Abstract
Purpose
Current smoking is a risk factor for osteoporosis (Op), but few data are available regarding the passive smoke impact on Op susceptibility. This cross-sectional study aimed to evaluate the association between the smoking habits and Op in community-dwelling women undergoing dual-energy X-ray absorptiometry (DXA).
Methods
On 01/06/2018, general practitioners from “COMEGEN” Medical Cooperative, Naples, Italy, selected the medical records from the last 10 years of women who had a measurement of bone mineral density performed and simultaneously completed a questionnaire about their smoking behaviour and their cohabiters’. The binary logistic regression analysis was used to estimate the role of passive smoke on the risk of Op, adjusting for age and body mass index (BMI).
Results
Among 10,616 subjects, 3942 were currently smokers [CS; mean age 69.4 ± 10.4 years; BMI 27.0 ± 4.9 kg/m2], 873 were passive smokers (PS; mean age 67.8 ± 11.6 years; BMI 27.0 ± 4.9 kg/m2) and 5781 were never smokers (NS; mean age 67.8 ± 11.6 years; body mass index (BMI) 27.0 ± 4.9 kg/m2). Of all, 8562 women (mean age 70.3 ± 10.2 yrs; BMI 27.0 ± 4.9 kg/m2) received the Op diagnosis. PS showed an increased Op risk compared to NS [odds ratio (OR) 1.38 (1.14–1.67)] and comparable to CS [OR 1.02 (0.84–1.24)].
Conclusion
The study results demonstrate an association between passive smoke and Op in community-dwelling women already presenting with susceptibility to Op according to Italian essential assistance levels, suggesting that passive and active smoke are equivalent Op risk factors in women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Smoke has a detrimental impact on human health, either as active or as passive smoking. It poses heavy health- and economic-related consequences worldwide [1,2,3]. Environmental tobacco smoke (ETS), also known as passive smoke, is defined by the World Health Organization (WHO) includes materials in the air originating from burned or heated tobacco. Exposure continues after smoking stops and includes second- and third-hand smoke. Second-hand smoke includes smoke released by actively burning tobacco products, while third-hand smoke refers to particles re-suspended in air. Sources include furniture, clothing, and smokers’ breath [4, 5].All forms of ETS are detrimental to health. Children are at particular risk, not being able to control their environments and re-volatizing third-hand smoke particles through active play and exploration [4, 5]. Smoke is a well-known risk factor for complex and multifactorial disorders, including lung, breast, and cervical cancers, cardiovascular diseases, and dementia [6,7,8,9]. It is currently responsible for over 600,000 deaths every year and the large majority of passive smoking deaths occur prematurely [5]. Based on these dramatic data, the WHO took a close focus on the smoking prevention [10] as well as the Italian National Institute of Health (in Italian, Istituto Superiore di Sanità, ISS) that, considering the WHO directives, published the guidelines for the prevention of health damage related to second-hand smoke [11].
Osteoporosis (Op) is the most common chronic metabolic bone disease worldwide and it is clinically defined by a reduced bone mineral density (BMD), estimated by dual-energy X-ray absorptiometry (DXA), resulting in increased risk of fragility fractures [12]. In 2010, Op affected 22 million European women and 5.5 million European men, and the economic burden of incidental and prior fragility fractures was estimated at € 37 billion [12, 13]. Considering the significant human and economic load of Op, the Italian Ministry of Health established a plan to prevent fragility fractures via the essential assistance levels, by determining the BMD by DXA in community-dwelling individuals, already presenting with an increased risk of Op, as: i) men and women of any age in the presence of a major risk factor (previous fragility fractures; radiological finding of osteoporosis; chronic therapies rising the risk of osteoporosis; diagnosed diseases, with higher risk of osteoporosis [14]), ii) post-menopausal women in the presence of a major risk factor (family history of bone fracture at age lower than 75 years; body mass index < 19 kg/m2; menopause at age lower than 45 years [14]), iii) postmenopausal women and men over the age of 60 years in the presence of at least three or more minor risk factors for Op (age > 65 years, family history of severe osteoporosis; amenorrhoea > 6 months in premenopausal age; inadequate calcium intake; vitamin D deficiency; smoking more than 20 cigarettes/day; alcohol abuse [> 60 g/day][14]).
