Abstract
Objective
The imbalance of gut microbiota has been linked to manifold endocrine diseases, but the association with Graves’ disease (GD) is still unclear. The purpose of this study was to investigate the correlation between human gut microbiota and clinical characteristics and thyroidal functional status of GD.
Methods
14 healthy volunteers (CG) and 15 patients with primary GD (HG) were recruited as subjects. 16SrDNA high-throughput sequencing was performed on IlluminaMiSeq platform to analyze the characteristics of gut microbiota in patients with GD. Among them, the thyroid function of 13 patients basically recovered after treatment with anti-thyroid drugs (oral administration of Methimazole for 3–5 months). The fecal samples of patients after treatment (TG) were sequenced again, to further explore and investigate the potential relationship between dysbacteriosis and GD.
Results
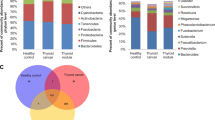
In terms of alpha diversity index, the observed OTUs, Simpson and Shannon indices of gut microbiota in patients with GD were significantly lower than those in healthy volunteers (P < 0.05).The difference of bacteria species was mainly reflected in the genus level, in which the relative abundance of Lactobacillus, Veillonella and Streptococcus increased significantly in GD. After the improvement of thyroid function, a significant reduction at the genus level were Blautia, Corynebacter, Ruminococcus and Streptococcus, while Phascolarctobacterium increased significantly (P < 0.05). According to Spearman correlation analysis, the correlation between the level of thyrotropin receptor antibody (TRAb) and the relative abundance of Lactobacillus and Ruminococcus was positive, while Synergistetes and Phascolarctobacterium showed a negative correlation with TRAb. Besides, there were highly significant negative correlation between Synergistetes and clinical variables of TRAb, TPOAb and TGAb (P < 0.05, R < − 0.6).
Conclusions
This study revealed that functional status and TRAb level in GD were associated with composition and biological function in the gut microbiota, with Synergistetes and Phascolarctobacterium protecting the thyroid probably, while Ruminococcus and Lactobacillus may be novel biomarkers of GD.




Similar content being viewed by others
References
Burcelin R, Nicolas S, Blasco-Baque V (2016) Microbiotes and metabolic diseases: the bases for therapeutic strategies. Med Sci (Paris) 32:952–960. https://doi.org/10.1051/medsci/20163211010
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W et al (2012) Host-gut microbiota metabolic interactions. Science 336:1262–1267. https://doi.org/10.1126/science.1223813
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK (2014) Specialized metabolites from the microbiome in health and disease. Cell Metab 20:719–730. https://doi.org/10.1016/j.cmet.2014.10.016
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F et al (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60. https://doi.org/10.1038/nature11450
Diamant M, Blaak EE, de Vos WM (2011) Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev 12:272–281. https://doi.org/10.1111/j.1467-789X.2010.00797.x
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. https://doi.org/10.1038/nature05414
Fei N, Zhao L (2013) An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 7:880–884. https://doi.org/10.1038/ismej.2012.153
Bervoets L, Van Hoorenbeeck K, Kortleven I, Van Noten C, Hens N, Vael C et al (2013) Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog 5:10. https://doi.org/10.1186/1757-4749-5-10
Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW et al (2013) Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring) 21:E607–E615. https://doi.org/10.1002/oby.20466
Xu P, Li M, Zhang J, Zhang T (2012) Correlation of intestinal microbiota with overweight and obesity in Kazakh school children. BMC Microbiol 12:283. https://doi.org/10.1186/1471-2180-12-283
Ferrer M, Ruiz A, Lanza F, Haange SB, Oberbach A, Till H et al (2013) Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environ Microbiol 15:211–226. https://doi.org/10.1111/j.1462-2920.2012.02845.x
Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL et al (2010) Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59:3049–3057. https://doi.org/10.2337/db10-0253
Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI et al (2011) Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 94:58–65. https://doi.org/10.3945/ajcn.110.010132
Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C et al (2010) Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18:190–195. https://doi.org/10.1038/oby.2009.167
Yun Y, Kim HN, Kim SE, Heo SG, Chang Y, Ryu S et al (2017) Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol 17:151. https://doi.org/10.1186/s12866-017-1052-0
Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Martinez JA (2019) Diet, Gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr 10:S17–S30. https://doi.org/10.1093/advances/nmy078
