Abstract
Purpose
The specific indications of somatostatin analogs (SSAs) in patients with neuroendocrine tumor (NET) emerged over the time. The objective of this review is to summarize and discuss the most relevant data concerning long-acting SSAs in NET.
Methods
A narrative review was performed including publications focusing on therapy with the long-acting octreotide, lanreotide, and pasireotide in patients with NET.
Results
Long-acting SSAs confirm to be a manageable and widely used tool in patients with NET. Both long-acting octreotide and lanreotide are safe as the short-acting formulations, while patient compliance and adherence is further improved. Together with some randomized phase-3 trials, many retrospective and prospective studies have been performed in the last 20 years revealing a variable but substantial impact on progression free survival, not only in gastroenteropancreatic but also in lung and unknown primary NETs. The most frequent tumor response to SSAs is stable disease, but an objective response can be observed, more frequently by using high-dose schedules and in MEN1-related pancreatic NETs. Low tumor burden, low tumor grade (G1 and low G2), good performance status and use as first-line therapy are the main predictive factors to SSAs in NET patients. Pasireotide has been evaluated in few studies. This compound remains a promising SSA and would deserve to be further evaluated as a potential additional indication in NET therapy.
Conclusions
Long-acting SSAs are an effective and safe initial therapy of patients with well differentiated NET, allowing tumor growth as well as symptoms control for long-time in selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatostatin analogs (SSAs) have been initially developed as short-acting octapeptide analogs of the native somatostatin and first employed for the treatment of neuroendocrine neoplasms (NENs) about 35 years ago. They were indicated in patients with carcinoid syndrome and other less frequent NEN-related endocrine syndromes, such as glucagonoma, VIPoma, etc [1,2,3]. The short-acting subcutaneous formulation of octreotide (OCT) is characterized by an 8-h half-life, administered two/three times a day, each single-dose ranging 0.05 to 0.5 mg [3, 4]. However, some studies reported experiences with high SSA daily dose, either with OCT or the other octapeptide lanreotide (LAN) [5,6,7,8].
Subsequently long-acting (LA) slow-release formulations have been developed. In particular, LAN slow-release and OCT LAR were developed for intramuscular injection at the dose of 30 mg every 14 days and 10–30 mg every 28 days, respectively [9,10,11]. Finally, the autogel LAN formulation was developed for deep subcutaneous injection at the dose of 60–120 mg every 14–28 days [12]. These compounds rapidly replaced the short-acting formulations being more manageable tool for clinical practice [4]. Pharmacokinetic studies of LA SSAs highlighted that the mean time to reach maximum concentration (tmax) was 22 days for a dose of 20 mg OCT, 12.6 days for a dose of 60 mg OCT, while tmax was 2.4 days and 1.1 days for LAN 90 and 120 mg, respectively [13, 14]. Despite the different tmax, the steady-state concentration was satisfying for both agents, resulting in a standard dose of 10–30 mg every 28 days for OCT LAR, 30–60 mg every 14–28 days for slow-release LAN, 60–120 mg every 14–28 days for LAN autogel. These formulations dramatically changed the patient’s perspective, improving the patient’s compliance to therapy and allowing long-time stable treatment. This finally resulted in an increase of studies investigating SSA effectiveness and safety in NEN patients. The pharmacokinetic findings above reported also suggest these drugs to be dose-dependent, supporting their use in high dose schedules, at least in NEN patients refractory to standard doses. This approach is now accepted in the last NEN guidelines and will be described in this review in a dedicated paragraph.
LA SSAs represent nowadays the first-line treatments of low-grade advanced well differentiated NENs, the so called neuroendocrine tumors (NETs). Both LA OCT and LAN demonstrated to improve the rate of progression in randomized placebo-controlled trials [15,16,17]. Even before these trials, LA SSAs have been used in clinical practice, first in patients with NEN-related endocrine syndromes to control hormone hypersecretion and related symptoms, then also in non-functioning NETs, mostly G1-G2, as antiproliferative agents. These preliminary results have mostly been reported in several retrospective and few prospective studies (Table 1). More recently, other studies have been conducted to investigate the activity of LA SSAs in different conditions as NET with primary origin other than gastroenteropancreatic (GEP) or genetically determined NETs, as well as, also, focusing on above level schedules or new SSAs (as Pasireotide).
This review aims to summarize data on efficacy and safety of LA SSAs in patients with NET but also to report some peculiar aspects which are not frequently focused on.
