Abstract
Aim
To assess which parameters among hyperandrogenism (total testosterone—tT—or free androgen index—FAI), sex hormone binding globulin (SHBG) or body mass index (BMI) could better predict a worse metabolic profile in women with polycystic ovary syndrome (PCOS).
Methods
Five hundred and eighty-six women with PCOS and clinical or biochemical hyperandrogenism were included. Receiver Operating Characteristics (ROC) curves with tT, FAI, SHBG and BMI were performed for metabolic parameters and a cut-off with sensitivity and specificity was obtained for each parameter. The women were then divided into three groups and compared according to their BMI.
Results
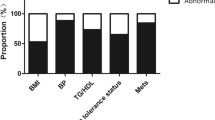
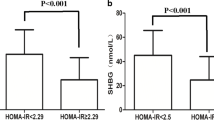
Based on the ROC curves, tT proved not to be a good predictor of metabolic alterations. FAI and SHBG resulted to be good predictors of some markers of metabolic damage. The area under the curves (AUC) of SHBG were greater than those of FAI. SHBG levels affects the values of homeostasis model assessment of insulin resistance (HOMA-IR), fasting insulin, high density lipoproteins (HDL), low density lipoproteins (LDL), and total cholesterol also when corrected for BMI. However, the highest AUCs of the ROC curves were observed when BMI was used, which was significantly related to all the metabolic parameters analyzed. Dividing women according to their BMI, BMI between 25.00 and 30.00 kg/m2 had a worse metabolic profile but still in a normal range, while BMI ≥ 30 kg/m2 women had a significant metabolic derangement.
Discussion
BMI is a good predictor factor of metabolic changes in PCOS women at any age, and obesity is associated to the appearance of metabolic complications. Overweight and obese PCOS women should be addressed to perform a complete metabolic assessment.


Similar content being viewed by others
Data availability
Data will be made available by the authors upon reasonable request.
References
Bozdag G, Mumusoglu S, Zengin D et al (2016) The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 31:2841–2855. https://doi.org/10.1093/humrep/dew218
The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004
Apridonidze T, Essah PA, Iuorno MJ, Nestler JE (2005) Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:1929–1935. https://doi.org/10.1210/jc.2004-1045
Wekker V, van Dammen L, Koning A et al (2020) Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update 26:942–960. https://doi.org/10.1093/humupd/dmaa029
Randeva HS, Tan BK, Weickert MO et al (2012) Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev 33:812–841. https://doi.org/10.1210/er.2012-1003
van der Ham K, Louwers YV, Laven JSE (2022) Cardiometabolic biomarkers in women with polycystic ovary syndrome. Fertil Steril 117:887–896. https://doi.org/10.1016/J.FERTNSTERT.2022.03.008
Osibogun O, Ogunmoroti O, Michos ED (2020) Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc Med 30:399–404. https://doi.org/10.1016/J.TCM.2019.08.010
Teede H, Misso M, Costello M et al (2018) International evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol. https://doi.org/10.1111/cen.13795
Teede HJ, Tay CT, Laven JJE et al (2023) Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab 00:1–23. https://doi.org/10.1210/CLINEM/DGAD463
Sanchez-Garrido MA, Tena-Sempere M (2020) Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab 35:100937. https://doi.org/10.1016/j.molmet.2020.01.001
Fruzzetti F, Perini D, Lazzarini V et al (2009) Adolescent girls with polycystic ovary syndrome showing different phenotypes have a different metabolic profile associated with increasing androgen levels. Fertil Steril 92:626–634. https://doi.org/10.1016/j.fertnstert.2008.06.004
Bil E, Dilbaz B, Cirik DA et al (2016) Metabolic syndrome and metabolic risk profile according to polycystic ovary syndrome phenotype. J Obstet Gynaecol Res 42:837–843. https://doi.org/10.1111/JOG.12985