Bahn CR, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I et al (2011) Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid 21:593–646. https://doi.org/10.1089/thy.2010.0417
Rees SB, McLachlan SM, Furmaniak J (1988) Autoantibodies to the thyrotropin receptor. Endocr Rev 9:106–121. https://doi.org/10.1210/edrv-9-1-106
Zhou L, Li X, Ahmed A, Wu D, Liu L, Qiu J et al (2014) Gut microbe analysis between hyperthyroid and healthy individuals. Curr Microbiol 69:675–680. https://doi.org/10.1007/s00284-014-0640-6
Ishaq HM, Mohammad IS, Shahzad M, Ma C, Raza MA, Wu X et al (2018) Molecular alteration analysis of human gut microbial composition in Graves’ disease patients. Int J Biol Sci 14:1558–1570. https://doi.org/10.7150/ijbs.24151
Shi TT, Xin Z, Hua L, Zhao RX, Yang YL, Wang H et al (2019) Alterations in the intestinal microbiota of patients with severe and active Graves’ orbitopathy: a cross-sectional study. J Endocrinol Invest 42:967–978. https://doi.org/10.1007/s40618-019-1010-9
Hagerty SL, Hutchison KE, Lowry CA, Bryan AD (2020) An empirically derived method for measuring human gut microbiome alpha diversity: demonstrated utility in predicting health-related outcomes among a human clinical sample. PLoS ONE 15:e229204. https://doi.org/10.1371/journal.pone.0229204
Covelli D, Ludgate M (2017) The thyroid, the eyes and the gut: a possible connection. J Endocrinol Invest 40:567–576. https://doi.org/10.1007/s40618-016-0594-6
Young VB (2012) The intestinal microbiota in health and disease. Curr Opin Gastroenterol 28:63–69. https://doi.org/10.1097/MOG.0b013e32834d61e9
De Leo S, Lee SY, Braverman LE (2016) Hyperthyroidism. Lancet 388:906–918. https://doi.org/10.1016/S0140-6736(16)00278-6
Chen LY, Zhou B, Chen ZW, Fang LZ (2010) Case report: recurrent severe vomiting due to hyperthyroidism. J Zhejiang Univ Sci B 11:218–220. https://doi.org/10.1631/jzus.B0900371
Ebert EC (2010) The thyroid and the gut. J Clin Gastroenterol 44:402–406. https://doi.org/10.1097/MCG.0b013e3181d6bc3e
You M, Mo S, Watt RM, Leung WK (2013) Prevalence and diversity of Synergistetes taxa in periodontal health and disease. J Periodontal Res 48:159–168. https://doi.org/10.1111/j.1600-0765.2012.01516.x
Chen B, Sun L, Zhang X (2017) Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. J Autoimmun 83:31–42. https://doi.org/10.1016/j.jaut.2017.03.009
Lopez P, de Paz B, Rodriguez-Carrio J, Hevia A, Sanchez B, Margolles A et al (2016) Th17 responses and natural IgM antibodies are related to gut microbiota composition in systemic lupus erythematosus patients. Sci Rep 6:24072. https://doi.org/10.1038/srep24072
Omenetti S, Pizarro TT (2015) The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Front Immunol 6:639. https://doi.org/10.3389/fimmu.2015.00639
Wu F, Guo X, Zhang J, Zhang M, Ou Z, Peng Y (2017) Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp Ther Med 14:3122–3126. https://doi.org/10.3892/etm.2017.4878
Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT (2018) Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr Physiol 8:1091–1115. https://doi.org/10.1002/cphy.c170050
Park J, Lee J, Yeom Z, Heo D, Lim YH (2017) Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals. Sci Rep 7:14520. https://doi.org/10.1038/s41598-017-15163-5
Shimizu J, Kubota T, Takada E, Takai K, Fujiwara N, Arimitsu N et al (2018) Propionate-producing bacteria in the intestine may associate with skewed responses of IL10-producing regulatory T cells in patients with relapsing polychondritis. PLoS ONE 13:e203657. https://doi.org/10.1371/journal.pone.0203657
Chua HH, Chou HC, Tung YL, Chiang BL, Liao CC, Liu HH et al (2018) Intestinal dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology 154:154–167. https://doi.org/10.1053/j.gastro.2017.09.006
Rocha-Ramirez LM, Perez-Solano RA, Castanon-Alonso SL, Moreno GS, Ramirez PA, Garcia GM et al (2017) Probiotic lactobacillus strains stimulate the inflammatory response and activate human macrophages. J Immunol Res 2017:4607491. https://doi.org/10.1155/2017/4607491
Ferrari SM, Ruffilli I, Elia G, Ragusa F, Paparo SR, Patrizio A et al (2019) Chemokines in hyperthyroidism. J Clin Transl Endocrinol 16:100196. https://doi.org/10.1016/j.jcte.2019.100196
Duraes C, Moreira CS, Alvelos I, Mendes A, Santos LR, Machado JC et al (2014) Polymorphisms in the TNFA and IL6 genes represent risk factors for autoimmune thyroid disease. PLoS ONE 9:e105492. https://doi.org/10.1371/journal.pone.0105492
Inoue N, Watanabe M, Morita M, Tatusmi K, Hidaka Y, Akamizu T et al (2011) Association of functional polymorphisms in promoter regions of IL5, IL6 and IL13 genes with development and prognosis of autoimmune thyroid diseases. Clin Exp Immunol 163:318–323. https://doi.org/10.1111/j.1365-2249.2010.04306.x
Anvari M, Khalilzadeh O, Esteghamati A, Momen-Heravi F, Mahmoudi M, Esfahani SA et al (2010) Graves’ disease and gene polymorphism of TNF-alpha, IL-2, IL-6, IL-12, and IFN-γ. Endocrine 37:344–348. https://doi.org/10.1007/s12020-010-9311-y
