Abstract
Background
Parathyroid gland (PG) is an endocrine organ which may display different immunohistochemical stainings with chief cells and oxyphilic cells in normal as well as hyperplasic/tumoral lesions.
Purpose
In this study, we aimed to identify the demographic properties and diagnostic value of the GATA3 antibody, which is a transcription factor in addition to PTH, and of PAX-8 (monoclonal and polyclonal) antibody.
Methods
We have analyzed in detail the cellular components and staining intensities of 46 adenomas all of which contained parathyroid rims, 12 hyperplasia and 5 adjacent non-neoplastic thyroidectomy materials (63 patients, 114 tissues).
Results
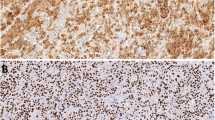
While no staining was identified in the thyroid tissue, cytoplasmic PTH immunoreactivity was observed in all (100%) normal parathyroid tissues, rim of PGs and hyperplasia, and in 43/46 cases (93.4%) of adenomas. Adenoma and hyperplasia were less stained than normal PG (p < 0.05). We detected GATA3 staining in all cases except for the thyroid (100%). Weak positivity (1+) was most apparent in adenoma cases (p < 0.05). Monoclonal PAX-8 immunoreactivity was not identified in any normal parathyroid tissue and rim of PG but positive immunoreactivity was detected in 83.3% of hyperplasia cases (10/12), 84.8% of adenoma (39/46) and 100% of thyroid tissues (5/5) (p < 0.05). However, polyclonal PAX-8 immunoreactivity was detected in one normal parathyroid tissue (1/5) and seven (7/46) rim of PGs. In cases of hyperplasia and adenoma, positive immunoreactivity was 75% (9/12) and 74% (34/46), respectively.
Conclusion
In conclusion, we have observed that PTH and GATA3 constitute a much more reliable and sensitive marker for parathyroid and are stained less in adenomas. While monoclonal PAX-8 (MRQ-50) never stains normal parathyroid and rim of PGs, it may help in the differential diagnosis of proliferated parathyroid lesions as a considerably sensitive and relatively specific marker by staining hyperplasic parathyroid, adenomas and the thyroid.





Similar content being viewed by others
References
Lloyd RV (1990) Parathyroid glands. In: Lloyd RV (ed) Endocrine pathology. Springer, New York, pp 71–83
Rosai J (2004) Parathyroid glands. In: Rosai J (ed) Rosai and Ackerman’s surgical pathology, 9th edn. Mosby, New York, pp 595–619
Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, Chirieac LR, Lis R, Loda M, Hornick JL, Drapkin R, Hirsch MS (2011) A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol 35:816–826
Ozcan A, Shen SS, Hamilton C et al (2011) PAX 8 expression in nonneoplastic tissues, primary tumors, and metastatic tumors:a comprehensive immunohistochemical study. Mod Pathol 24:751–764
Ordonez NG (2012) Value of PAX 8 immunostaining in tumor diagnosis:a review and update. Adv Anat Pathol 19:140–151
Erickson LA, Mete O (2018) Immunohistochemistry in diagnostic parathyroid pathology. Endocrine Pathol 29(2):113–129
Apel RL, Asa SL (2002) The parathyroid glands. In: Volsi VA, Asa SL (eds) Endocrine pathology. Churchill Livingstone, Philadelphia, pp 103–147
Tomita T (1999) Immunocytochemical staining patterns for parathyroid hormone and chromogranin in parathyroid hyperplasia, adenoma and carcinoma. Endocr Pathol 10:145–156
Ritter CS, Haughey BH, Miller B, Brown AJ (2012) Differential gene expression by oxyphil and chief cells of human parathyroid glands. J Clin Endocrinol Metab 97(8):E1499–E1505
Nonaka D (2011) Study of parathyroid transcription factor Gcm2 expression in parathyroid lesions. Am J Surg Pathol 35:145–151
Marchiori E, Pelizzo MR, Herten M, Townsend DM, Rubello D, Boschin IM (2017) Specifying the molecular pattern of sporadic parathyroid tumorigenesis-The Y282D variant of the GCM2 gene. Biomed Pharmacother 92:843–848
Nonaka D, Wang BY, Edmondson D et al (2013) A study of GATA3 and PHOX2B expression in tumors of the autonomic nervous system. Am J Surg Pathol 37:1236–1241
Ordonez NG (2014) Value of GATA3 immunostaining in the diagnosis of parathyroid tumors. Appl Immunohistochem Mol Morphol 22:756–761