Currently, cigarette smoking is already considered a significant Op risk factor [14], since it inversely correlates with BMD in both elderly men and women, in perimenopausal women, and even in young men [15]. However, few data are available regarding the association between ETS and Op, preventing national health-care systems from considering ETS as a real risk factor for Op. The aim of this cross-sectional study was to evaluate the Op prevalence in community-dwelling women current smoker (CS), passive smokers (PS) and never smokers (NS) of European ancestry, subjected to a DXA evaluation of BMD, according to Italian EALs. NS women were dichotomized into those exposed to PS and those not exposed to ETS according to smoking habits of their cohabitants. All enrolled patients were resident in the Naples 1 district of the National Health System (in Italian, Azienda Sanitaria Locale, ASL, Naples 1) in the Campania region (Italy), a geographic area with high environmental air pollution [16].
Methods
This cross-sectional retrospective study was based on the clinical records of all patients followed by the general practitioners (GPs) referring to the “COMEGEN” (COoperativa di MEdicina GENerale in Italian) Medical Cooperative operating within the urban area of ASL Naples 1 (40°50′N 14°15′E). As of June 1, 2018, the participating GPs selected, among their patients, women who had simultaneously undergone an evaluation of BMD by DXA [International Classification of Diseases—9th revision (ICD9) code 8898] because of clinically suspected of Op according to the Italian Ministry of Health prevention plan and completed a questionnaire that included queries on their smoking behaviour and their cohabiter’s between June 1, 2008 and May 31, 2018. In case of more than one contextual assessment of BMD by DXA and of smoking habits questionnaire in the lapse of time considered, only the data referring to the first contextual assessment were collected. From all the medical records, other than DXA results and answers to smoking questionnaire, data regarding weight, height, waist circumference, body mass index (BMI), age, sex, estimated glomerular filtration rate (eGFR, estimated by a standard formula [17]), and any pharmacological treatments were collected as part of clinical practice. Since that, data regarding the number of daily cigarettes smoked were not available for this study. Informed consent was obtained from all individual participants included in the study, also as part of clinical practice. All data were collected in an electronic file. This study is part of the SIMON (metabolic syndrome, osteoporosis, and nephrolithiasis; SIndrome Metabolica, Osteoporosi e Nefrolitiasi in Italian) protocol [18,19,20], which was approved by the ASL Naples 1 Ethical Committee, protocol number 0018508/2018.
Op diagnosis
The Op diagnosis was based on the following criteria: (1) T-score value measured by DXA ≤ − 2.5 in the lumbar spine, total hip or femoral neck, according to the WHO diagnostic criteria [21]; (2) fragility fractures, only diagnosed by radiograph images (ICD9 codes 73,310 to 73,319); (3) personal history of anti-osteoporotic treatment according to Italian Medicine Agency prescriptive criteria (in Italian, Agenzia Italiana del FArmaco—AIFA) [22]. Subjects with a lumbar, total hip or femoral T-score > − 2.5 and a personal history negative for clinical fragility fractures and anti-osteoporotic treatment were considered as not osteoporotic women.
Smoking questionnaire and smoking habits classification
The smoking questionnaires included the following questions: (a) Have you ever smoked? Eventually, when did you initiate? (b) Have you ever smoked? Eventually, when did you stop? (c) Do your cohabitants or co-workers smoke in your presence? The smoking habits of the population were defined according to the smoking questionnaire replies. Firstly, the population was asked about current and lifetime smoking habits, smoking initiation, and eventual age at smoking cessation (question a and/or b). In case the reply to question b was positive and the subjects reported to have stopped smoking habits from more than 12 months, they were excluded from the study. Then, subjects that replied positively to question a were then classified as current smokers (CS), because of a regular consumption of cigarettes reported (duration > 6 months) at the time of examination; if not, as non-smokers. In case the subject was defined as CS, the last query was not administered. Instead, non-smokers were further asked if there were smokers living in the participant's family currently or during childhood or at workplace (question c). If the responses were affirmative to any of the previous questions, non-smokers were classified as PS. Otherwise, if subjects were non-smokers and they never had a smoker as relative or as a co-worker, the responses to all the questions were negative (a, b and c) and they were classified as never smokers (NS).
