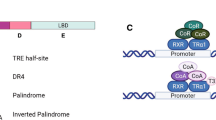
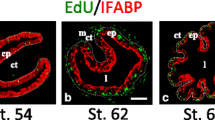
Abstract
Thyroid hormone is a major determinant of tissue functions in vivo. The deiodinase family controls the tissue-specific activation or inactivation of intracellular thyroid hormones. Precise control of the T3-dependent transcriptional program is required by multiple cell systems, including the stem cell. In this context, the identification of a close connection between thyroid hormones and different signal pathways involved in the control of stem cell functions suggested that the deiodinases may play a role in the definition of stem cell biology and physiology. Stem cells have an unlimited self-renewal capacity and the potential to differentiate into different types of mature cells. Deciphering how all these events are achieved, how the T3 signal is controlled and integrated in stem cells and their niches, and how it can impact on them is essentially unknown and represents a challenge for coming years. In this review, I will explore the role played by the deiodinases in the modulation of the TH signal in stem cells of adult tissues, namely muscle and intestine, and how their actions control the delicate balance among self-renewal, proliferation and differentiation. Elucidation of the molecular mechanisms presiding thyroid hormone action in stem cells may reveal therapeutic potential, for example in the fields of regenerative diseases and cancer.



Similar content being viewed by others
References
Marsili A, Zavacki AM, Harney JW, Larsen PR (2011) Physiological role and regulation of iodothyronine deiodinases: a 2011 update. J Endocrinol Invest 34(5):395–407. doi:10.1007/BF03347465
Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (2002) Biochemistry, cellular and molecular biology and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89
Canettieri G, Franchi A, Sibilla R, Guzman E, Centanni M (2004) Functional characterisation of the CRE/TATA box unit of type 2 deiodinase gene promoter in a human choriocarcinoma cell line. J Mol Endocrinol 33(1):51–58
Canettieri G, Franchi A, Guardia MD, Morantte I, Santaguida MG, Harney JW, Larsen PR, Centanni M (2008) Activation of thyroid hormone is transcriptionally regulated by epidermal growth factor in human placenta-derived JEG3 cells. Endocrinology 149(2):695–702. doi:10.1210/en.2007-0779
Luongo C, Ambrosio R, Salzano S, Dlugosz AA, Missero C, Dentice M (2014) The sonic hedgehog-induced type 3 deiodinase facilitates tumorigenesis of basal cell carcinoma by reducing Gli2 inactivation. Endocrinology 155(6):2077–2088. doi:10.1210/en.2013-2108
Dentice M, Luongo C, Ambrosio R, Sibilio A, Casillo A, Iaccarino A, Troncone G, Fenzi G, Larsen PR, Salvatore D (2012) beta-Catenin regulates deiodinase levels and thyroid hormone signaling in colon cancer cells. Gastroenterology 143(4):1037–1047. doi:10.1053/j.gastro.2012.06.042
Baldwin KM, Haddad F (2001) Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol 1985 90(1):345–357
Simonides WS, van Hardeveld C (2008) Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid 18(2):205–216. doi:10.1089/thy.2007.0256
McIntosh LM, Pernitsky AN, Anderson JE (1994) The effects of altered metabolism (hypothyroidism) on muscle repair in the mdx dystrophic mouse. Muscle Nerve 17(4):444–453. doi:10.1002/mus.880170413
Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102(6):777–786
Cornelison DD, Wold BJ (1997) Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 191(2):270–283. doi:10.1006/dbio.1997.8721
Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A (1994) Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn 199(4):326–337. doi:10.1002/aja.1001990407
Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM (1995) Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165(2):307–312. doi:10.1002/jcp.1041650211
Burkin DJ, Kaufman SJ (1999) The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res 296(1):183–190
Gnocchi VF, White RB, Ono Y, Ellis JA, Zammit PS (2009) Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One 4(4):e5205. doi:10.1371/journal.pone.0005205
Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151(6):1221–1234
McLoon LK, Wirtschafter J (2003) Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol Vis Sci 44(5):1927–1932
Dentice M, Marsili A, Ambrosio R, Guardiola O, Sibilio A, Paik JH, Minchiotti G, DePinho RA, Fenzi G, Larsen PR, Salvatore D (2010) The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest 120(11):4021–4030. doi:10.1172/JCI43670
Gibson MC, Schultz E (1982) The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec 202(3):329–337. doi:10.1002/ar.1092020305
Accili D, Arden KC (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117(4):421–426
Hu P, Geles KG, Paik JH, DePinho RA, Tjian R (2008) Codependent activators direct myoblast-specific MyoD transcription. Dev Cell 15(4):534–546. doi:10.1016/j.devcel.2008.08.018
Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S (2012) A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148(1–2):112–125. doi:10.1016/j.cell.2011.11.049
Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S (2012) A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30(2):243–252. doi:10.1002/stem.775
Schmalbruch H, Lewis DM (2000) Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve 23(4):617–626
Ten Broek RW, Grefte S, Von den Hoff JW (2010) Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol 224(1):7–16. doi:10.1002/jcp.22127
Bentzinger CF, Wang YX, Rudnicki MA (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. doi:10.1101/cshperspect.a008342
Lepper C, Partridge TA, Fan CM (2011) An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138(17):3639–3646. doi:10.1242/dev.067595
Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G (2011) Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138(17):3625–3637. doi:10.1242/dev.064162
Rudnicki MA, Le Grand F, McKinnell I, Kuang S (2008) The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73:323–331. doi:10.1101/sqb.2008.73.064
Brack AS, Rando TA (2012) Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell 10(5):504–514. doi:10.1016/j.stem.2012.04.001
Dhawan J, Rando TA (2005) Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15(12):666–673. doi:10.1016/j.tcb.2005.10.007
Dentice M, Ambrosio R, Damiano V, Sibilio A, Luongo C, Guardiola O, Yennek S, Zordan P, Minchiotti G, Colao A, Marsili A, Brunelli S, Del Vecchio L, Larsen PR, Tajbakhsh S, Salvatore D (2014) Intracellular inactivation of thyroid hormone is a survival mechanism for muscle stem cell proliferation and lineage progression. Cell metabolism 20(6):1038–1048. doi:10.1016/j.cmet.2014.10.009
Aranda A, Martinez-Iglesias O, Ruiz-Llorente L, Garcia-Carpizo V, Zambrano A (2009) Thyroid receptor: roles in cancer. Trends Endocrinol Metab 20(7):318–324. doi:10.1016/j.tem.2009.03.011
Khurana TS, Davies KE (2003) Pharmacological strategies for muscular dystrophy. Nat Rev Drug Discov 2(5):379–390. doi:10.1038/nrd1085
McArdle A, Helliwell TR, Beckett GJ, Catapano M, Davis A, Jackson MJ (1998) Effect of propylthiouracil-induced hypothyroidism on the onset of skeletal muscle necrosis in dystrophin-deficient mdx mice. Clin Sci (Lond) 95(1):83–89
King DB, Entrikin RK (1991) Thyroidal involvement in the expression of avian muscular dystrophy. Life Sci 48(9):909–916
McIntosh LM, Anderson JE (1995) Hypothyroidism prolongs and increases mdx muscle precursor proliferation and delays myotube formation in normal and dystrophic limb muscle. Biochem Cell Biol 73(3–4):181–190
Anderson JE, Liu L, Kardami E (1994) The effects of hyperthyroidism on muscular dystrophy in the mdx mouse: greater dystrophy in cardiac and soleus muscle. Muscle Nerve 17(1):64–73. doi:10.1002/mus.880170109
Cooper RN, Butler-Browne GS, Mouly V (2006) Human muscle stem cells. Curr Opin Pharmacol 6(3):295–300. doi:10.1016/j.coph.2006.01.007
Schreiber AM, Cai L, Brown DD (2005) Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci USA 102(10):3720–3725. doi:10.1073/pnas.0409868102
Cai L, Brown DD (2004) Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol 266(1):87–95
Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A (2011) The development of the adult intestinal stem cells: insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci 1(1):30. doi:10.1186/2045-3701-1-30
Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, Artavanis-Tsakonas S, Louvard D (2009) Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci USA 106(15):6309–6314. doi:10.1073/pnas.0900427106
Catalano V, Dentice M, Ambrosio R, Luongo C, Carollo R, Benfante A, Todaro M, Stassi G, Salvatore D (2016) Activated thyroid hormone promotes differentiation and chemotherapeutic sensitization of colorectal cancer stem cells by regulating Wnt and BMP4 signaling. Cancer Res 76(5):1237–1244. doi:10.1158/0008-5472.CAN-15-1542
Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S (2005) Notch signals control the fate of immature progenitor cells in the intestine. Nature 435(7044):964–968. doi:10.1038/nature03589
Sirakov M, Boussouar A, Kress E, Frau C, Lone IN, Nadjar J, Angelov D, Plateroti M (2015) The thyroid hormone nuclear receptor TRalpha1 controls the Notch signaling pathway and cell fate in murine intestine. Development 142(16):2764–2774. doi:10.1242/dev.121962
Miladpour B, Rasti M, Owji AA, Mostafavipour Z, Khoshdel Z, Noorafshan A, Zal F (2016) Quercetin potentiates transdifferentiation of bone marrow mesenchymal stem cells into the beta cells in vitro. J Endocrinol Invest. doi:10.1007/s40618-016-0592-8
Acknowledgements
This work was supported in part by grants from Ministero Italiano dell’Università e Ricerca (MIUR) (Grant No. 2012Z3F7HE) and the Horizon 2020 research and innovation programme under grant agreement No 666869-THYRAGE. I am indebted to Jean Ann Gilder (Scientific Communication srl., Naples, Italy) for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author states the absence of any conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent.
Rights and permissions
About this article
Cite this article
Salvatore, D. Deiodinases and stem cells: an intimate relationship. J Endocrinol Invest 41, 59–66 (2018). https://doi.org/10.1007/s40618-017-0737-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-017-0737-4