Abstract
Background
Cardiovascular disease and obesity affect racial and ethnic minorities disproportionally. Public health research suggests that suboptimal diet is an important contributor to health disparities. Limited evidence points at an increased dietary acid load (DAL) in certain ethnic groups. DAL is determined by the balance of acidifying foods and alkaline foods, and elevated DAL scores have been associated with numerous chronic lifestyle-related conditions. The present analysis investigated DAL scores among ethnic groups in the USA.
Methods
Using cross-sectional data from the National Health and Nutrition Examination surveys (NHANES, 2007–2016), we contrasted several markers of DAL (potential renal acid load (PRAL) and net endogenous acid production (NEAP)) between Non-Hispanic Whites, Non-Hispanic Blacks, Mexican Americans, Other Hispanics, and Other Race. The comparison included crude scores and adjusted scores following multivariate linear regression.
Results
The sample for this analysis comprised 19,565 participants, which may be extrapolated to represent 156,116,471 United States Americans. When compared to Non-Hispanic Whites, Non-Hispanic Blacks and Mexican Americans had significantly higher crude DAL scores. PRALR was highest in Mexican Americans (20.42 (0.61) mEq/day), followed by Non-Hispanic Blacks (17.47 (0.42) mEq/day). Crude NEAPF was highest in Non-Hispanic Blacks (64.66 (0.43) mEq/day), and almost 9 mEq/day higher compared to Non-Hispanic Whites (55.78 (0.39) mEq/day). Multivariate linear regression adjusting for confounders revealed comparable interracial DAL differences.
Conclusions
We found significant DAL differences across the investigated ethnic groups. Whether these differences potentially play a role in population health inequity in the USA will be subject to additional research.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Health equity is a frequently cited goal of public health in the USA. In recent years, public health leaders have placed greater emphasis on achieving equity in population health [1,2,3], and implemented numerous approaches to combat the medical and social burden resulting from racial and ethnic disparities [4]. Nevertheless, a recent study by Zimmermann and Anderson suggested a clear lack of progress on health equity during the past 25 years [3].
For racial and ethnic minorities in the USA, health disparities take on many forms, including (but not limited to) higher premature death rates and a higher incidence of chronic diseases [5]. Cardiovascular disease (CVD), obesity, and obesity-related conditions affect racial and ethnic minorities disproportionally [6,7,8].
Non-Hispanic Blacks, for example, have a substantially higher CVD mortality compared with other races/ethnic groups [9]. The age-adjusted mortality rate from CVD of Black men in 2018 was 260 per 100,000, compared to 206 per 100,000 in Non-Hispanic White men. Moreover, all-cause mortality rates among Black populations was 24% higher than among White populations in the 30 most populous United States cities [10]. Analyses of maternal mortality disparities revealed that the maternal mortality rate for Non-Hispanic Black women was 3.55 times that for non-Hispanic White women [11].
Minority groups also share a disproportionate risk of obesity and obesity-related diseases. Odds of obesity were shown to be significantly higher in Non-Hispanic Blacks (OR = 1.97) and Hispanics (OR = 1.81) compared to Non-Hispanic Whites [12].
Socioeconomic factors, fewer health-promoting resources, differences in social norms, lifestyle-related aspects, genetics and psychological factors have been discussed as determinants of obesity and CVD disparities [13, 14, 16]. Public health research shows that suboptimal diet is another important contributor to health disparities among racial and ethnic minorities [17]. Non-Hispanic Blacks and Hispanics tend to have a greater prevalence of poor diet scores than Non-Hispanic Whites [18]. Analyses by Brown et al. suggested that in US adults without prevalent cardiovascular disease, the number of Non-Hispanic Blacks with a poor diet was greater than the number of Non-Hispanic Whites by a magnitude of 6.8 to 11.7% percentage points from 1988 to 2010 [6].
Very limited evidence points at an increased dietary acid load (DAL) in certain ethnic groups [19]. DAL is determined by the balance of acidifying foods (including red meat and cheese) and alkaline foods (such as vegetables, fruits, and legumes) [20]. Diet can markedly affect acid–base status [21], and an altered acid–base homeostasis in the form of an acidifying diet has been shown to modulate molecular activity, including adrenal glucocorticoid secretion, adipocyte cytokine signaling, and altered insulin sensitivity [22]. An alkaline diet, to the contrary, has been associated with a reduction of morbidity and mortality from chronic diseases [23].