Exclusion criteria
Pre-established exclusion criteria were all the known causes of secondary Op, apart from cigarette smoking, such as: age lower than 40 years, ever diagnosis of malabsorption syndromes (ICD9 codes 5793 to 5799), rheumatoid arthritis (ICD9 code 7140), long-term immobilization, moderate to severe chronic kidney disease [estimated glomerular filtration rate < 60 ml/min/1.73 m2 [17] (ICD9 codes 5853–5859, 586 and 6393)], hyperthyroidism (ICD9 codes 24,200–24291), primary hyperparathyroidism (ICD9 codes 25,200–25208), hypoparathyroidism (ICD9 code 2521), Cushing’s syndrome (ICD9 code 2550), chronic liver disease (ICD9 codes 5710–5719), pituitary tumours (ICD9 codes 1943, 2273, 2370), surgical history of terminal ileal resection (ICD9 code 4562), gastrectomy or small bowel bypass (ICD9 codes 430–4499), bilateral ovariosalpingectomy (ICD9 code 6551, 6553, 6561, 6563), eating disorders (ICD9 codes 3071 and 30,750–30759), alcoholism (ICD9 codes 30,390–30393), regular use of gonadotropin-releasing hormone agonist, glucocorticoids, anticonvulsants, heparin, vitamin A and cytotoxic agents for a current or past diagnosis of cancer. Subjects with history of Op treatment not compliant to AIFA prescriptive criteria were also excluded [22]. Past smokers were also excluded. This latter was defined as individuals who had smoked during their lifetime but not in the last 12 months, prior to the time of the baseline examination [23].
Statistical analysis
Statistical analysis was performed using IBM SPSS (Statistical Package for Social Science), version 25 (IBM, Armonk, NY, USA). In univariate analyses, statistical comparisons were based on the Student’s t test for continuous variables and on the Chi-squared test for dichotomous variables. The binary logistic regression analysis was used to estimate the role of SHS on the risk of Op, adjusting for age and BMI. The collinearity among variables included in the models was assessed. The analysis did not detect any collinearity among variables (tolerance: 0.94, variance inflation factor 1.0). All statistical tests were two-tailed. The results are reported as mean (standard deviation – SD), or absolute, or percentages or as odds ratio (OR) and 95% confidence interval (95% CI). A p value < 0.05 was considered significant.
Results
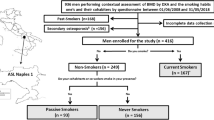
From June 1, 2008, to May 31, 2018, 10,616 community-dwelling women were submitted to DXA and simultaneously completed the self-administered questionnaire regarding their smoking behaviours and their cohabiter’s (Fig. 1). Subjects underwent a DXA examination according to clinical criteria, as reported in Table 1. 3962 of them were classified as CS, and 6654 as non-smokers. Among the non-smokers, 873 were classified as PS and 5781 as NS, according to the smoking habit classification previously exposed (Fig. 1). Some clinical characteristics of CS, PS and NS are reported in Table 2. PS showed a lower mean age compared to NS and CS.
Study flowchart. The study population is highlighted in grey. ASL Na 1: Local Health Unit (in Italian: Azienda Sanitaria Locale) Naples 1. BMD: bone mineral density assessed by dual-energy X-ray absorptiometry. DXA: dual-energy X-ray absorptiometry. §: subjects with malabsorption syndromes [International Classification of Diseases-9th revision (ICD9) codes 5793 to 5799], rheumatoid arthritis (ICD9 code 7140), long-term immobilization, moderate to severe chronic kidney disease [estimated glomerular filtration rate < 60 ml/min/1.73 m2 [17] (ICD9 codes 5853–5859, 586 and 6393], hyperthyroidism (ICD9 codes 24,200–24291), primary hyperparathyroidism (ICD9 codes 25,200–25208), hypoparathyroidism (ICD9 code 2521), Cushing’s syndrome (ICD9 code 2550), chronic liver disease (ICD9 codes 5710–5719), bilateral ovariosalpingectomy (ICD9 code 6551, 6553, 6561, 6563), pituitary tumours (ICD9 codes 1943, 2273, 2370), surgical history of terminal ileal resection (ICD9 code 4562), gastrectomy or small bowel bypass (ICD9 codes 430–4499), eating disorders (ICD9 codes 3071 and 30,750–30759), alcoholism (ICD9 codes 30,390–30393), regular use of gonadotropin-releasing hormone agonist, glucocorticoids, anticonvulsants, heparin, vitamin A and cytotoxic agents, were excluded from the study
In the entire study population, according to criteria previously reported, 8562 women (mean age 70.3 ± 10.2 yrs; BMI 27.0 ± 4.9 kg/m2; eGFR 77.7 ± 12.1 ml/min/1.73 m2) were diagnosed with Op. The remaining 2054 women who did not receive the Op diagnosis (mean age 64.9 ± 11.7 yrs; BMI 27.3 ± 5.1 kg/m2, eGFR 76.1 ± 12.3 ml/min/1.73 m2) were considered as controls. As depicted in Fig. 2, the Op prevalence in CS was significantly higher compared to NS [odd ratio (OR) 1.40 (95% CI: 1.26–1.56)]. Likewise, the Op prevalence in PS was significantly higher compared to NS [OR 1.38 (95% CI: 1.14–1.67)], but not different compared to CS [OR 1.02 (95% CI: 0.84–1.24)]. All these differences remained significant also after correction for age, BMI, eGFR, family history of bone fractures, inadequate calcium intake, vitamin D deficiency, and alcohol abuse as shown in Table 3.