Methods
This narrative review was performed for available prospective, retrospective and review articles, published up to April 2023 in PubMed. Data were extracted from the text and from the tables of the manuscript. The keyword search used included “somatostatin analogues and neuroendocrine tumors”, “somatostatin analogues and neuroendocrine neoplasms”, “somatostatin analogues and carcinoid syndrome”, “somatostatin analogues and inherited tumor syndromes”, “somatostatin analogues and MEN1”, “octreotide and neuroendocrine tumors”, “lanreotide and neuroendocrine neoplasms”, “octreotide and neuroendocrine neoplasms”, “lanreotide and neuroendocrine neoplasms”, “octreotide and inherited tumor syndromes”, “lanreotide and inherited tumor syndromes”, “octreotide and MEN1”, “lanreotide and MEN1”, “pasireotide and neuroendocrine tumors”, “pasireotide and neuroendocrine neoplasms”. The articles were selected on the basis of relevance of title and abstract in the topic.
Results
Efficacy and tolerability of octreotide and lanreotide
The main advantage associated with LA formulations has been to obtain a manageable tool for long-term therapy. Improved patient compliance has been reported in patients treated with LA formulations as compared to the short-acting ones, even if associated with a similar drug profile in terms of both effectiveness and safety [18,19,20].
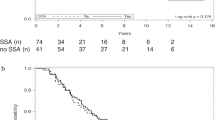
SSA effectiveness in NET patients has been observed regardless from their anatomic origin, either to control hormonal symptoms or to exert antiproliferative activity [21,22,23]. The most robust data have been obtained in gastroenteropancreatic (GEP) NETs and in particular in ileal and pancreatic tumors which were investigated in randomized phase 3 trials [15, 16]. OCT LAR at the dose of 30 mg every 28 days demonstrated a significant improvement of the median time to progression vs placebo in patients with advanced midgut NET (14.3 vs 6 months, HR 0.34, p <0.0001) [15]. Notably, in this study 95.3% of the included patients had Ki-67 values up to 2%. LAN autogel at the dose of 120 mg every 28 days was associated with a significant improvement of the median PFS as compared to placebo in patients with advanced entero-pancreatic NET (not reached vs 18 months, HR 0.47, p <0.001) [16]. Among the 101 patients treated with LAN autogel, 69 cases (68%) had a Ki-67 of 0-2 % and 32 cases (32%) a Ki-67 up to 10%. A subsequent open-label extension study reported a median PFS of 38.5 months in LAN-LAN subgroup and a time to death or subsequent progression of 19 months in those treated with LAN at the time of progression [17]. If these studies definitively clarified the antiproliferative activity of LA OCT and LAN in low grade GEP NETs, on the other hand they demonstrated a satisfying safety profile with a low rate of treatment discontinuation, consistent with long-term treatment and good quality of life [15,16,17]. Many other non-randomized studies investigated the role of LA SSAs in real world, demonstrating variable results but confirming the activity of these compounds not only in GEP but also in lung NETs, as well as in metastatic tumors with unknown primary site, even though at a lower extent (Table 1). More in deep, non-randomized studies were highly variable for study design, patient population, SSA type and schedule. The median PFS ranged from 12.9 to 89 months mainly depending on tumor stage, grade and growth rate as well as previous treatments. The study reporting the highest PFS included mainly low-grade tumors (G1 and G2 NET in 79% of cases) and both localized and metastatic tumor stage [24]. The objective response rate varied at a lesser extent ranging from 0 to 15%, which was a partial response in the vast majority of cases. The most frequent type of response to SSA was stable disease, ranging from 45 to 89% (Table 1). Unfortunately, the development of resistance to SSA could occur and this issue should be taken into account in patients’ management. However, the molecular mechanisms involved in this complex phenomenon have not been elucidated, so far.
LA SSAs are safe as well documented not only in controlled trials but also in real-world studies [25]. Both treatment discontinuation and dose adjustment are unfrequently needed [26]. Gastrointestinal abnormalities are the most frequently reported side effects in this setting. They are generally mild to moderate and include abdominal cramps, steatorrhea and altered digestion [27]. One relevant but poorly investigated cause of gastrointestinal disorders as consequence of SSA therapy is the exocrine pancreatic insufficiency, which needs to be recognized and treated [28]. However, the most relevant side-effect to manage in patients treated with SSAs is cholelithiasis, ranging from biliary sludge to gallstones, which can be complicated by biliary colic, cholecystitis or cholangitis [29]. The rate of biliary disorders in patients with advanced GEP NET is not increased in LA as compared to short-acting SSAs [30]. This finding is also true for the whole spectrum of toxicity which does not differ between different SSA formulations [18]. The safety profile is not different between OCT and LAN. However, from a practical point of view, LAN was reported to be easier to manage than OCT and less frequently complicated by pain and technical problems, because of the autogel formulation with a comfortable device and a fast administration [31, 32].
