Abstract
Aim
Xenin is a peptide of the neurotensin/xenopsin/xenin family secreted from gastric cells and other tissues. The first aim of this study was to investigate the serum xenin and ghrelin levels in obese children and compare the patients with healthy controls. The second aim was to compare the xenin levels in patients with nonalcoholic fatty liver disease (NAFLD) and also with insulin resistance with the patients without these complications.
Methods
62 obese adolescents (27 with NAFLD) and 32 healthy controls were enrolled in the study. Obesity was defined as a body mass index exceeding the 95th percentile for the patients’ age and sex. NAFLD was diagnosed via ultrasonographic examination. The insulin resistance was calculated by a homeostasis model assessment (HOMA-IR) index. Serum xenin and ghrelin levels were assessed by enzyme-linked immunosorbent assay.
Results
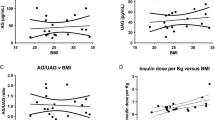
The mean serum xenin concentration was significantly higher in obese adolescents than the healthy peers (68.15 ± 0.63 vs 16.54 ± 0.07 pg/mL, p = 0.000). Serum xenin levels were not different between the patients with and without NAFLD and also between the patients with and without IR (p > 0.05). There was a positive correlation between xenin levels and relative weight (r = 0.663, p < 0.001) and HOMA-IR (r = 0.612, p < 0.001). Ghrelin was negatively correlated with relative weight (r = −0.283, p < 0.05).
Conclusion
In this study, serum xenin levels of both groups of obese patients were found higher than controls. On the other hand, xenin levels were not different in patients with and without NAFLD. High levels of xenin may be in relation with obesity.
Similar content being viewed by others
References
Arslan N, Büyükgebiz B, Öztürk Y, Çakmakçi H (2005) Fatty liver in obese children: prevalence and correlation with anthropometric measurements and hyperlipidemia. Turk J Pediatr 47:23–27
Demircioğlu F, Koçyiğit A, Arslan N, Cakmakçi H, Hizli S, Tuncel SA (2008) Intima-media thickness of carotid artery and susceptibility to atherosclerosis in obese children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 47:68–75
Iughetti L, De Simone M, Verrotti A, Iezzi ML, Predieri B, Bruzzi P, Bernasconi S, Balli F, Bedogni G (2008) Thirty-year persistence of obesity after presentation to a pediatric obesity clinic. Ann Hum Biol 35:439–448
Murphy KG, Bloom SR (2006) Gut hormones and the regulation of energy homeostasis. Nature 444:854–859
Reinehr T, Roth CL, Alexy U, Kersting M, Kiess W, Andler W (2005) Ghrelin levels before and after reduction of overweight due to a low-fat high-carbohydrate diet in obese children and adolescents. Int J Obes (Lond) 29:362–368
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719
Shen C, Yu T, Tang ZH, Wu KM (2013) Changes in ghrelin and obestatin levels before and after a meal in children with simple obesity and anorexia. Horm Res Paediatr 79:341–346
Kyrgios I, Galli-Tsinopoulou A, Stylianou C (2013) Ghrelin-leptin network influences serum chitinase 3-like protein 1 (YKL-40) levels in obese prepubertal children. Regul Pept 183C:69–73
Arrigo T, Gitto E, Ferraù V, Munafò C, Alibrandi A, Marseglia GL, Salpietro A, Miraglia Del Giudice M, Leonardi S, Ciprandi G, Salpietro C (2012) Effect of weight reduction on leptin, total ghrelin and obestatin concentrations in prepubertal children. J Biol Regul Homeost Agents 26(1 Suppl):S95–S103
Yuksel H, Sogut A, Yilmaz O, Onur E, Dinc G (2012) Role of adipokines and hormones of obesity in childhood asthma. Allergy Asthma Immunol Res 4:98–103
Lee J, Yoon J, Lee JA, Lee SY, Shin CH, Yang SW (2012) Urinary 6-sulfatoxymelatonin level in girls and its relationship with obesity. Korean J Pediatr 55:344–349
Feurle GE, Hamscher G, Kusiek R, Meyer HE, Metzger JW (1992) Identification of xenin, a xenopsin-related peptide, in the human gastric mucosa and its effect on exocrine pancreatic secretion. J Biol Chem 267:22305–22309
Hamscher G, Meyer HE, Metzger JW, Feurle GE (1995) Distribution, formation, and molecular forms of the peptide xenin in various mammals. Peptides 16:791–797
van de Sande-Lee S, Cardoso AR, Garlipp CR, Chaim EA, Pareja JC, Geloneze B, Velloso LA (2013) Cerebrospinal fluid xenin levels during body mass reduction: no evidence for obesity-associated defective transport across the blood-brain barrier. Int J Obes (Lond) 37:416–419
Anlauf M, Weihe E, Hartschuh W, Hamscher G, Feurle GE (2000) Localization of xenin-immunoreactive cells in the duodenal mucosa of humans and various mammals. J Histochem Cytochem 48:1617–1626