Subjects undergoing DXA because of reported fractures were 250 (6.3%) among CS, 62 (7.1%) among PS and 244 (4.2%) among NS. CS presented a higher risk of fractures compared to NS [OR 1.58 (95% I.C. 1.27–1.83), p < 0.05], but not compared to PS [OR 0.88 (95% I.C. 0.66–1.17), p > 0.05]. In turn, PS presented a significant higher risk of fractured compared to NS [OR 1.68 (95% I.C. 1.28–2.20), p < 0.05]. No statistically significant difference was found in the prevalence of vitamin D deficiency among the three groups: subjects with vitamin D deficiency were 2127 (53.7%) in the CS group, 511 (58.5%) in PS and 3000 (51.9) in NS. Also, the prevalence of family history of bone fractures, inadequate calcium intake and alcohol abuse among the three study groups was not statistically significant.
Discussion
The study results underline the presence of a significant association between passive smoking and Op in non-smoker community-dwelling women of European ancestry and suggest that CS and PS women have a similar increased risk of Op and fracture compared to NS.
Bone structure may be altered by exposure to smoke. In particular, increased levels of cross-links of the N-terminal collagen (NTx) were found in CS subjects [24], highlighting the changes in the composition of collagen fibres and in their cross-linking, which may affect the strength and resilience of the bone material. Therefore, any smoking-related increase in bone turnover can cause skeletal fragility [25].
ETS is generated by tobacco products’ combustion and it is a complex mixture of over 4000 compounds [2, 3]. These include more than 40 known or suspected human carcinogens, such as 4-aminobiphenyl,2-naphthylamine, benzene, nickel and various polycyclic aromatic hydrocarbons (PAHs) and N-nitrosamines. Furthermore, there are also several irritants in this mixture, such as ammonia, nitrous oxide, sulphur dioxide and aldehydes, and cardiovascular toxicants, such as carbon monoxide and nicotine [2, 3]. All these substances influence bone turnover through different pathways. In particular, experimental models showed that aldehydes can induce intense oxidative stress and inflammatory reactions. Excessive oxidative stress impairs osteoblast function by suppressing differentiation and inducing apoptosis. Additionally, oxidative stress also increased the responsiveness of osteoclast precursors to the receptor activator of nuclear factor-κB ligand (RANKL) signalling cascade and further stimulated the production of osteoclastogenic cytokines [26]. Meanwhile, PAHs suppress bone resorption by osteoclasts and bone synthesis by osteoblasts [27]. Another experimental model proved that nicotine administration can elevate RANKL and decrease osteoprotegerin levels in serum to stimulate osteoclast genesis. On the contrary, interrupting administration of nicotine to mice increased bone mass and decreased levels of the bone resorption markers [28].
The mechanisms underlying the association between passive smoke and Op are not well known, but its impact on bone mass may be even stronger in PS than CS, because of the mains and side stream smoke that composes the ETS mixture [29, 30]. Notably, side-stream smoke is formed from smouldering of cigarettes or other paraphernalia between puffs, while mainstream smoke is emitted at the mouthpiece during a puff, then exhaled by a smoker [6]. In addition, passive smoking accounts also for exposure to third-hand smoke. Third-hand smoke is defined as “residual tobacco smoke pollutants that remain on surfaces and in dust after tobacco has been smoked” [31]. In third-hand smoke, the exposure happens through dust ingestion, dermal absorption and inhalation due to residual tobacco smoke pollutants on surfaces such as floors, counters and walls. Moreover, residual tobacco smoke can persist even for months after smoking [31]. It is also clear that living in a geographical area with a high environmental air pollution can also affect the association between second- or third-hand smoke and BMD reduction [16].
