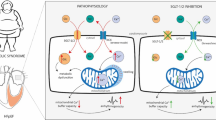
Abstract
Background
The association between obesity and some cardiovascular complications such as heart failure (HF) is well established, and drugs affecting adiposity are supposed to be promising treatments for these conditions. The sodium-glucose cotransporter-2 inhibitors (SGLT2i) are antidiabetic drugs showing benefits in patients with HF, despite the underlying mechanisms have not been completely understood yet. SGLT2i are supposed to promote systemic effects, such as triglycerides mobilization, through the enhancement of fibroblast growth factor-21 (FGF-21) activity. So, in this study, we evaluated the effects of dapagliflozin treatment on FGF-21 and related receptors (FGF-Rs) gene expression and on lipid content in myocardial tissue in an animal model of genetically induced obesity to unravel possible metabolic mechanisms accounting for the cardioprotection of SGLT2i.
Methods
Six-week-old C57BL/6J wild-type mice and B6.V-LEP (ob/ob) mice were randomly assigned to the control or treatment group (14 animals/group). Treatment was based on the administration of dapagliflozin 0.15 mg/kg/day for 4 weeks. The gene expression of FGF-21 and related receptors (FGF-R1, FGF-R3, FGF-R4, and β-klotho co-receptor) was assessed at baseline and after treatment by real-time PCR. Similarly, cardiac triglycerides concentration was measured in the control group and treated animals.
Results
At baseline, FGF-21 mRNA expression in the heart did not differ between lean and obese ob/ob mice. Dapagliflozin administration significantly increased heart FGF-21 gene expression, but only in ob/ob mice (p < 0.005). Consistently, when measuring the amount of triglycerides in the cardiac tissue, SGLT2i treatment reduced the lipid content in obese ob/ob mice, while no significant effects were observed in treated lean animals (p < 0.001). The overall expression of the FGF-21 receptors was only minimally affected by dapagliflozin treatment both in obese ob/ob mice and in lean controls.
Conclusions
Dapagliflozin administration increases FGF-21gene expression and reduces triglyceride content in myocardial tissue of ob/ob mice, while no significant effect was observed in lean controls. These results might help understand the cardiometabolic effects of SGLT2i inducing increased FGF-21 synthesis while reducing lipid content in cardiomyocytes as a possible expression of the switch to different energy substrates. This mechanism could represent a potential target of SGLT2i in obesity-related heart diseases.





Similar content being viewed by others
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- NAFLD:
-
Non-alcoholic fatty liver disease
- HFpEF:
-
Heart failure with preserved ejection fraction
- GLP-1:
-
Glucagon-like peptide 1
- SGLT2i:
-
Sodium-glucose cotransporter-2 inhibitors
- FGF-21:
-
Fibroblast growth factor-21
- NIH:
-
National Institute of Health
- FGF-R(s):
-
Fibroblast growth factor-21 receptor(s)
- BMI:
-
Body mass index
References
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A (2017) GBD 2015 obesity collaborators health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377(1):13–27. https://doi.org/10.1056/NEJMoa1614362
Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL (2019) Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 381(25):2440–2450. https://doi.org/10.1056/NEJMsa1909301
Schnurr TM, Jakupović H, Carrasquilla GD, Ängquist L, Grarup N, Sørensen TIA, Tjønneland A, Overvad K, Pedersen O, Hansen T, Kilpeläinen TO (2020) Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: a case-cohort study. Diabetologia 63(7):1324–1332. https://doi.org/10.1007/s00125-020-05140-5
Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B (2016) Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev 17(6):510–519. https://doi.org/10.1111/obr.12407
Piché ME, Tchernof A, Després JP (2020) Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 126(11):1477–1500. https://doi.org/10.1161/CIRCRESAHA.120.316101
Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE (2011) Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 4(3):324–331. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959890
Alex L, Russo I, Holoborodko V, Frangogiannis NG (2018) Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 315(4):H934–H949. https://doi.org/10.1152/ajpheart.00238.2018
Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271. https://doi.org/10.1016/j.jacc.2013.02.092
Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ, Hamdani N (2016) Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail 4(4):312–324. https://doi.org/10.1016/j.jchf.2015.10.007
Schilling JD, Machkovech HM, Kim AH, Schwendener R, Schaffer JE (2012) Macrophages modulate cardiac function in lipotoxic cardiomyopathy. Am J Physiol Heart Circ Physiol 303(11):H1366–H1373. https://doi.org/10.1152/ajpheart.00111.2012
Higashikuni Y, Tanaka K, Kato M, Nureki O, Hirata Y, Nagai R, Komuro I, Sata M (2013) Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through interleukin-1β upregulation via nuclear factor κB activation. J Am Heart Assoc 2(6):e000267. https://doi.org/10.1161/JAHA.113.000267
Si Y, Feng Z, Liu Y, Fan W, Shan W, Zhang Y, Shi F, Xing E, Sun L (2023) Inflammatory biomarkers, angiogenesis and lymphangiogenesis in epicardial adipose tissue correlate with coronary artery disease. Sci Rep 13(1):2831. https://doi.org/10.1038/s41598-023-30035-x
Wu CK, Lee JK, Hsu JC, Su MM, Wu YF, Lin TT, Lan CW, Hwang JJ, Lin LY (2020) Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur J Heart Fail 22(3):445–454. https://doi.org/10.1002/ejhf.1617
Benn M, Marott SCW, Tybjærg-Hansen A, Nordestgaard BG (2023) Obesity increases heart failure incidence and mortality: observational and Mendelian randomization studies totalling over 1 million individuals. Cardiovasc Res 118(18):3576–3585. https://doi.org/10.1093/cvr/cvab368
Lin Y, Cai Z, Yuan J, Liu H, Pang X, Chen Q, Tang X, Geng Q, Dong S (2022) Effect of pharmacological treatment on outcomes of heart failure with preserved ejection fraction: an updated systematic review and network meta-analysis of randomized controlled trials. Cardiovasc Diabetol 21(1):237. https://doi.org/10.1186/s12933-022-01679-2
Abdesselam I, Pepino P, Troalen T, Macia M, Ancel P, Masi B, Fourny N, Gaborit B, Giannesini B, Kober F, Dutour A, Bernard M (2015) Time course of cardiometabolic alterations in a high fat high sucrose diet mice model and improvement after GLP-1 analog treatment using multimodal cardiovascular magnetic resonance. J Cardiovasc Magn Reson 6(17):95. https://doi.org/10.1186/s12968-015-0198-x
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, EMPEROR-Preserved Trial Investigators (2021) Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 385(16):1451–1461. https://doi.org/10.1056/NEJMoa2107038
Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C, Dreyfuss JM, Pan H, Tangcharoenpaisan Y, Morningstar J, Gerszten R, Patti ME (2019) SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 4(5):e123130. https://doi.org/10.1172/jci.insight.123130
Chen MZ, Chang JC, Zavala-Solorio J, Kates L, Thai M, Ogasawara A, Bai X, Flanagan S, Nunez V, Phamluong K, Ziai J, Newman R, Warming S, Kolumam G, Sonoda J (2017) FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/βKlotho complex in non-adipocytes. Mol Metab 6(11):1454–1467. https://doi.org/10.1016/j.molmet.2017.09.003
Withaar C, Meems LMG, Markousis-Mavrogenis G, Boogerd CJ, Silljé HHW, Schouten EM, Dokter MM, Voors AA, Westenbrink BD, Lam CSP, de Boer RA (2021) The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc Res 117(9):2108–2124. https://doi.org/10.1093/cvr/cvaa256
Gaborit B, Ancel P, Abdullah AE, Maurice F, Abdesselam I, Calen A, Soghomonian A, Houssays M, Varlet I, Eisinger M, Lasbleiz A, Peiretti F, Bornet CE, Lefur Y, Pini L, Rapacchi S, Bernard M, Resseguier N, Darmon P, Kober F, Dutour A (2021) Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: the EMPACEF study. Cardiovasc Diabetol 20(1):57. https://doi.org/10.1186/s12933-021-01237-2