Predictive factors of response
Among the pathologic factors, somatostatin receptor (SSTR) expression is expected to play a central role to predict response to LA SSAs in NET patients. However, few evidences are available regarding the SSTR status in relation to SSA therapy. In a work by Kasajima et al. including 38 NEN patients, SSTR type 2 (SSTR2) expression evaluated through HER2-Score was associated with response to SSA treatment (p = 0.045) [33]. Volante et al. reported a positive correlation between immunohistochemical SSTR2A score 2/3, as defined by tumor cell membrane immunostaining, and response to treatment with octreotide LAR [34]. Conversely, a negative predictive role has been identified for poorly differentiated morphology [35], as well as higher tumor grade [36, 37]. The Ki-67 index, the main proliferative marker in NET, has been reported to be predictive for survival and progression outcomes in NET patients treated with SSA [24, 38]. Guidelines consider a threshold of 10% to suggest SSA therapy in advanced NETs [22], on the basis of Clarinet trial. A 10% cut-off is also supported by some retrospective studies [39, 40], while a 5% cut-off has been reported in other studies on GEP and lung NETs [24, 41].
With regards to clinical factors, a worse patient performance status [38], as well as the tumor growth rate at baseline have been identified as negative predictors of response to SSA [42]. In this context, the GETNE-TRASGU tool, elaborated by the Spanish Group of Neuroendocrine and Endocrine Tumors (GETNE) from the data of 535 patients with GEP-NENs receiving SSA treatment, has suggested that the presence of symptoms, the extent of liver involvement, the presence of bone and peritoneal metastases and the progression status, are all negative factors [43]. In the same work, also higher neutrophil-to-lymphocyte ratio and higher alkaline phosphatase levels have been identified as negative predictive factors for SSA treated patients [43]. Among the circulating biomarkers, conflicting data are available on chromogranin A (CgA). Few studies report the decrease of CgA after SSA therapy as a positive predictive factor [44, 45]. A post hoc analysis of the Phase 3 CLARINET study detected a correlation between CgA response to SSA and patients’ outcomes [45]. Interestingly, in this study a decrease of 5-hydroxyindoleacetic acid (5-HIAA) levels was associated to an improved PFS in SSA-treated patients. Forest plot analysis confirmed a correlation of 5‐HIAA reduction being favorable for SSA [45]. Finally, positive SSTR status on somatostatin receptor imaging (SRI) has failed to demonstrate to be predictive of response to SSA [46]. By a practical point of view, to check SRI positivity is recommended but not necessary for antiproliferative LA SSA therapy in NET [23]. On the other hand, it should be underlined that the optimal candidates for SSA therapy are well differentiated low-grade NETs, which are commonly SSTR2-positive and therefore expected to be SRI-positive.
High-dose somatostatin analogs
Traditionally, a SSA dose increase is implemented to control refractory symptoms related to specific endocrine syndromes, such as carcinoid syndrome, glucagonoma etc. However, a role in tumor growth control has been recently recognized in the ENETS guidelines [47]. A non-randomized clinical trial dedicated to investigate above-label dose of LA SSAs has been only recently published in patients with pancreatic or midgut NETs treated with LAN 120 mg every two weeks, after progression with LAN at standard dose, reporting a median PFS of 8.3 months and 5.6 months, respectively [48]. Few phases 3 trials on digestive NETs with tumor progression or uncontrolled carcinoid syndrome on standard SSA therapy reported data on high-dose OCT as control arm. In particular, in the pasireotide trial OCT 40 mg monthly resulted in a mPFS of 6.8 months, while in the Netter-1 trial, OCT 60 mg monthly resulted in a mPFS of 8.4 months [49, 50]. No randomized trial focusing on high-dose SSAs has been performed. However, high-dose schedules with SSAs are widely used in clinical practice and studies on this topic dated from more than twenty years ago, firstly investigating short-acting agents then LA formulations. A conclusion shared by all studies is that adverse events are not increased by the increase of the dose. Regardless from SSA and type of schedule, high-dose treatments show similar safety profiles as standard treatments [23, 26, 51, 52]. Less definitive findings are reported about the efficacy outcomes of this approach. A recent metanalysis reported low rates of antiproliferative effect with high-dose after failure of SSAs at standard doses [53]. Most of data are obtained on low-grade GEP NETs. In this setting, median PFS ranges about 25–30 months in patients with radiologic or symptomatic progression under therapy with SSAs at standard doses. An objective tumor response was found in 4–14% of patients, according to different studies, patient and tumor characteristics, SSA schedule (Table 2). A large retrospective Italian Multicentric Study, performed on a homogeneous population of G1–G2 GEP NETs progressing on standard SSA treatments, highlighted median PFS 31 months, objective response 8.4%, stable disease 75.7% [54]. The early use in the therapeutic sequence was the only predictive factor of response to high-dose SSA. These findings are in line with a previous retrospective study comparing four different therapeutic sequences in GEP and lung G1-G2 NETs progressing on standard doses. The sequence with above-standard SSA in second-line was equally effective as the other sequences with everolimus, chemotherapy and PRRT respectively [55]. According to these studies as well as to the Clarinet forte trial [48], the increased dose density is the commonest schedule, although dose intensity is a further possibility to take in account. An ultra-high-dose LA OCT (160 mg every 28 days) has been tested in patients with progressive ileal NET in progression after standard doses, but this compound has not been further developed and commercialized [56]. In summary, high-doses seem to be a reliable and safe approach to late the progression and switch to more aggressive treatments. However, the real antiproliferative efficacy of this approach is still debated and needs to be better investigated in dedicated prospective trials. At now, dose frequency increase is the best schedule in clinical practice. The best candidate for high-dose SSA therapy reflects the same identikit of the candidate to standard SSA therapy. In particular, a Ki-67 cut-off of 5% is a recognized predictive factor both for standard and high doses [24, 57]. On the contrary, tumors with Ki-67 >5% but also high growth rate and tumor burden suggest different approach (PRRT, everolimus, chemotherapy) after failure of SSA at standard doses.