Another element to consider is represented by the half-time of cotinine, the predominant catabolite of nicotine usually used as biomarker for exposure to tobacco smoke that has been found to be significantly higher in serum, urine and saliva of PS compared to CS [31]. The serum cotinine has also been demonstrated to negatively associate with the vitamin D concentration in community-dwelling population [32, 33], partially clarifying the reasoning behind our result. In this regard, it must be noted that our population is not representative of a community-dwelling one, since it has been selected according to EAL criteria to be subjected to DXA, accounting for a high prevalence of vitamin D deficiency among the three groups.
Consistent with our results, the association between passive smoking and low BMD was observed in different ages and ethnic groups worldwide [31, 32, 34]. In two retrospective studies, passive smoking has been inversely associated with phalangeal BMD in adults [35] and with total hip and femoral neck BMD in 154 premenopausal women [36]. In a prospective study, the exposure to passive smoking in childhood was an important determinant of reduced BMD, density and strength indices measured 28 years later in adulthood [37].
One further last observation is represented by the tangled interaction between ETS, BMI and Op. The latter are notoriously intertwined with one another: BMI is indeed inversely associated with Op risk [38], whereas smoke presents with a direct association [39]. The joint effect of ETS and BMI is a well-known pattern for the occurrence and the worsening of other conditions, such as asthma [40, 41], tumour and cancer-related death [42], hypertension [43], gout [44] and abnormalities in glucose metabolism [45]. Our results confirm that Op risk related to ETS dramatically increases when BMI is included in the multivariate model in PS, but not to the same extent in CS. The low dose and nonmonotonicity of some chemical compounds from side smoke in PS can be one reason for the different result observed in PS and CS [46]. It must also be considered that side stream smoke from passive smoking is a low-temperature oxygen-poor environment that contains higher concentrations of ammonia and nitric oxides, which are smaller than the ones produced during the act of smoking [47]. Further studies will be needed to better clarify these observations.
Some strengths and limits of this study are strictly related to the use of administrative databases [34]. Their use warrants a high sample size with reasonable costs, and, in effect, we enrolled a large and very homogeneous population. In addition, the sensitivity and specificity of Op diagnosis based on administrative health database are very affordable, especially when the prescription of anti-osteoporotic drugs is used as diagnostic criteria. Finally, the study protocol excludes the more prevalent causes of secondary Op. All data collected for this study are integral part of the usual clinical management of the adult subjects registered in the Italian National Health System. On the other hand, the use of administrative data does not allow evaluating if the anatomical site of DXA examination showing pathological BMD finding (i.e. lumbar, femoral or ulnar site) influences the study results, or the number of cigarette smoked per day. We are also not able to obtain data regarding some factors that can influence the BMD (such as vitamin D status, physical activity, dietary habits and socio-economics factors) of enrolled subjects, or the duration of the exposure to second- or third-hand smoke. Moreover, we used data obtained with different types of DXA machines, albeit all measurements were carried out in clinical centres affiliated with the Italian Health National System (in Italian Sistema Sanitario Nazionale) and therefore each clinical centre is subjected to unique quality controls (International Organization for Standardization—ISO 9001). Another limit of the study is its cross-sectional and retrospective nature, which impairs establish cause–effect relationships between passive smoking and Op. Actually, to our knowledge, this is the first study based on women population of European ancestry.
A double-edged sword may be represented by the generalizability of the study results. Indeed, our study population is not representative of all community-dwelling women, but only of those undergoing DXA evaluation of BMD. The examined women are just representative for those that already present a higher risk of Op and fragility fractures, being selected according to the Italian Ministry of Health prevention plan, based in turn on the WHO criteria. On the other hand, our selection criteria provided a population that presented already with a higher risk of Op than the general public and it miss to recognize other significant differences among the three groups, such as BMI and vitamin D deficiency. Nevertheless, the high prevalence of Op provides also with the opportunity of studying specific risk factors, such as passive smoke. The selection criteria and the high environmental air pollution are responsible for the high prevalence of Op observed in the entire study population (more than 80%).
In conclusion, despite these limits, our study results suggest, that PS showed an Op risk similar to the one observed in CS and significantly higher to the risk observed in NS. Finally, active and passive smoking could be two equivalent Op risk factors, but further prospective studies, using different study methodologies, are desirable to expand our knowledge in this intriguing and epidemiological very important research setting. This study sends the provocative message that the inclusion of exposure to ETS as an Op risk factor is needed and that it may affect the screening programs.