Dasari D, Goyal SG, Penmetsa A, Sriram D, Dhar A (2023) Canagliflozin protects diabetic cardiomyopathy by mitigating fibrosis and preserving the myocardial integrity with improved mitochondrial function. Eur J Pharmacol 15(949):175720. https://doi.org/10.1016/j.ejphar.2023.175720
Jhuo SJ, Lin YH, Liu IH, Lin TH, Wu BN, Lee KT, Lai WT (2023) Sodium glucose cotransporter 2 (SGLT2) inhibitor ameliorate metabolic disorder and obesity induced cardiomyocyte injury and mitochondrial remodeling. Int J Mol Sci 24(7):6842. https://doi.org/10.3390/ijms24076842
Hiruma S, Shigiyama F, Hisatake S, Mizumura S, Shiraga N, Hori M, Ikeda T, Hirose T, Kumashiro N (2021) A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early-stage type 2 diabetes: the ASSET study. Cardiovasc Diabetol 20(1):32. https://doi.org/10.1186/s12933-021-01228-3
Hsu JC, Wang CY, Su MM, Lin LY, Yang WS (2019) Effect of empagliflozin on cardiac function, adiposity, and diffuse fibrosis in patients with type 2 diabetes mellitus. Sci Rep 9(1):15348. https://doi.org/10.1038/s41598-019-51949-5
Cowie MR, Fisher M (2020) SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 17(12):761–772. https://doi.org/10.1038/s41569-020-0406-8
Solomon SD, Vaduganathan M, Claggett BL, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Belohlavek J, Chiang CE, Willem Borleffs CJ, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, AlcocerGamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O’Meara E, Kerr Saraiva JF, Tereschenko SN, Thierer J, Vardeny O, Verma S, Vinh PN, Wilderäng U, Zaozerska N, Lindholm D, Petersson M, McMurray JJV (2022) Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail. 10(3):184–197. https://doi.org/10.1016/j.jchf.2021.11.006
Adamson C, Kondo T, Jhund PS, de Boer RA, Cabrera Honorio JW, Claggett B, Desai AS, AlcocerGamba MA, Al Habeeb W, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Langkilde AM, Lindholm D, Bachus E, Litwin SE, Martinez F, Petersson M, Shah SJ, Vaduganathan M, Nguyen Vinh P, Wilderäng U, Solomon SD, McMurray JJV (2022) Dapagliflozin for heart failure according to body mass index: the DELIVER trial. Eur Heart J 43(41):4406–4417. https://doi.org/10.1093/eurheartj/ehac481
Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, Fox CS (2013) Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. ArteriosclerThrombVasc Biol 33(4):863–870. https://doi.org/10.1161/ATVBAHA.112.301009
Huynh K, Ayers C, Butler J, Neeland I, Kritchevsky S, Pandey A, Barton G, Berry JD (2022) Association between thigh muscle fat infiltration and incident heart failure: the health ABC study. JACC Heart Fail 10(7):485–493. https://doi.org/10.1016/j.jchf.2022.04.012
Spearman JV, Renker M, Schoepf UJ, Krazinski AW, Herbert TL, De Cecco CN, Nietert PJ, Meinel FG (2015) Prognostic value of epicardial fat volume measurements by computed tomography: a systematic review of the literature. Eur Radiol 25(11):3372–3381. https://doi.org/10.1007/s00330-015-3765-5
Haider A, Possner M, Messerli M, Bengs S, Osto E, Maredziak M, Portmann A, Fiechter M, Giannopoulos AA, Treyer V, Gaisl T, von Felten E, Patriki D, Benz DC, Fuchs TA, Gräni C, Pazhenkottil AP, Buechel RR, Kaufmann PA, Gebhard C (2019) Quantification of intrathoracic fat adds prognostic value in women undergoing myocardial perfusion imaging. Int J Cardiol 1(292):258–264. https://doi.org/10.1016/j.ijcard.2019.04.092
Nerlekar N, Muthalaly RG, Wong N, Thakur U, Wong DTL, Brown AJ, Marwick TH (2018) Association of volumetric epicardial adipose tissue quantification and cardiac structure and function. J Am Heart Assoc 7(23):e009975. https://doi.org/10.1161/JAHA.118.009975
Brandt-Jacobsen NH, Jürgens M, Hasbak P, Gaede P, Rossing P, Rasmussen JJ, Andersen CF, Forman JL, Faber J, Inzucchi SE, Gustafsson F, Schou M, Kistorp C (2023) Reduction of cardiac adipose tissue volume with short-term empagliflozin treatment in patients with type 2 diabetes: a substudy from the SIMPLE randomized clinical trial. Diabetes ObesMetab 25(3):844–855. https://doi.org/10.1111/dom.14933