NET in patients with inherited tumor syndromes
No specific indications are available for therapy with LA SSAs in NETs associated to inherited syndromes. Up to 10% of NENs can occur in the context of hereditary syndromes, such as multiple endocrine neoplasia type 1 (MEN1), multiple endocrine neoplasia type 2 (MEN2), multiple endocrine neoplasia type 4 (MEN4), Von Hippel–Lindau (VHL), neurofibromatosis type 1 (NF1), and tuberous sclerosis [58], the most frequent localization being the pancreas [58, 59]. MEN1 pancreatic NETs are the most frequent genetic NENs. These represent autosomal dominant inherited tumors, characterized by multifocality, very low grade and high SSTR expression. MEN1-related pancreatic NETs appear as optimal candidate to SSA therapy even at localized non-metastatic stage. According to the available evidences the objective tumor response was obtained with LA SSA in 10-33% of MEN-1-related pancreatic and duodenal NET patients (Table 3). A recent review systematically analyzed the efficacy and safety of SSA treatment in patients with MEN1-related pancreatic NETs [60]. Overall, 20 studies comprehensives of 105 MEN1 patients were included. Tumor response to SSAs was higher in MEN1-related NETs as compared to the sporadic counterpart. Specifically, stable disease (SD) was found in 75.6% of cases, while an objective response occurred in 12.7%. In particular, 8.9% of patients showed a partial response (PR), 3.8% a complete response (CR). No significant differences were observed in terms of efficacy between OCT and LAN. The only MEN1-dedicated prospective study with SSAs at standard dose evaluated LAN 120 mg monthly vs active surveillance. LAN was able to induce an objective tumor response in 17.4% and stable disease in 65.2% of patients with MEN1 pancreatic NETs <2 cm, while the median PFS was not reached vs 40 months in the group of patients without treatment [61].
Different reasons have been proposed to explain the better therapeutic response to SSAs observed in MEN1 than in sporadic pancreatic NETs. First, these patients showed a high rate of early stage and localized disease. Second, they are well differentiated low-grade tumors, with Ki-67 index usually <5% and high SSTR2 expression. Third, functioning NETs are more frequent in MEN1. All these characteristics make MEN1-related pancreatic NETs highly sensitive to SSA treatment [60].
Finally, in NEN associated with MEN2, NF1, VHL, and tuberous sclerosis data on the efficacy of SSAs are scarce and derive generally from case reports [62, 63]. In absence of specific indications, NETs associated with these syndromes can be treated in analogy with the MEN1-related NETs.
Pasireotide
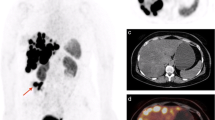
Pasireotide is a second generation SSA which has binding affinity for SSTR1, 2, 3, and 5. Pasireotide is available in a short-acting formulation for subcutaneous (SC) injection, which is administered twice daily, and a LA formulation for intramuscular (IM) injection, which is given every 28 days. Both formulations exhibit similar pharmacokinetic and pharmacodynamic properties and have a comparable safety profile [64, 65]. LA pasireotide is a promising therapy for patients with NET, especially those refractory or resistant to other SSAs [65] (Figure 1).