References
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health (2014) The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US)
Rojas-Rueda D, Morales-Zamora E, Alsufyani WA, Herbst CH, AlBalawi SM, Alsukait R, Alomran M (2021) Environmental risk factors and health: an umbrella review of meta-analyses. Int J Environ Res Public Health 18(2):704. https://doi.org/10.3390/ijerph18020704
Nogueira SO, Fernández E, Driezen P, Fu M, Tigova O, Castellano Y, Mons U, Herbeć A, Kyriakos CN, Demjén T, Trofor AC, Przewoźniak K, Katsaounou PA, Vardavas CI, Fong GT, EUREST-PLUS Consortium (2022) Secondhand Smoke Exposure in European Countries with different smoke-free legislation: findings from the EUREST-PLUS ITC Europe surveys. Nicotine Tob Res 24(1):85–92. https://doi.org/10.1093/ntr/ntab157
Öberg M, Woodward A, Jaakkola MS, Peruga A, Prüss-Ustün A (2011) Global estimate of the burden of disease from second-hand smoke. World Health Organization, Geneva
Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A (2011) Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 377(9760):139–146. https://doi.org/10.1016/S0140-6736(10)61388-8
Chen C, Huang YB, Liu XO, Gao Y, Dai HJ, Song FJ, Li WQ, Wang J, Yan Y, Wang PS, Wang YG, Chen KX (2014) Active and passive smoking with breast cancer risk for Chinese females: a systematic review and meta-analysis. Chin J Cancer 33(6):306–316. https://doi.org/10.5732/cjc.013.10248
Su B, Qin W, Xue F, Wei X, Guan Q, Jiang W, Wang S, Xu M, Yu S (2018) The relation of passive smoking with cervical cancer: a systematic review and meta-analysis. Medicine (Baltimore) 97(46):e13061. https://doi.org/10.1097/MD.0000000000013061
Lv X, Sun J, Bi Y, Xu M, Lu J, Zhao L, Xu Y (2015) Risk of all-cause mortality and cardiovascular disease associated with secondhand smoke exposure: a systematic review and meta-analysis. Int J Cardiol 199:106–115. https://doi.org/10.1016/j.ijcard.2015.07.011
Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E (2017) Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta-analyses. Alzheimers Dement 13(4):406–418. https://doi.org/10.1016/j.jalz.2016.07.152
Burki TK (2021) WHO releases latest report on the global tobacco epidemic. Lancet Oncol 22(9):1217. https://doi.org/10.1016/S1470-2045(21)00464-2
Dogliotti E, Achene L, Beccaloni E et al (2019) Linee guida per la valutazione di impatto sanitario (DL.vo 104/2017) (in Italian) Roma: Istituto Superiore di Sanità (Rapporti ISTISAN 19/9)
Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8(1):136. https://doi.org/10.1007/s11657-013-0136-1
World Health Organization (2003) Prevention and management of osteoporosis. World Health Organ Tech Rep Ser 921:1–164
Nuti R, Brandi ML, Checchia G, Di Munno O, Dominguez L, Falaschi P, Fiore CE, Iolascon G, Maggi S, Michieli R, Migliaccio S, Minisola S, Rossini M, Sessa G, Tarantino U, Toselli A, Isaia GC (2019) Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med 14(1):85–102. https://doi.org/10.1007/s11739-018-1874-2
Yoon V, Maalouf NM, Sakhaee K (2012) The effects of smoking on bone metabolism. Osteoporos Int 23(8):2081–2092. https://doi.org/10.1007/s00198-012-1940-y
Adami G, Cattani G, Rossini M, Viapiana O, Olivi P, Orsolini G, Bertoldo E, Fracassi E, Gatti D, Fassio A (2022) Association between exposure to fine particulate matter and osteoporosis: a population-based cohort study. Osteoporos Int 33(1):169–176. https://doi.org/10.1007/s00198-021-06060-9
Levey AS, Inker LA, Coresh J (2014) GFR estimation: from physiology to public health. Am J Kidney Dis 63(5):820–834. https://doi.org/10.1053/j.ajkd.2013.12.006
Rendina D, D’Elia L, Evangelista M, De Filippo G, Giaquinto A, Abate V, Barone B, Piccinocchi G, Prezioso D, Strazzullo P (2020) Metabolic syndrome is associated to an increased risk of low bone mineral density in free-living women with suspected osteoporosis. J Endocrinol Invest 44(6):1321–1326. https://doi.org/10.1007/s40618-020-01428-w
