Abstract
Background
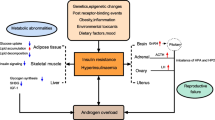
Insulin resistance is a common feature among women with polycystic ovary syndrome (PCOS), especially in those patients with hyperandrogenism and chronic anovulation. PCOS women are at risk for developing metabolic syndrome, impaired glucose tolerance and type II diabetes mellitus (DM II).
Objective
The aim of this review is to explore the existing knowledge of the interplay between androgen excess, pancreatic β-cell function, non-alcoholic fatty liver disease (NAFLD), intra-abdominal and subcutaneous (SC) abdominal adipocytes in PCOS, providing a better comprehension of the molecular mechanisms of diabetologic interest.
Methods
A comprehensive MEDLINE® search was performed using relevant key terms for PCOS and DM II.
Results
Insulin-induced hyperandrogenism could impair pancreatic β-cell function, the SC abdominal adipocytes’ lipid storage capacity, leading to intra-abdominal adipocyte hypertrophy and lipotoxicity, which in turn promotes insulin resistance, and could enhance NAFLD. Fetal hyperandrogenism exposure prompts to metabolic disorders. Treatment with flutamide showed to partially reverse insulin resistance.
Conclusions
Metabolic impairment seems not to be dependent only on the total fat mass content and body weight in women with PCOS and might be ascribed to the androgen excess.

Similar content being viewed by others
Abbreviations
- A:
-
Androstenedione
- AACE:
-
American Association of Clinical Endocrinology
- ACE:
-
American College of Endocrinology
- BMI:
-
Body mass index
- DHEAS:
-
Dehydroepiandrosterone sulfate
- DHT:
-
Di-hydro-testosterone
- DM II:
-
Type II diabetes mellitus
- DNMT3:
-
De novo methyl-transferase 3
- FFA:
-
Free fatty acids
- FPIR:
-
First phase insulin response
- IGT:
-
Impaired glucose tolerance
- LDL-R:
-
LDL receptor
- MetS:
-
Metabolic syndrome
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatoepatitis
- NIH:
-
National Institutes of Health
- 17αOH-P:
-
17α-Hydroxy-progesterone
- OGTT:
-
Oral glucose tolerance test
- PCOS:
-
Polycystic ovary syndrome
- SC:
-
Subcutaneous
- SHBG:
-
Sex hormone-binding globulin
- SSPG:
-
Steady-state plasma glucose
- T:
-
Testosterone
References
Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K (2012) Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 97(1):28–38
Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, American Association of Clinical Endocrinologists (AACE), American College of Endocrinology (ACE), Androgen Excess and PCOS Society (AES) (2015) American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract 21(11):1291–1300
Barry JA, Azizia MM, Hardiman PJ (2014) Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Updat 20(5):748–758
Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, American Association of Clinical Endocrinologists (AACE), American College of Endocrinology (ACE), Androgen Excess and PCOS Society (AES) (2015) American Association of Clinical Endocrinologists, American College of Endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract 21(12):1415–1426
Diamanti-Kandarakis E, Kouli C, Alexandraki K, Spina G (2004) Failure of mathematical indices to accurately assess insulin resistance in lean, overweight, or obese women with polycystic ovary syndrome. JCEM 89(3):1273–1276
Cassar S, Misso ML, Hopkins WG, Shaw CS, Teede HJ, Stepto NK (2016) Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod 31:2619–2631
Gambieri A, Patton L, Altieri P, Pagotto U, Pizzi C, Manzoli L, Pasquali R (2012) Polycystic ovary syndrome is a risk factor for type 2 diabetes: results from a long-term prospective study. Diabetes 61:2369–2374
Dunaif A (1997) Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800
Malin SK, Kirwan JP, Sia CL, Gonzàles F (2015) Pancreatic β-cell dysfunction in polycystic ovary syndrome: role of hyperglycemia-induced nuclear factor-kB activation and systemic inflammation. Am J Physiol Endocrinol Metab 308:E770–E777
Rosenfield RL, Barnes RB, Ehrmann DA (1994) Studies of the nature of 17-hydroxyprogesterone hyperresponsiveness to gonadotropin-releasing hormone agonist challenge in functional ovarian hyperandrogenism. JCEM 79(6):1686–1692
Mansour A, Hosseini S, Larijani B, Mohajeri-Tehrani MR (2016) Nutrients as novel therapeutic approaches for metabolic disturbances in polycystic ovary syndrome. EXCLI J 15:551–564
Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, De Gendt K, Kim SH, Wu H, Zhang H, Verhoeven G, Katzenellenbogen JA, Mauvais-Jarvis F (2016) Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab 23(5):837–851
