. J Bone Miner Res 35(9):1711–1717
Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO et al (2015) Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 100(8):2807–2831
Nowotny HF, Braun L, Vogel F, Bidlingmaier M, Reincke M, Tschaidse L et al (2022) 11-Oxygenated C19 steroids are the predominant androgens responsible for hyperandrogenemia in Cushing’s disease. Eur J Endocrinol 187(5):663–673
Fleseriu M, Biller BMK, Bertherat J, Young J, Hatipoglu B, Arnaldi G et al (2022) Long-term efficacy and safety of osilodrostat in Cushing’s disease: final results from a Phase II study with an optional extension phase (LINC 2). Pituitary 25(6):959–970
Valassi E, Santos A, Yaneva M, Toth M, Strasburger CJ, Chanson P et al (2011) The European Registry on Cushing’s Syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 165(3):383–392
Vogel F, Braun LT, Rubinstein G, Zopp S, Kunzel H, Strasding F et al (2020) Persisting muscle dysfunction in Cushing’s syndrome despite biochemical remission. J Clin Endocrinol Metab 105(12):e4490–e4498
Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Sen K et al (2014) High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin Endocrinol (Oxf) 80(2):261–269
Newell-Price J, Pivonello R, Tabarin A, Fleseriu M, Witek P, Gadelha MR et al (2020) Use of late-night salivary cortisol to monitor response to medical treatment in cushing’s disease. Eur J Endocrinol 182(2):207–217
Acknowledgements
We thank all the investigators, nurses, study coordinators and patients who participated in the trial. We also thank Rebecca Helson, PhD of Mudskipper Business Ltd for medical editorial assistance with this manuscript.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. This study was funded by Novartis Pharma AG; however, as of 12 July 2019, osilodrostat is an asset of Recordati. Financial support for medical editorial assistance was provided by Recordati.
Author information
Authors and Affiliations
Contributions
RP, MF, JNP, AS, and BMKB, as part of the academic investigator steering committee, and AMP contributed to the design of the study alongside the funder. RP, MF, JNP, AS, RAF, PK, AT, TCB, EBG, and BMKB all enrolled patients in the study. Data were collected by investigators of the LINC 3 Study Group using the funder’s data management systems. AP and the funder’s statistical team analysed the data. All authors were involved in drafting/revising the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of interests
RP has received research funding from Recordati AG, Corcept Therapeutics, Strongbridge Biopharma, and Neurocrine Biosciences and served as a consultant for Corcept Therapeutics, Recordati AG, Crinetics Pharmaceuticals, and H Lundbeck A/S. MF reports grants to her university and occasional scientific consulting fees from Recordati Rare Diseases, Sparrow, and Xeris (Strongbridge); she served as a member of the LINC 3 steering committee. JNP reports research grants and consultancy payments to his university from Crinetics, Diurnal, HRA Pharma, and Recordati Rare Diseases. AS reports serving as a speaker and consultant for Recordati; he also served as a member of the LINC 3 steering committee. RAF reports research grants from Strongbridge and Corcept Therapeutics and consultancy fees from Recordati Rare Diseases and Corcept Therapeutics. PK has no conflicts of interest to disclose. AT reports research contracts and consulting and conference fees from HRA Pharma, Ipsen-Biotech, Novartis, and Recordati Rare Diseases. TCB reports institutional research support from Sandoz and Pfizer and consultancy and lectureship fees from Novartis, Ipsen, Strongbridge, Pfizer, and Recordati Rare Diseases. EBG reports research grants to her hospital from Xeris (Strongbridge), Recordati Rare Diseases, Sparrow, and Corcept Therapeutics and personal consulting fees from Xeris (Strongbridge). AP is an employee of Recordati. AMP was an employee of Recordati when the analyses were conducted. BMKB reports research grants to her institution from Novartis, Strongbridge, and Millendo and occasional consulting honoraria from HRA Pharma, Novartis, Recordati Rare Diseases, Sparrow, and Xeris (Strongbridge); she served on the LINC 3 steering committee.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, with an independent ethics committee/institutional review board at each site approving the study protocol.
Informed consent
Patients provided written informed consent to participate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pivonello, R., Fleseriu, M., Newell-Price, J. et al. Improvement in clinical features of hypercortisolism during osilodrostat treatment: findings from the Phase III LINC 3 trial in Cushing's disease. J Endocrinol Invest 47, 2437–2448 (2024). https://doi.org/10.1007/s40618-024-02359-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-024-02359-6