Yao et al. in a phase I study, demonstrated that the maximum tolerated dose for pasireotide in patients with advanced NETs is 120 mg, at which dose bradycardia events reached 31% compared to 0% at the 80 mg dose. The same authors highlighted encouraging effects of pasireotide on PFS and disease control, as well as on reduction of tumor markers (CgA, NSE, IGF-1). The pharmacokinetic profile of LA pasireotide in patients with NET revealed that this compound maintains steady plasma concentrations over the 28-day dosing interval with no evidence of accumulation in the body [66]. Furthermore, pasireotide was generally well-tolerated despite a high rate of adverse effects. The most common adverse events of any grade observed were hyperglycemia, fatigue, but also diarrhea and nausea [66]. Focusing specifically on hyperglycemia, in this study this side effects accounted for 79.3% of cases, with grade 3–4 in 10.3% of patients (a grade 3–4 was reported in 15.4% of cases with LA pasireotide 80 mg and in 6.3% of patients treated with LA pasireotide 120 mg). A good profile of safety and tolerability of LA pasireotide at a maximum dose of 60 mg has been then reported in patients with GEP-NET [49]. In a phase II prospective clinical trial, LA pasireotide 60 mg was evaluated in 29 NET patients as first-line systemic therapy. In this study 13 patients had a low-grade NET and 16 an intermediate grade tumor. The median PFS was 11 months (95% CI 7.6–16 months) [67]. A rate of 4% of patients experienced a partial response, 60% a stable disease and 36% progressive disease. In line with previous studies, adverse effects were mild to moderate, with high prevalence of hyperglycemia (65%), followed by diarrhea (14%) [67]. In a phase III study, two different SSA therapies, LA pasireotide (60 mg) and OCT (40 mg), were compared in patients with metastatic NET and carcinoid symptoms refractory to first-generation SSAs. The included patients had a well differentiated tumor in 77% of cases in the LA pasireotide arm and in 84% of cases enrolled in OCT arm. The study was halted at an interim analysis following a data monitoring committee recommendation due to a low predictive probability of showing superiority of pasireotide over OCT for carcinoid symptoms control. However, pasireotide group had a longer median PFS as compared to OCT (11.8 versus 6.8 months, p=0.045), suggesting a better antiproliferative activity of pasireotide [49].
Conclusions
LA OCT and LAN are an effective and safe tool for long-term therapy of patients with NET. In the last twenty years many evidences have been reported not only from selected patients included in randomized trials but also from real-world patients included in different types of retrospective and prospective studies. These compounds impact significantly on progression free survival and disease control rate, despite the variable entity of the response observed, according to study design, SSA schedule, study population and tumor characteristics. When used in patients with good performance status, with low-tumor burden and low-grade NETs, even better if with Ki-67 index ≤5%, and as first-line therapy, SSAs ensure prolonged progression free survival and a low but not negligible objective response rate, together with good control of endocrine symptoms and life quality, while severe adverse events and treatment discontinuation are rarely reported. In the same setting, SSAs administered at high doses, more easily with a dose-frequency increase, seem to be an effective second-line therapy in NETs progressing on standard doses. In the peculiar setting of MEN1, SSAs seem to be even more effective than in sporadic NETs. Finally, pasireotide remains a promising SSA and would deserve to be further evaluated as a potential additional indication in NET therapy.
References
Bauer W, Briner U, Doepfner W et al (1982) SMS 201–995: a very potent and selective octapeptide analog of somatostatin with prolonged action. Life Sci 31:1133–1140
Öberg K (1993) Chemotherapy and biotherapy in neuroendocrine tumors. Curr Opin Oncol 5:110–120
Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ (1996) Octreotide. N Engl J Med 334:246–254
Oberg K, Kvols L, Caplin M et al (2004) Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 15(6):966–973
Anthony L, Johnson D, Hande K et al (1993) Somatostatin analogue phase I trials in neuroendocrine neoplasms. Acta Oncol 32:217–223
di Bartolomeo M, Bajetta E, Buzzoni R et al (1996) Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. a study by the Italian trials in medical Oncology group. Cancer 77:402–408
Eriksson B, Renstrup J, Imam H, Oberg K (1997) High-dose treatment with lanreotide of patients with advanced neuroendocrine gastrointestinal tumors: clinical and biological effects. Ann Oncol 8:1041–1044