Giusti C (2019) The Th1 chemokine MIG in Graves’ disease: a narrative review of the literature. Clin Ter 170:e285–e290. https://doi.org/10.7417/CT.2019.2149
Shiau MY, Huang CN, Yang TP, Hwang YC, Tsai KJ, Chi CJ et al (2007) Cytokine promoter polymorphisms in Taiwanese patients with Graves’ disease. Clin Biochem 40:213–217. https://doi.org/10.1016/j.clinbiochem.2006.11.009
Matsuno H, Yudoh K, Katayama R, Nakazawa F, Uzuki M, Sawai T et al (2002) The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): a study using a human RA/SCID mouse chimera. Rheumatology (Oxford) 41:329–337. https://doi.org/10.1093/rheumatology/41.3.329
Gonzalez-Diaz SN, Sanchez-Borges M, Rangel-Gonzalez DM, Guzman-Avilan RI, Canseco-Villarreal JI, Arias-Cruz A (2020) Chronic urticaria and thyroid pathology. World Allergy Organ J 13:100101. https://doi.org/10.1016/j.waojou.2020.100101
Lou J, Jiang Y, Rao B, Li A, Ding S, Yan H et al (2020) Fecal microbiomes distinguish patients with autoimmune hepatitis from healthy individuals. Front Cell Infect Microbiol 10:342. https://doi.org/10.3389/fcimb.2020.00342
Ganesh BB, Bhattacharya P, Gopisetty A, Prabhakar BS (2011) Role of cytokines in the pathogenesis and suppression of thyroid autoimmunity. J Interferon Cytokine Res 31:721–731. https://doi.org/10.1089/jir.2011.0049
Makki K, Deehan EC, Walter J, Backhed F (2018) The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23:705–715. https://doi.org/10.1016/j.chom.2018.05.012
Arif N, Sheehy EC, Do T, Beighton D (2008) Diversity of Veillonella spp. from sound and carious sites in children. J Dent Res 87:278–282. https://doi.org/10.1177/154405910808700308
Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL et al (2002) Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40:1001–1009. https://doi.org/10.1128/jcm.40.3.1001-1009.2002
Mashima I, Theodorea CF, Thaweboon B, Thaweboon S, Nakazawa F (2016) Identification of veillonella species in the tongue biofilm by using a novel one-step polymerase chain reaction method. PLoS ONE 11:e157516. https://doi.org/10.1371/journal.pone.0157516
Marriott D, Stark D, Harkness J (2007) Veillonella parvula discitis and secondary bacteremia: a rare infection complicating endoscopy and colonoscopy? J Clin Microbiol 45:672–674. https://doi.org/10.1128/JCM.01633-06
Shah A, Panjabi C, Nair V, Chaudhry R, Thukral SS (2008) Veillonella as a cause of chronic anaerobic pneumonitis. Int J Infect Dis 12:e115–e117. https://doi.org/10.1016/j.ijid.2008.03.018
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14. https://doi.org/10.1093/intimm/dxh186
Egland PG, Palmer RJ, Kolenbrander PE (2004) Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci USA 101:16917–16922. https://doi.org/10.1073/pnas.0407457101
van den Bogert B, Meijerink M, Zoetendal EG, Wells JM, Kleerebezem M (2014) Immunomodulatory properties of Streptococcus and Veillonella isolates from the human small intestine microbiota. PLoS ONE 9:e114277. https://doi.org/10.1371/journal.pone.0114277
Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T et al (2013) The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 4:1829. https://doi.org/10.1038/ncomms2852
Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S et al (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108:8030–8035. https://doi.org/10.1073/pnas.1016088108
Ozato N, Saito S, Yamaguchi T, Katashima M, Tokuda I, Sawada K et al (2019) Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 5:28. https://doi.org/10.1038/s41522-019-0101-x
Benitez-Paez A, Gomez DPE, Lopez-Almela I, Moya-Perez A, Codoner-Franch P, Sanz Y (2020) Depletion of blautia species in the microbiota of obese children relates to intestinal inflammation and metabolic phenotype worsening. mSystems. https://doi.org/10.1128/mSystems.00857-19
Funding
This study was funded by National Natural Science Foundation of China (No. 81774134 and No. 81873174); Natural Science Foundation of Jiangsu Province of China (No. BK20150558 and No. BK20171331); Postdoctoral Foundation of Jiangsu Province of China (No. 1501120C); Jiangsu Province 333 Talent Funding Project (No. BRA2017595); Young Medical Key Talents Project of Jiangsu Province (No. QNRC2016902); Key Research and Development Plan Project of Jiangsu Province—Social Development Projects (No. BE2020701).
Author information
Authors and Affiliations
Contributions
CJ, SJQ and GP contributed to the conception and design of this study. Data preparation, sample collection, data collection and analysis were conducted by CJ, WW, GZH, HSS, LHY, ZP and LB The first draft of the paper was written by CJ, and all authors participated in revising the previous version of the manuscript. All the authors have read and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Wang, W., Guo, Z. et al. Associations between gut microbiota and thyroidal function status in Chinese patients with Graves’ disease. J Endocrinol Invest 44, 1913–1926 (2021). https://doi.org/10.1007/s40618-021-01507-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01507-6