Contemporary Western diets typically produce a total acid load ranging from 50 to 80 mEq per day [24, 25]. Crews et al. suggested that DAL is substantially higher in Non-Hispanic Blacks; however, their analysis was limited to patients with chronic kidney disease [19]. Whether there are also DAL differences among other racial and ethnic groups in the general population in the USA has not yet been analyzed in large population-based studies. A high DAL induces a low-grade metabolic acidosis state, which has been associated with the development of metabolic alterations such as insulin resistance, diabetes, and cardiovascular disease; conditions that are more frequently found in ethnic minorities in the USA [26]. In light of the numerous chronic conditions associated with pathologically elevated DAL scores [27,28,29,30], DAL is an important modifiable lifestyle risk factor.
In this context, we used data from the National Health and Nutrition Examination Surveys (NHANES) to investigate two main questions: (1) are there differences in DAL among racial/ethnic groups in the USA, and (2) which groups are disproportionally affected? In this study, we hypothesized that compared to Non-Hispanic Whites, other ethnic groups in the USA yield higher markers of DAL.
Methods
Study Population and Design
The present analysis is based on aggregated population-based cross-sectional data from the NHANES [31]. The NHANES is an ongoing cross-sectional survey in the United States of America that uses a complex, stratified, multistage probability sampling design [32]. It was designed to assess the health and nutritional status of non-institutionalized adults in the USA and is one of several large health‐related programs conducted by the National Center for Health Statistics in the USA.
NHANES study and survey protocols were approved by the Research Ethics Review Board of the National Center for Health Statistics, and written informed consent was obtained from all participants [33]. NHANES data is publicly available but without personal identifiable information. We carried out all methods in full accordance with relevant guidelines and regulations.
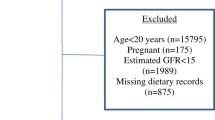
For this study, we included all participants aged 20 years or older who participated in the continuous NHANES survey cycles of 2007–2008 through 2015–2016 to increase the potential sample size for analyses stratified by population subgroups. We constructed appropriate sample weights for the combined NHANES cycles based on National Center for Health Statistics guidelines [34].
For the present analysis, we merged multiple NHANES modules of interest. Participant characteristics were obtained from the demographics module and included age, sex, race/ethnicity, marital status, annual household income, and education level. Nutrient intake data was obtained from the dietary interview component. This module used a computerized 24-h dietary recall method to estimate energy and nutrient intake for all participants. The examination protocol and the data collection methods may be obtained from the NHANES dietary interviewer’s procedure manual [35]. Anthropometric data was obtained from the body measures module and included height, weight, and body mass index (BMI).
Dietary Acid Load Markers
In the NHANES, nutritional assessment was conducted through two repeated 24-h dietary recalls [36]. This is a retrospective and quantitative method to gather information about foods and beverages consumed by the participants in the 24 h prior to their visit. For this analysis, we relied solely on dietary data from day 1, and calculated DAL based on nutrient intake data.
The methods for estimating DAL have been described in detail elsewhere [37,38,39]. In brief, we used the formulas by Remer et al. and Frassetto et al. to estimate DAL scores from nutrient intake [40, 41]. All formulas are commonly used in epidemiological and clinical research. Their performance and accuracy have been discussed in detail by Parmenter et al. [42]. Remer and Manz estimated potential renal acid load (PRALR) based on intestinal absorption rates of potassium, phosphate, magnesium, calcium, and protein [43]. Their formula is as follows:
This formula has been cross-validated and considers ionic dissociation and sulfur metabolism. Frassetto et al. estimated net endogenous acid production (NEAP) based on protein and potassium intake:
The third employed formula (NEAPR) also stems from the work of Remer et al. and uses anthropometric measures and nutrient quantities of cations and anions derived from dietary intake. NEAPR was estimated as follows:
OAest (mEq/day) was calculated as follows:
To estimate individual body surface area, we used the Du Bois and Du Bois formula [44]. Each of the aforementioned formulas has its strengths and limitations; thus, we decided to use them in this particular combination to allow for a more detailed DAL quantification [42].