Faiss S, Räth U, Mansmann U et al (1999) Ultra-high-dose lanreotide treatment in patients with metastatic neuroendocrine gastroenteropancreatic tumors. Digestion 60:469–476
Arnold R, Simon B, Wied M (2000) Treatment of neuroendocrine GEP tumours with somatostatin analogues. A review Digestion 62(Suppl 1):84–91
Wymenga AN, Eriksson B, Salmela PI et al (1999) Efficacy and safety of prolonged-release lanreotide in patients with gastrointestinal neuroendocrine tumors and hormone-related symptoms. J Clin Oncol 17(4):1111
Ricci S, Antonuzzo A, Galli L et al (2000) Long-acting depot lanreotide in the treatment of patients with advanced neuroendocrine tumors. Am J Clin Oncol 23:412–415
Lightman S (2002) Somatuline autogel: an extended release lanreotide formulation. Hosp Med 63(3):162–165
Astruc B, Marbach P, Bouterfa H et al (2005) Long-acting octreotide and prolonged-release lanreotide formulations have different pharmacokinetic profiles. J Clin Pharmacol 45:836–844
Chen T, Miller TF, Prasad P et al (2000) Pharmacokinetics, pharmacodynamics, and safety of microencapsulated octreotide acetate in healthy subjects. J Clin Pharmacol 40:475–481
Rinke A, Müller HH, Schade-Brittinger C et al (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27(28):4656–4663
Caplin ME, Pavel M, Ćwikła JB et al (2014) Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371(3):224–233
Caplin ME, Pavel M, Phan AT et al (2021) Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: final results of the CLARINET open-label extension study. Endocrine 71(2):502–513
Rubin J, Ajani J, Schirmer W et al (1999) Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol 17(2):600–606
O’Toole D, Ducreux M, Bommelaer G et al (2000) Treatment of carcinoid syndrome: a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer 88(4):770–776
Dogliotti L, Tampellini M, Stivanello M et al (2001) The clinical management of neuroendocrine tumors with long-acting repeatable (LAR) octreotide: comparison with standard subcutaneous octreotide therapy. Ann Oncol 12(Suppl 2):S105–S109
Pivonello R, Ferone D, Filippella M et al (2003) Role of somatostatin analogs in the management of non-functioning neuroendocrine tumors. J Endocrinol Invest 26(8 Suppl):82–88
Pavel M, O’Toole D, Costa F et al (2016) ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 103(2):172–185
La Salvia A, Modica R, Rossi RE et al (2023) Targeting neuroendocrine tumors with octreotide and lanreotide: key points for clinical practice from NET specialists. Cancer Treat Rev 117:102560
Faggiano A, Carratù AC, Guadagno E et al (2016) Somatostatin analogues according to Ki67 index in neuroendocrine tumours: an observational retrospective-prospective analysis from real life. Oncotarget 7(5):5538–5547
Öberg K, Lamberts SW (2016) Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer 23(12):R551–R566
Faggiano A, Lo Calzo F, Pizza G et al (2017) The safety of available treatments options for neuroendocrine tumors. Expert Opin Drug Saf 16(10):1149–1161
Bornschein J, Drozdov I, Malfertheiner P (2009) Octreotide LAR: safety and tolerability issues. Expert Opin Drug Saf 8(6):755–768
Panzuto F, Magi L, Rinzivillo M (2021) Exocrine pancreatic insufficiency and somatostatin analogs in patients with neuroendocrine neoplasia. Expert Opin Drug Saf 20(4):383–386
Brighi N, Lamberti G, Maggio I et al (2019) Biliary stone disease in patients receiving somatostatin analogs for neuroendocrine neoplasms a retrospective observational study. Dig Liver Dis 51(5):689–694
Trendle MC, Moertel CG, Kvols LK (1997) Incidence and morbidity of cholelithiasis in patients receiving chronic octreotide for metastatic carcinoid and malignant islet cell tumors. Cancer 79(4):830–834
Adelman D, Truong Thanh XM, Feuilly M et al (2020) Evaluation of nurse preferences between the lanreotide autogel new syringe and the octreotide long-acting release syringe: an international simulated-use study (PRESTO). Adv Ther 37(4):1608–1619
O’Toole D, Kunz PL, Webb SM et al (2022) PRESTO 2: an international survey to evaluate patients’ injection experiences with the latest devices/formulations of long-acting somatostatin analog therapies for neuroendocrine tumors or acromegaly. Adv Ther 40(2):671–690
Kasajima A, Papotti M, Ito W et al (2018) High interlaboratory and interobserver agreement of somatostatin receptor immunohistochemical determination and correlation with response to somatostatin analogs. Hum Pathol 72:144–152
Volante M, Brizzi MP, Faggiano A et al (2007) Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 20(11):1172–1182
Ter-Minassian M, Zhang S, Brooks NV et al (2017) Association between tumor progression endpoints and overall survival in patients with advanced neuroendocrine tumors. Oncologist 22(2):165–172