Tian S, Lin XH, Xiong YM, Liu ME, Yu TT, Lv M, Zhao W, Xu GF, Ding GL, Xu CM, Jin M, Feng C, Wu YT, Tan YJ, Gao Q, Zhang J, Li C, Ren J, Jin LY, Chen B, Zhu H, Zhang XY, Chen SC, Liu XM, Liu Y, Zhang JY, Wang L, Zhang P, Chen XJ, Jin L, Chen X, Meng YC, Wu DD, Lin H, Yang Q, Zhou CL, Li XZ, Wang YY, Xiang YQ, Liu ZW, Gao L, Chen LT, Pan HJ, Li R, Zhang FH, Xing LF, Zhu YM, Klausen C, Leung PC, Li JX, Sun F, Sheng JZ, Huang HF (2017) Prevalence of prediabetes risk in offspring born to mothers with hyperandrogenism. EBioMedicine 16:275–283
Makri E, Tziomalos K (2017) Prevalence, etiology and management of non-alcoholic fatty liver disease in patients with polycystic ovary syndrome. Minerva Endocrinol 42(2):122–131
Vassilatou E (2014) Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J Gastroenterol 20:8351–8363
Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S et al (2007) The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA 104:12587–12594
Jornayvaz FR, Samuel VT, Shulman GI (2010) The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and non-alcoholic fatty liver disease associated with the metabolic syndrome. Annu Rev Nutr 30:273–290
Baranova A, Tran TP, Afendy A, Wang L, Shamsaddini A, Mehta R et al (2013) Molecular signature of adipose tissue in patients with both non-alcoholic fatty liver disease (NAFLD) and polycystic ovarian syndrome (PCOS). J Transl Med 11:133
Macut D, Tziomalos K, Božić-Antić I, Bjekić-Macut J, Katsikis I, Papadakis E et al (2016) Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulation product in women with polycystic ovary syndrome. Hum Reprod 31:1347–1353
Croston GE, Milan LB, Marschke KB, Reichman M, Briggs MR (1997) Androgen receptor-mediated antagonism of estrogen-dependent low density lipoprotein receptor transcription in cultured hepatocytes. Endocrinology 138:3779–3786
Chen MJ, Chiu HM, Chen CL, Yang WS, Yang YS, Ho HN (2010) Hyperandrogenemia is independently associated with elevated alanine aminotransferase activity in young women with polycystic ovary syndrome. JCEM 95:3332–3341
Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N et al (2012) Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. JCEM 97:3709–3716
Abruzzese GA, Heber MF, Ferreira SR, Velez LM, Reynoso R, Pignataro OP, Motta AB (2016) Prenatal hyperandrogenism induces alterations that affect liver lipid metabolism. J Endocrinol 230(1):67–79
Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A (1987) Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. JCEM 65:499–507
Robinson S, Kiddy D, Gelding SV, Willis D, Niththyananthan R, Bush A et al (1993) The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf) 39:351–355
Dimitriadis GK, Kyrou I, Randeva HS (2016) Polycystic ovary syndrome as a proinflammatory state: the role of adipokines. Curr Pharm Des 22(36):5535–5546
Mannerås-Holm L, Leonhardt H, Kullberg J et al (2011) Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. JCEM 96:E304–E311
McLaughlin T, Lamendola C, Liu A, Abbasi F (2011) Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. JCEM 96:E1756–E1760
Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA, Okeya BL, Abbott DH, Chazenbalk GD (2016) Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. JCEM 101(11):4178–4188
Li H, Xu X, Wang X, Liao X, Li L, Yang G, Gao L (2016) Free androgen index and Irisin in polycystic ovary syndrome. J Endocrinol Invest 39(5):549–556
Mario FM, Graff SK, Spritzer PM (2017) Habitual physical activity is associated with improved anthropometric and androgenic profile in PCOS: a cross-sectional study. J Endocrinol Invest 40(4):377–384
Moghetti P, Tosi F, Castello R et al (1996) The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. JCEM 81:952–960
Wallace IR, McKinley MC, Bell PM, Hunter SJ (2013) Sex hormone binding globulin and insulin resistance. Clin Endocrinol (Oxf) 78:321–329
Le TN, Nestler JE, Strauss JF 3rd, Wickam EP 3rd (2012) Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab 23:32–40
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical approval
This article does not contain studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent.
Rights and permissions
About this article
Cite this article
Condorelli, R.A., Calogero, A.E., Di Mauro, M. et al. Androgen excess and metabolic disorders in women with PCOS: beyond the body mass index. J Endocrinol Invest 41, 383–388 (2018). https://doi.org/10.1007/s40618-017-0762-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-017-0762-3