Rendina D, D’Elia L, Evangelista M, De Filippo G, Giaquinto A, Barone B, Piccinocchi G, Prezioso D, Strazzullo P (2020) Osteoporosis is a predictive factor for nephrolithiasis in an adult free-living caucasian population from Southern Italy: a longitudinal retrospective study based on a general practice database. Calcif Tissue Int 107(5):446–452. https://doi.org/10.1007/s00223-020-00737-9
Rendina D, D’Elia L, De Filippo G, Abate V, Evangelista M, Giaquinto A, Barone B, Piccinocchi G, Prezioso D, Strazzullo P (2021) Metabolic syndrome is not associated to an increased risk of low bone mineral density in men at risk for osteoporosis. J Endocrinol Invest 45(2):309–315. https://doi.org/10.1007/s40618-021-01638-w
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9(8):1137–1141. https://doi.org/10.1002/jbmr.5650090802
Gazzetta Ufficiale Della Repubblica Italiana. Aggiornamento della Nota 79 (14 March 2017). https://www.aifa.gov.it/documents/20142/0/Determinazione_446-2017_agg_nota79.pdf Accessed 11 Oct 2022
Rendina D, De Palma D, De Filippo G, De Pascale F, Muscariello R, Ippolito R, Fazio V, Fiengo A, Benvenuto D, Strazzullo P, Galletti F (2014) Prevalence of simple nodular goiter and Hashimoto’s thyroiditis in current, previous, and never smokers in a geographical area with mild iodine deficiency. Horm Metab Res 47(3):214–219. https://doi.org/10.1055/s-0034-1387702
Oncken C, Prestwood K, Cooney JL, Unson C, Fall P, Kulldorff M, Raisz LG (2002) Effects of smoking cessation or reduction on hormone profiles and bone turnover in postmenopausal women. Nicotine Tob Res 4(4):451–458. https://doi.org/10.1080/1462220021000018399
Akhter MP, Lund AD, Gairola CG (2005) Bone biomechanical property deterioration due to tobacco smoke exposure. Calcif Tissue Int 77(5):319–326. https://doi.org/10.1007/s00223-005-0072-1
Zhu P, Xiong X, Chen C, Ran J (2022) Association of aldehyde exposure with bone mineral density in the national health and nutrition examination survey (NHANES 2013–2014). J Endocrinol Invest 45(11):2085–2096. https://doi.org/10.1007/s40618-022-01840-4
Ye Q, Xi X, Fan D, Cao X, Wang Q, Wang X, Zhang M, Wang B, Tao Q, Xiao C (2022) Polycyclic aromatic hydrocarbons in bone homeostasis. Biomed Pharmacother 146:112547. https://doi.org/10.1016/j.biopha.2021.112547
Kiyota Y, Muramatsu H, Sato Y, Kobayashi T, Miyamoto K, Iwamoto T, Matsumoto M, Nakamura M, Tateno H, Sato K, Miyamoto T (2020) Smoking cessation increases levels of osteocalcin and uncarboxylated osteocalcin in human sera. Sci Rep 10(1):16845. https://doi.org/10.1038/s41598-020-73789-4
Ko CH, Chan RL, Siu WS, Shum WT, Leung PC, Zhang L, Cho CH (2015) Deteriorating effect on bone metabolism and microstructure by passive cigarette smoking through dual actions on osteoblast and osteoclast. Calcif Tissue Int 96(5):389–400. https://doi.org/10.1007/s00223-015-9966-8
U.S. Department of Health and Human Services (2006) The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
Matt GE, Quintana PJ, Destaillats H, Gundel LA, Sleiman M, Singer BC, Jacob P, Benowitz N, Winickoff JP, Rehan V, Talbot P, Schick S, Samet J, Wang Y, Hang B, Martins-Green M, Pankow JF, Hovell MF (2011) Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect 119(9):1218–1226. https://doi.org/10.1289/ehp.1103500
Yuan L, Ni J (2022) The association between tobacco smoke exposure and vitamin D levels among US general population, 2001–2014: temporal variation and inequalities in population susceptibility. Environ Sci Pollut Res Int 29(22):32773–32787. https://doi.org/10.1007/s11356-021-17905-5
Nwosu BU, Kum-Nji P (2018) Tobacco smoke exposure is an independent predictor of vitamin D deficiency in US children. PLoS ONE 13(10):e0205342. https://doi.org/10.1371/journal.pone.0205342
Akerblom HK, Uhari M, Pesonen E, Dahl M, Kaprio EA, Nuutinen EM, Pietikäinen M, Salo MK, Aromaa A, Kannas L (1991) Cardiovascular risk in young Finns. Ann Med 23(1):35–39. https://doi.org/10.3109/07853899109147928
Holmberg T, Bech M, Curtis T, Juel K, Grønbæk M, Brixen K (2011) Association between passive smoking in adulthood and phalangeal bone mineral density: results from the KRAM study–the Danish Health Examination Survey 2007–2008. Osteoporos Int 22(12):2989–2999. https://doi.org/10.1007/s00198-010-1506-9