Laskaratos FM, Armeni E, Shah H et al (2019) Predictors of antiproliferative effect of lanreotide autogel in advanced gastroenteropancreatic neuroendocrine neoplasms. Endocrine 67(1):233–242
Kang J, Yoo C, Hwang HS et al (2019) Efficacy and safety of lanreotide in Korean patients with metastatic, well-differentiated gastroenteropancreatic-neuroendocrine tumors: a retrospective analysis. Invest New Drugs 37(4):763–770
Özaslan E, Karaca H, Koca S et al (2017) Comparison of survival with somatostatin analog and chemotherapy and prognostic factors for treatment in 165 advanced neuroendocrine tumor patients with Ki-67 20% or less. Anticancer Drugs 28(2):222–229
Jann H, Denecke T, Koch M et al (2013) Impact of octreotide long-acting release on tumour growth control as a first-line treatment in neuroendocrine tumours of pancreatic origin. Neuroendocrinology 98(2):137–143
Lenotti E, Alberti A, Spada F et al (2021) Outcome of patients with metastatic lung neuroendocrine tumors submitted to first line monotherapy with somatostatin analogs. Front Endocrinol (Lausanne) 12:669484
Martín-Richard M, Massutí B, Pineda E et al (2013) Antiproliferative effects of lanreotide autogel in patients with progressive, well-differentiated neuroendocrine tumours: a Spanish, multicentre, open-label, single arm phase II study. BMC Cancer 20(13):427
Dromain C, Pavel ME, Ruszniewski P et al (2019) Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer 19(1):66
Carmona-Bayonas A, Jiménez-Fonseca P, Lamarca Á et al (2019) Prediction of progression-free survival in patients with advanced, well-differentiated, neuroendocrine tumors being treated with a Somatostatin Analog: The GETNE-TRASGU Study. J Clin Oncol 37(28):2571–2580
Buil-Bruna N, Dehez M, Manon A et al (2016) Establishing the Quantitative relationship between Lanreotide Autogel®, chromogranin a, and progression-free survival in patients with Nonfunctioning gastroenteropancreatic neuroendocrine tumors. AAPS J 18(3):703–712
Pavel ME, Phan AT, Wolin EM et al (2019) Effect of lanreotide Depot/Autogel on urinary 5-hydroxyindoleacetic acid and plasma chromogranin a biomarkers in nonfunctional metastatic enteropancreatic neuroendocrine tumors. Oncologist 24(4):463–474
Pavel M, Öberg K, Falconi M et al (2020) Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 31(7):844–860
Pavel M, Valle JW, Eriksson B et al (2017) ENETS consensus guidelines for the standards of care in neuroendocrine neoplasms systemic therapy biotherapy and novel targeted agents. Neuroendocrinology 105(3):266–280
Pavel M, Ćwikła JB, Lombard-Bohas C et al (2021) Efficacy and safety of high-dose lanreotide autogel in patients with progressive pancreatic or midgut neuroendocrine tumours: CLARINET FORTE phase 2 study results. Eur J Cancer 157:403–414
Wolin EM, Jarzab B, Eriksson B et al (2015) Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther 9:5075–5086
Strosberg J, El-Haddad G, Wolin E et al (2017) Phase 3 Trial of <sup>177</sup>Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 376(2):125–135
Broder MS, Beenhouwer D, Strosberg JR et al (2015) Gastrointestinal neuroendocrine tumors treated with high dose octreotide-LAR: a systematic literature review. World J Gastroenterol 21(6):1945–1955
Chan DL, Ferone D, Albertelli M et al (2017) Escalated-dose somatostatin analogues for antiproliferative effect in GEPNETS: a systematic review. Endocrine 57(3):366–375
Panzuto F, Ricci C, Rinzivillo M et al (2022) The antiproliferative activity of high-dose somatostatin analogs in gastro-entero-pancreatic neuroendocrine tumors: a systematic review and meta-analysis. J Clin Med 11(20):6127
Lamberti G, Faggiano A, Brighi N et al (2020) Nonconventional doses of somatostatin analogs in patients with progressing well-differentiated neuroendocrine tumor. J Clin Endocrinol Metab 105(1):194–200
Faggiano A, Di Maio S, Mocerino C et al (2019) Therapeutic sequences in patients with grade 1–2 neuroendocrine tumors (NET): an observational multicenter study from the ELIOS group. Endocrine 66(2):417–424
Welin SV, Janson ET, Sundin A et al (2004) High-dose treatment with a long-acting somatostatin analogue in patients with advanced midgut carcinoid tumours. Eur J Endocrinol 151(1):107–112
Diamantopoulos LN, Laskaratos FM, Kalligeros M et al (2021) Antiproliferative effect of above-label doses of somatostatin analogs for the management of gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology 111(7):650–659
Lewis MA (2020) Hereditary syndromes in neuroendocrine tumors. Curr Treat Options Oncol 21(6):50
Ruggeri RM, Benevento E, De Cicco F et al (2023) Neuroendocrine neoplasms in the context of inherited tumor syndromes: a reappraisal focused on targeted therapies. J Endocrinol Invest 46(2):213–234
La Salvia A, Sesti F, Grinzato C et al (2021) Somatostatin analogue therapy in MEN1-related pancreatic neuroendocrine tumors from evidence to clinical practice: a systematic review. Pharmaceuticals (Basel) 14(10):1039