Biernacka-Bartnik A, Kocełak P, Owczarek AJ et al (2022) Prediction of insulin resistance and impaired fasting glucose based on sex hormone-binding globulin (SHBG) levels in polycystic ovary syndrome. Int J Endocrinol 2022:1–6. https://doi.org/10.1155/2022/6498768
Di Stasi V, Maseroli E, Rastrelli G et al (2021) SHBG as a marker of NAFLD and metabolic impairments in women referred for oligomenorrhea and/or hirsutism and in women with sexual dysfunction. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2021.641446
Glueck CJ, Goldenberg N (2019) Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism 92:108–120. https://doi.org/10.1016/J.METABOL.2018.11.002
Peña AS, Witchel SF, Hoeger KM et al (2020) Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med 18:72. https://doi.org/10.1186/s12916-020-01516-x
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. https://doi.org/10.1093/clinchem/18.6.499
Hosmer DW, Lemeshow S, Sturdivant RX (2013) Applied logistic regression. Chapter 5: assessing the fit of the model, 3rd edn. Wiley
Genazzani AD, Prati A, Santagni S et al (2012) Differential insulin response to myo-inositol administration in obese polycystic ovary syndrome patients. Gynecol Endocrinol 28:969–973. https://doi.org/10.3109/09513590.2012.685205
Mandrekar JN (2010) Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 5:1315–1316. https://doi.org/10.1097/JTO.0B013E3181EC173D
Armanini D, Boscaro M, Bordin L, Sabbadin C (2022) Controversies in the pathogenesis, diagnosis and treatment of PCOS: focus on insulin resistance, inflammation, and hyperandrogenism. Int J Mol Sci. https://doi.org/10.3390/IJMS23084110
Rosner W, Auchus RJ, Azziz R et al (2007) Utility, limitations, and pitfalls in measuring testosterone: an endocrine society position statement. J Clin Endocrinol Metab 92:405–413. https://doi.org/10.1210/jc.2006-1864
Hahn S, Kuehnel W, Tan S et al (2007) Diagnostic value of calculated testosterone indices in the assessment of polycystic ovary syndrome. Clin Chem Lab Med 45:5. https://doi.org/10.1515/CCLM.2007.031
Mueller A, Dittrich R, Cupisti S et al (2006) Is it necessary to measure free testosterone to assess hyperandrogenemia in women? The role of calculated free and bioavailable testosterone. Exp Clin Endocrinol Diabetes 114:182–187. https://doi.org/10.1055/S-2006-924062
Zhang B, Fan Y, Wang Y et al (2021) Comparison of bioelectrical body and visceral fat indices with anthropometric measures and optimal cutoffs in relation to hypertension by age and gender among Chinese adults. BMC Cardiovasc Disord. https://doi.org/10.1186/S12872-021-02100-8
Liu P, Ma F, Lou H, Liu Y (2013) The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health. https://doi.org/10.1186/1471-2458-13-629
Almasi MH, Barzin M, Serahati S et al (2022) Body composition assessment by bioelectrical impedance analysis in prediction of cardio-metabolic risk factors: Tehran lipid and glucose study (TLGS). Iran J Public Health 51:851–859. https://doi.org/10.18502/IJPH.V51I4.9246
Funding
No funding was required for the study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. FF and TF participated in study, execution, analysis, manuscript drafting, and critical discussion. EB and FB participated in study execution. MT participated in manuscript drafting and critical discussion.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Research involving human participants and/or animals
All procedures were performed in accordance with the ethical standards of the Committee on Institutional Human Experimentation, and with the Helsinki Declaration of 1975, as revised in 1983.
Informed consent
All patients gave informed consent for data to be collected and for their anonymous data to be used for clinical publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fruzzetti, F., Fidecicchi, T., Benelli, E. et al. Body mass index is a good predictor of metabolic abnormalities in polycystic ovary syndrome. J Endocrinol Invest 47, 927–936 (2024). https://doi.org/10.1007/s40618-023-02210-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02210-4