Blum M, Harris SS, Must A, Phillips SM, Rand WM, Dawson-Hughes B (2002) Household tobacco smoke exposure is negatively associated with premenopausal bone mass. Osteoporos Int 13(8):663–668. https://doi.org/10.1007/s001980200090
Juonala M, Pitkänen N, Tolonen S, Laaksonen M, Sievänen H, Jokinen E, Laitinen T, Sabin MA, Hutri-Kähönen N, Lehtimäki T, Taittonen L, Jula A, Loo BM, Impivaara O, Kähönen M, Magnussen CG, Viikari JSA, Raitakari OT (2019) Childhood exposure to passive smoking and bone health in adulthood: the cardiovascular risk in Young Finns Study. JCEM 104(6):2403–2411. https://doi.org/10.1210/jc.2018-02501
Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C (1999) Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res 14(9):1622–1627. https://doi.org/10.1359/jbmr.1999.14.9.1622
Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2005) Smoking and fracture risk: a meta-analysis. Osteoporos Int 16(2):155–162. https://doi.org/10.1007/s00198-004-1640-3
Helevä A, Murtomäki A, Huhtala H, Bousquet J, Luukkainen A, Karjalainen J, Lemmetyinen R, Haukka J, Torkki P, Nuutinen M, Toppila-Salmi S (2023) Risk factors of NSAID-exacerbated respiratory disease: a population-based study. Clin transl Allergy 13(8):e12296. https://doi.org/10.1002/clt2.12296
Wu TD, Brigham EP, Peng R, Koehler K, Rand C, Matsui EC, Diette GB, Hansel NN, McCormack MC (2018) Overweight/obesity enhances associations between secondhand smoke exposure and asthma morbidity in children. J Allergy Clin Immunol 6(6):2157-2159.e55. https://doi.org/10.1016/j.jaip.2018.04.020
Ha L, Tran A, Bui L, Giovannucci E, Mucci L, Song M, Le PD, Hoang M, Tran H, Kim G, Pham T (2023) Proportion and number of cancer cases and deaths attributable to behavioral risk factors in Vietnam. Int J Cancer 153(3):524–538. https://doi.org/10.1002/ijc.34549
Gu H, Hao L, Li M, Li J (2023) Joint effect of overweight/obesity and tobacco exposure on hypertension in children aged 6–17 years: a cross-sectional study. Front Pediatr 11:1188417. https://doi.org/10.3389/fped.2023.1188417
Kurniasari MD, Karwur FF, Rayanti RE, Dharmana E, Rias YA, Chou KR, Tsai HT (2021) Second-hand smoke and its synergistic effect with a Body-Mass Index of >24.9 kg/m2 increase the risk of gout arthritis in Indonesia. Int J Environ Res Public Health 18(8):4324. https://doi.org/10.3390/ijerph18084324
Kermah D, Shaheen M, Pan D, Friedman TC (2017) Association between secondhand smoke and obesity and glucose abnormalities: data from the National Health and Nutrition Examination Survey (NHANES 1999–2010). BMJ Open Diabetes Res Care 5(1):e000324. https://doi.org/10.1136/bmjdrc-2016-000324
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455. https://doi.org/10.1210/er.2011-1050
Institute of Medicine (US) Committee on Secondhand Smoke Exposure and Acute Coronary Events. Secondhand Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence. Washington (DC): National Academies Press (US); 2010. 2, Evaluating Exposure to Secondhand Smoke. Available from: https://www.ncbi.nlm.nih.gov/books/NBK219569
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. This was an unfunded project. All authors declare that there had no support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and informed consent
Written informed consent has been obtained from each patient or subject after full explanation of the purpose and nature of all used procedures. The study protocol was approved by the ASL Napoli 1 Ethical Committee, protocol number 0018508/2018. Informed consent was obtained from all individual participants included in the study.
Research involving human and animal participants
All procedures performed in this study, which involves human participants, were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vergatti, A., Abate, V., Giaquinto, A. et al. Role of active and environmental tobacco smoke on susceptibility to osteoporosis in women undergoing dual-X-ray absorptiometry. J Endocrinol Invest 47, 937–946 (2024). https://doi.org/10.1007/s40618-023-02211-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02211-3