Faggiano A, Modica R, Lo Calzo F et al (2020) Lanreotide therapy vs active surveillance in MEN1-related pancreatic neuroendocrine tumors < 2 centimeters. J Clin Endocrinol Metab 105(1):78–84
Mahler C, Verhelst J, de Longueville M et al (1990) Long-term treatment of metastatic medullary thyroid carcinoma with the somatostatin analogue octreotide. Clin Endocrinol (Oxf) 33(2):261–269
Martin S, Fica S, Parfeni O et al (2020) Somatostatinoma and Neurofibromatosis Type 1-a case report and review of the literature. Diagnostics (Basel) 10(9):620
Chen X, Shen G, Jiang J et al (2014) Pharmacokinetics and safety of subcutaneous pasireotide and intramuscular pasireotide long-acting release in Chinese male healthy volunteers: a phase I, single-center, open-label, randomized study. Clin Ther 36(8):1196–1210
Vitale G, Dicitore A, Sciammarella C et al (2018) Pasireotide in the treatment of neuroendocrine tumors: a review of the literature. Endocr Relat Cancer 25(6):R351–R364
Yao JC, Chan JA, Mita AC et al (2017) Phase I dose-escalation study of long-acting pasireotide in patients with neuroendocrine tumors. Onco Targets Ther 10:3177–3186
Cives M, Kunz PL, Morse B et al (2015) Phase II clinical trial of pasireotide long-acting repeatable in patients with metastatic neuroendocrine tumors. Endocr Relat Cancer 22(1):1–9
Panzuto F, Di Fonzo M, Iannicelli E et al (2006) Long-term clinical outcome of somatostatin analogues for treatment of progressive, metastatic, well-differentiated entero-pancreatic endocrine carcinoma. Ann Oncol 17(3):461–466
Khan MS, El-Khouly F, Davies P et al (2011) Long-term results of treatment of malignant carcinoid syndrome with prolonged release Lanreotide (Somatuline Autogel). Aliment Pharmacol Ther 34(2):235–242
Anthony L, Vinik AI (2011) Evaluating the characteristics and the management of patients with neuroendocrine tumors receiving octreotide LAR during a 6-year period. Pancreas 40(7):987–994
Bianchi A, De Marinis L, Fusco A et al (2011) The treatment of neuroendocrine tumors with long-acting somatostatin analogs: a single center experience with lanreotide autogel. J Endocrinol Invest 34(9):692–697
Wang Y, Wang W, Jin K et al (2017) Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in Chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol Lett 13(3):1165–1174
Sullivan I, Le Teuff G, Guigay J et al (2017) Antitumour activity of somatostatin analogues in sporadic, progressive, metastatic pulmonary carcinoids. Eur J Cancer 75:259–267
Satapathy S, Mittal BR, Sood A et al (2021) 177Lu-DOTATATE Plus Radiosensitizing Capecitabine versus octreotide Long-acting release as first-line systemic therapy in advanced grade 1 or 2 gastroenteropancreatic neuroendocrine tumors: a single-institution experience. JCO Glob Oncol 7:1167–1175
Ferolla P, Faggiano A, Grimaldi F et al (2012) Shortened interval of long-acting octreotide administration is effective in patients with well-differentiated neuroendocrine carcinomas in progression on standard doses. J Endocrinol Invest 35(3):326–331
Shojamanesh H, Gibril F, Louie A et al (2002) Prospective study of the antitumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinoma. Cancer 94(2):331–343
Ramundo V, Del Prete M, Marotta V et al (2014) Impact of long-acting octreotide in patients with early-stage MEN1-related duodeno-pancreatic neuroendocrine tumours. Clin Endocrinol 80(6):850–855
Cioppi F, Cianferotti L, Masi L et al (2017) The LARO-MEN1 study: a longitudinal clinical experience with octreotide long-acting release in patients with multiple endocrine neoplasia type 1 syndrome. Clin Cases Miner Bone Metab 14(2):123–130
Oleinikov K, Uri I, Jacob H et al (2020) Long-term outcomes in MEN-1 patients with pancreatic neuroendocrine neoplasms: an Israeli specialist center experience. Endocrine 68(1):222–229
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. The research was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no financial or non-financial competing interests to disclose.
Ethical approval
The authors have no ethical conflict to disclose. The study was performed in accordance with the principles of the Declaration of Helsinki.
Human and animals rights
The study does not involve human participants and/or animals.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lecture of the Lifetime achievement award of the Italian Society of Endocrinology “Premio SIE alla carriera 2022”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Faggiano, A. Long-acting somatostatin analogs and well differentiated neuroendocrine tumors: a 20-year-old story. J Endocrinol Invest 47, 35–46 (2024). https://doi.org/10.1007/s40618-023-02170-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02170-9