Alexiou C, Zimmermann JP, Schick RR, Schusdziarra V (1998) Xenin–a novel suppressor of food intake in rats. Brain Res 800:294–299
Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG (2009) Peripheral and central administration of xenin and neurotensin suppress food intake in rodents. Obesity (Silver Spring) 17:1135–1143
Taylor AI, Irwin N, McKillop AM, Patterson S, Flatt PR, Gault VA (2010) Evaluation of the degradation and metabolic effects of the gut peptide xenin on insulin secretion, glycaemic control and satiety. J Endocrinol 207:87–93
Cline MA, Nandar W, Rogers JO (2007) Xenin reduces feed intake by activating the ventromedial hypothalamus and influences gastrointestinal transit rate in chicks. Behav Brain Res 179:28–32
Leckstrom A, Kim ER, Wong D, Mizuno TM (2009) Xenin, a gastrointestinal peptide, regulates feeding independent of the melanocortin signaling pathway. Diabetes 58:87–94
Wice BM, Reeds DN, Tran HD, Crimmins DL, Patterson BW, Dunai J, Wallendorf MJ, Ladenson JH, Villareal DT, Polonsky KS (2012) Xenin-25 amplifies GIP-mediated insulin secretion in humans with normal and impaired glucose tolerance but not type 2 diabetes. Diabetes 61:1793–1800
Wice BM, Wang S, Crimmins DL, Diggs-Andrews KA, Althage MC, Ford EL, Tran H, Ohlendorf M, Griest TA, Wang Q, Fisher SJ, Ladenson JH, Polonsky KS (2010) Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J Biol Chem 285:19842–19853
Schiavo-Cardozo D, Lima MM, Pareja JC, Geloneze B (2013) Appetite-regulating hormones from the upper gut: disrupted control of xenin and ghrelin in night workers. Clin Endocrinol (Oxf) 79:807–811
Mrózek B, Tomasik PJ, Wędrychowicz A, Wójcik M, Skoczeń S, Fyderek K, Starzyk J, Sztefko K (2012) Plasma xenin concentrations in children. Pediatr Endocrinol Diabetes Metab 18:5–8
Szulińska M, Piorunek T, Suliburska J, Pupek-Musialik D, Kupsz J, Drzymala-Czyż S, Bogdański P (2013) Evaluation of insulin resistance, tumor necrosis factor alpha, and total antioxidant status in obese patients smoking cigarettes. Eur Rev Med Pharmacol Sci 17:1916–1922
Azzalini L, Ferrer E, Ramalho LN, Moreno M, Domínguez M, Colmenero J, Peinado VI, Barberà JA, Arroyo V, Ginès P, Caballería J, Bataller R (2010) Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology 51:1567–1576
Cole TJ, Lobstein T (2012) Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 7:284–294
Tanner JM, Whitehouse RH (1976) Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 51:170–179
National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents (1996) Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics 98:649–658
Quin SF, Gosink BB (1985) Characteristic sonographic signs of hepatic fatty infiltration. Am J Roentgenol 145:753–755
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Valerio G, Licenziati MR, Iannuzzi A, Franzese A, Siani P, Riccardi G, Rubba P (2006) Insulin resistance and impaired glucose tolerance in obese children and adolescents from Southern Italy. Nutr Metab Cardiovasc Dis 16:279–284
Martin CM, Gault VA, McClean S, Flatt PR, Irwin N (2012) Degradation, insulin secretion, glucose-lowering and GIP additive actions of a palmitate-derivatised analogue of xenin-25. Biochem Pharmacol 84:312–319
Pedrosa C, Oliveira BM, Albuquerque I, Simões-Pereira C, Vaz-de-Almeida MD, Correia F (2010) Obesity and metabolic syndrome in 7-9 years-old Portuguese schoolchildren. Diabetol Metab Syndr 2:40
Pedrosa C, Oliveira BM, Albuquerque I, Simões-Pereira C, Vaz-de-Almeida MD, Correia F (2011) Metabolic syndrome, adipokines and ghrelin in overweight and obese schoolchildren: results of a 1-year lifestyle intervention programme. Eur J Pediatr 170:483–492
Wilasco MI, Goldani HA, Dornelles CT, Maurer RL, Kieling CO, Porowski M, Silveira TR (2012) Ghrelin, leptin and insulin in healthy children: relationship with anthropometry, gender, and age distribution. Regul Pept 173:21–26
Bellone S, Prodam F, Savastio S, De Rienzo F, Demarchi I, Trovato L, Petri A, Rapa A, Aimaretti G, Bona G (2012) Acylated and unacylated ghrelin levels in normal weight and obese children: influence of puberty and relationship with insulin, leptin and adiponectin levels. J Endocrinol Invest 35:191–197
Conflict of interest
The authors report no declaration of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arslan, N., Sayin, O. & Tokgoz, Y. Evaluation of serum xenin and ghrelin levels and their relationship with nonalcoholic fatty liver disease and insulin resistance in obese adolescents. J Endocrinol Invest 37, 1091–1097 (2014). https://doi.org/10.1007/s40618-014-0160-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-014-0160-z