Betts G, Beckett E, Nonaka D (2014) GATA3 shows differential immunohistochemical expression across thyroid and parathyroid lesions. Histopathology 65:288–290
Takada N, Hirokawa M, Suzuki A, Higuchi M, Kuma S, Miyauchi A (2016) Diagnostic value of GATA-3 in cytological identification of parathyroid tissues. Endocr J 63(7):621–626
Weissferdt A, Kalhor N, Liu H, Rodriguez J, Fujimoto J, Tang X, Wistuba II, Moran CA (2014) Thymic neuroendocrine tumors (paraganglioma and carcinoid tumors): a comparative immunohistochemical study of 46 cases. Hum Pathol. 45(12):2463–2470
Dahl E, Koseki H, Balling R (1997) PAX genes and organogenesis. Bioassays 9:755–765
Plachov D, Chowdhury K, Walther C et al (1990) PAX 8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 110:643–651
Poleev A, Fickenscher H, Mundlos S et al (1992) PAX 8, a human paired box gene: isolation and expression in developing thyroid, kidney and Wilms’ tumors. Development 116:611–623
Tacha D, Zhou D, Cheng L (2011) Expression of PAX-8 in normal and neoplastic tissues: a comprehensive immunohistochemical study. Appl Immunohistochem Mol Morphol 19:293–299
Liau JY, Tsai JH, Jeng YM, Kuo KT, Huang HY, Liang CW, Yang CY (2016) The diagnostic utility of PAX8 for neuroendocrine tumors: an immunohistochemical reappraisal. Appl Immunohistochem Mol Morphol 24:57–63
Bombi JA, Nadal A, Muftoz J, Cardesa A (1993) Ultrastructural pathology of parathyroid glands in hyperparathyroidism: a report of 69 cases. Ultrastruct Pathol 17:567–582
Mnaymneh L, Kimura N (1984) The parathyroid adenoma. A histopathologic definition with a study of 172 cases of primary hyperparathyroidism. Am J Pathol 115:70–83
Li-Volsi VA (1985) Pathology of parathroid glands. In: Barnes L (ed) Surgical pathology of the head and neck. Marcel DekKer, New York, pp 1487–1654
Williams JG, Wheeler MH, Aston JP, Brown RC, Woodhead JS (1992) The relationship between adenoma weight and intact (l-84) parathyroid hormone level in primary hyperparathyroidism. Am J Surg 163:301–304
Grimelius L, Akerström G, Johansson H, Lundqvist H (1978) Estimation of parenchymal cell content of human parathyroid glands using the image analyzing computer technique. Am J Pathol 93(3):793–800
Kroll TG, Sarraf P, Pecciarini L et al (2000) PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma. Science 289:135
Clifton-Bligh R, Robinson BG (2003) Detection of the PAX8-PPAR gamma fusion oncogene in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab 88(354–357):7–1360
Long KB, Srivastana A, Hirsch MS et al (2010) PAX 8 expression in well-diffrentiated pancreatic endocrine tumors: correlation with clinicopathologic features and comparison with gastrointestinal and pulmonary carcinoid tumors. Am J Surg Pathol 34:723–729
Duan K, Mete O (2016) Algorithmic approach to neuroendocrine tumors in targeted biopsies: practical applications of immunohistochemical markers. Cancer Cytopathol 124:871–884
Moretti L, Medeiros LJ, Kunkalla K et al (2012) N-terminal PAX8 polyclonal antibody shows cross-reactivity with N-terminal region of PAX5 and is responsible for reports of PAX8 positivity in malignant lymphomas. Mod Pathol 25:231–236
Acknowledgments
We would like to thank our surgeons, endocrinologists, radiologists, biochemists and nuclear medicine specialists, who work together as an endocrine unit in our hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of ınterest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical Statement Institutional REB, IEC approvals were obtained. The study was approved by the Clinical Trial Ethics Committee (Dr. Sadi Konuk Hospital, number 2020/132).
Informed consent
Fort his type of study formal constent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altınay, S., Erözgür, B., Dural, A.C. et al. Monoclonal/polyclonal PAX-8, PTH and GATA3 immunohistochemistry in parathyroid lesions. J Endocrinol Invest 44, 1997–2008 (2021). https://doi.org/10.1007/s40618-021-01518-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01518-3