Abstract
Background
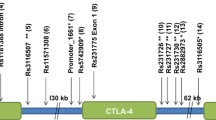
In a previous work, we found linkage and association of type 1 diabetes (T1D) to a 12 known gene region at chromosome 2p25 in Colombian families. Here, we present further work on this candidate region.
Materials and methods
Seventeen SNPs located on the 12 candidate genes, in 100 familial trios set, were tested by ARMS–tetraprimer–PCR or PCR–RFLP. Five extra SNPs in the vicinity of rs10186193 were typed. A replica phase included 97 novel familial trios, in whom diabetes-related auto-antibodies (AABs) were tested in sera of the patients. In addition to transmission disequilibrium tests, haplotype analyses were carried out using the unphased software.
Results
SNP rs10186193 (at RNASEH1 gene) showed association with T1D (P = 0.005). The additional five SNPs revealed that rs7607888 (P = 2.03 × 10−7), rs55981318 (P = 0.018), and rs1136545 (P = 1.93 × 10−9) were also associated with T1D. Haplotype analysis showed association for rs55981318–rs10186193 (P = 0.0005), rs7563960–rs7607888 (P = 0.0007), rs7607888–rs1136545 (P = 9.21 × 10−10), and rs1136545–rs11538545 (P = 6.67 × 10−8). In contrast, the new set of 97 familial trios tested for SNPs rs55981318, rs10186193, and rs7607888 did not support the previous finding; however, by combining the sample (197 trios), evidence of association of T1D with rs55981318 and rs7607888 was conclusive. In addition, a two-loci haplotype analysis of the combined sample showed significant association of RNASEH1 with T1D (P = 3.1 × 10−5).
Conclusion
In conclusion, our analyses suggest that RNASEH1 gene variants associate with susceptibility/protection to T1D in Colombia.

Similar content being viewed by others
References
ADA (2016) Classification and diagnosis of diabetes. Diabetes Care 39:S13–S22
Knip M (2012) Descriptive epidemiology of type 1 diabetes—is it still in? Diabetologia 55(5):1227–1230
Pundziute-Lyckå A, Dahlquist G, Nyström L, Arnqvist H, Björk E, Blohmé G, Bolinder J, Eriksson JW, Sundkvist G, Ostman J (2002) The incidence of type I diabetes has not increased but shifted to a younger age at diagnosis in the 0–34 years group in Sweden 1983–1998. Diabetologia 45:783–791
Tuomilehto J (2013) The emerging global epidemic of type 1 diabetes. Curr Diabetes Rep 13:795–804
Barnett AH, Eff C, Leslie RD, Pyke DA (1981) Diabetes in identical twins. A study of 200 pairs. Diabetologia 20:87–93
Ziegler A-G, Pflueger M, Winkler C, Achenbach P, Akolkar B, Krischer JP, Bonifacio E (2011) Accelerated progression from islet autoimmunity to diabetes is causing the escalating incidence of type 1 diabetes in young children. J Autoimmun 37:3–7
Laitinen OH, Honkanen H, Pakkanen O et al (2014) Coxsackievirus B1 is associated with induction of cell autoimmunity that portends type 1 diabetes. Diabetes 63:446–455
Tuomilehto J, Podar T, Tuomilehto-Wolf E, Virtala E (1995) Evidence for importance of gender and birth cohort for risk of IDDM in offspring of IDDM parents. Diabetologia 38:975–982
Gao S, Jia S, Hessner MJ, Wang X (2012) Predicting disease-related subnetworks for type 1 diabetes using a new network activity score. OMICS 16:566–578
Fløyel T, Kaur S, Pociot F (2015) Genes affecting β-cell function in type 1 diabetes. Curr Diabetes Rep 15:97
Bottini N, Vang T, Cucca F, Mustelin T (2006) Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Semin Immunol 18:207–213
Královicová J, Gaunt TR, Rodriguez S, Wood PJ, Day INM, Vorechovsky I (2006) Variants in the human insulin gene that affect pre-mRNA splicing: is − 23HphI a functional single nucleotide polymorphism at IDDM2? Diabetes 55:260–264
Marron MP, Raffel LJ, Garchon HJ et al (1997) Insulin-dependent diabetes mellitus (IDDM) is associated with CTLA4 polymorphisms in multiple ethnic groups. Hum Mol Genet 6:1275–1282
Todd JA, Walker NM, Cooper JD et al (2007) Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 39:857–864