Bamba R, Okamura T, Hashimoto Y, Majima S, Senmaru T, Ushigome E, Nakanishi N, Asano M, Yamazaki M, Takakuwa H, Hamaguchi M, Fukui M (2022) Extracellular lipidome change by an SGLT2 inhibitor, luseogliflozin, contributes to prevent skeletal muscle atrophy in db/db mice. J Cachexia Sarcopenia Muscle 13(1):574–588. https://doi.org/10.1002/jcsm.12814
Takada S, Sabe H, Kinugawa S (2022) Treatments for skeletal muscle abnormalities in heart failure: sodium-glucose transporter 2 and ketone bodies. Am J Physiol Heart Circ Physiol 322(2):H117–H128. https://doi.org/10.1152/ajpheart.00100.2021
von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, Potthoff MJ (2016) FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab 23(2):335–343. https://doi.org/10.1016/j.cmet.2015.12.003
Lindström P (2007) The physiology of obese-hyperglycemic mice [ob/ob mice]. ScientificWorldJournal 29(7):666–685. https://doi.org/10.1100/tsw.2007.117
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ et al (2005) FGF-21 as a novel metabolic regulator. J Clin Investig 115:1627–1635. https://doi.org/10.1172/JCI23606
Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V, Haluzik M (2009) Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 71(3):369–375. https://doi.org/10.1111/j.1365-2265.2008.03502.x
Packer M (2022) Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation 146(18):1383–1405. https://doi.org/10.1161/CIRCULATIONAHA.122.061732
Kosugi R, Nakatani E, Okamoto K, Aoshima S, Arai H, Inoue T (2019) Effects of sodium-glucose cotransporter 2 inhibitor (dapagliflozin) on food intake and plasma fibroblast growth factor 21 levels in type 2 diabetes patients. Endocr J 66(8):677–682. https://doi.org/10.1507/endocrj.EJ19-0013
Yamakage H, Tanaka M, Inoue T, Odori S, Kusakabe T, Satoh-Asahara N (2020) Effects of dapagliflozin on the serum levels of fibroblast growth factor 21 and myokines and muscle mass in Japanese patients with type 2 diabetes: a randomized, controlled trial. J Diabetes Investig 11(3):653–661. https://doi.org/10.1111/jdi.13179
Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, Miliotis T, Forsberg GB, Risérus U, Lind L, Oscarsson J (2018) Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia 61(9):1923–1934. https://doi.org/10.1007/s00125-018-4675-2
Funding
Research relating to this abstract was funded by a non-conditioning grant from AstraZeneca (476/2016), in charge to R.V.
Author information
Authors and Affiliations
Contributions
ADV, MR, and RV designed the study and wrote the manuscript; MC and MG performed the experiments and the statistics; MV and PF proofread and checked the entire manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
P.F. is involved as advisory board member and speaker for AstraZeneca, Eli Lilly, Boehringer Ingelheim, Novo, and Bayer.
Ethical approval and consent to participate
As reported in the Methods section, the procedures involving animal care followed institutional guidelines that comply with national and international laws and policies (European Economic Community Council Directive 86/609, OJ L 358, 1 Dec.2012, 1987; NIH Guide for the Care and Use of Laboratory Animals, NIH Publication no. 85–23, 1985). The study design was approved by the Ethics Committee of the University of Padova for the care and use of laboratory animals (CEASA protocol number 58/2018-PR, approved by the Ethics Committee of the University of Padova, Italy). All experiments followed committee guidelines and the institutional protocol for animal care that was approved by the Italian Ministry of Health (58/2018-PR).
Consent for publication
Not applicable.
Informed consent
For this type of study, consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Di Vincenzo, A., Crescenzi, M., Granzotto, M. et al. Treatment with dapagliflozin increases FGF-21 gene expression and reduces triglycerides content in myocardial tissue of genetically obese mice. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-023-02273-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40618-023-02273-3