Alcina A, Fedetz M, Ndagire D et al (2009) IL2RA/CD25 gene polymorphisms: uneven association with multiple sclerosis (MS) and type 1 diabetes (T1D). PLoS One 4:e4137
Rodríguez A, Alfaro JM, Balthazar V, Pineda Trujillo N (2015) Association analysis of PTPN22, CTLA4 and IFIH1 genes with type 1 diabetes in Colombian families. J Diabetes 7:402–410
Pineda-Trujilo N, Uribe F, Montoya F et al (2010) Chromosome region 2p25 is linked and associated with type 1 diabetes in Colombia. J Genet 89:457–461
Filippi C, von Herrath M (2005) How viral infections affect the autoimmune process leading to type 1 diabetes. Cell Immunol 233:125–132
Günther C, Kind B, Reijns MAM et al (2015) Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J Clin Investig 125:413–424
Flodström-Tullberg M, Hultcrantz M, Stotland A, Maday A, Tsai D, Fine C, Williams B, Silverman R, Sarvetnick N (2005) RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J Immunol 174:1171–1177
Ye S, Dhillon S, Ke X, Collins AR, Day IN (2001) An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res 29:E88
O’Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266
Dudbridge F (2008) Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum Hered 66:87–98
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265
Chang CC, Huang CN, Chuang LM (1998) Autoantibodies to thyroid peroxidase in patients with type 1 diabetes in Taiwan. Eur J Endocrinol 139:44–48
Gutiérrez-achury J, Balthazar-gonzález V, Bedoya-berrío G, Ruíz-linares A, Uribe-londoño F, Alfaro JM, Pineda-Trujillo N (2009) Association of the TPO gene in Colombian families with type 1 diabetes. Iatreia 22:323–329
Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M (2004) Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47:225–239
Jenson AB, Rosenberg HS, Notkins AL (1980) Pancreatic islet-cell damage in children with fatal viral infections. Lancet 2:354–358
ten Asbroek ALMA, van Groenigen M, Jakobs ME, Koevoets C, Janssen B, Baas F (2002) Ribonuclease H1 maps to chromosome 2 and Has at least three pseudogene loci in the human genome. Genomics 79:818–823
Crow YJ, Chase DS, Lowenstein Schmidt J et al (2015) Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A 167A:296–312
Rice GI, del Toro Duany Y, Jenkinson EM et al (2014) Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 46:503–509
Tanaka D, Nagashima K, Sasaki M, Yamada C, Funakoshi S, Akitomo K, Takenaka K, Harada K, Koizumi A, Inagaki N (2011) GCKR mutations in Japanese families with clustered type 2 diabetes. Mol Genet Metab 102:453–460
Barrett JC, Clayton DG, Concannon P et al (2009) Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 41:703–707
Qiu Y-H, Deng F-Y, Li M-J, Lei S-F (2014) Identification of novel risk genes associated with type 1 diabetes mellitus using a genome-wide gene-based association analysis. J Diabetes Investig 5:649–656
Gravel S, Zakharia F, Moreno-Estrada A et al (2013) Reconstructing Native American migrations from whole-genome and whole-exome data. PLoS Genet 9:e1004023
Acknowledgements
We are very grateful to the patients and their families for participating in this study. This study was funded by a Colciencias Grant # 1115-343-19156 and a CODI-Universidad de Antioquia Grant # 8704-2449.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We do not have a conflict of interest to declare, related to this study.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments.
Informed Consent
Informed consent was obtained from all participants included in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pineda-Trujillo, N., Rodríguez-Acevedo, A., Rodríguez, A. et al. RNASEH1 gene variants are associated with autoimmune type 1 diabetes in Colombia. J Endocrinol Invest 41, 755–764 (2018). https://doi.org/10.1007/s40618-017-0797-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-017-0797-5