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: age ≥ 20 years, available demographic data, plausible energy intake data (based on Willett’s criteria) [45], available anthropometric data, and plausible DAL scores. Participants that indicated consumption of a special diet (e.g. a low-carbohydrate diet, gluten-free diet) were excluded to reduce the likelihood of selection bias. Special diet status was assessed based on the NHANES “DRQSDIET” variable, inquiring about special diets for weight loss or other health reasons [37].
Statistical Analysis
We used the STATA 14 statistical software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) for our statistical analysis.
Histograms, box plots, and subpopulation summary statistics were used to check for normality of the data. Normally distributed variables were described with their mean and standard error in parenthesis, whereas categorical variables were described with their weighted proportions and standard error in parenthesis. We estimated standard errors using Taylor series linearization to account for the complex NHANES sampling design. Appropriate sample weights were used to account for differential nonresponse and/or non-coverage, to adjust for planned oversampling of some groups, and to generate weighted percentages and means that are representative of the noninstitutionalized civilian population. Since we appended five different NHANES cycles (2007–2008 through 2015–2016), we generated a 10‐year weight for dietary data (wtdrd10yr = wtdrd1/5).
Based on the most recent NCHS data presentation standards for proportions [46], we checked all weighted proportions for potential unreliability using the user-written post-estimation command “kg_nchs” in Stata [47]. Unreliable proportions were flagged with superscript letters.
Stata’s Rao–Scott test (a design-adjusted version of the Pearson chi-square test) and multivariate linear regression analyses (followed by adjusted Wald tests and Stata’s margins function) were used to test for potential differences between races/ethnicities. All multivariate linear regression models were constructed based on the recommendations of West, Berglund, and Heeringa [48].
In a first step, we conducted exploratory bivariate analyses to investigate potential candidate predictors with a significant relationship with DAL scores (PRALR and NEAPF). We included only predictor variables of scientific relevance and with a bivariate relationship of significance < 0.25 with the response variables in the initial model. Using t-tests for individual coefficients and Wald tests for multiple coefficients, we verified the importance of the included predictor variables and assessed potential changes in all predictor variables in the multivariate model. Finally, we tried to improve the overall fit of the model using established survey data analyses techniques [48]. Post regression, marginsplots were used to graph statistics from fitted models. We used a p-value < 0.05 as a cutoff for statistical significance.
Results
The total sample for this analysis comprised 19,565 participants, which may be extrapolated to represent 156,116,471 United States Americans. The unweighted numbers of participants were as follows: n = 2989 Mexican Americans, n = 1951 Other Hispanics, n = 8617 Non-Hispanic Whites, n = 4031 Non-Hispanic Blacks, and n = 1977 Other Race (including multi-racial).
Table 1 shows sample characteristics by race/ethnicity. Mean age of Non-Hispanic Whites was significantly higher than for the other groups 49.01 (0.32). Significant intergroup differences were also found with regard to the weighted proportions of males and females (Table 1). The weighted proportion of obese participants was significantly higher in Mexican Americans (43.38% (1.19)) and Non-Hispanic Blacks (44.58% (1.27)). Significant intergroup differences in the weighted proportions were also found regarding marital status, educational level, and annual household income.
Table 2 displays nutrient and total energy intake by race/ethnicity. Significant intergroup differences were found across all 5 groups. Mexican Americans had the highest total energy intake (2262.30 (21.98) kcal/day) and the highest total protein intake (89.22 (1.03) g/day). The lowest protein intake was found in Non-Hispanic Blacks (78.66 (0.66)). The latter also had a significantly lower total potassium intake (2351.63 (21.55) mg/day) compared to the other groups. Moreover, Non-Hispanic Blacks also had the lowest magnesium (264.54 (2.93) mg/day) and phosphorus intake (1233.90 (9.58) mg/day). Sodium intake exceeded 3000 mg/day in all five groups (Table 2).
Table 3 shows crude dietary acid load scores by race/ethnicity. Mexican Americans had the highest mean PRALR scores (20.42 (0.61) mEq/day), followed by Non-Hispanic Blacks (17.47 (0.42) mEq/day). A comparable sequence was found with regard to NEAPR. Mexican Americans had the highest mean NEAPR scores (64.64 (0.67) mEq/day), followed by Non-Hispanic Blacks (63.82 (0.47) mEq/day). For NEAPF, the highest average scores were found in Non-Hispanic Blacks (64.66 (0.43) mEq/day), followed by Mexican Americans (61.70 (0.69) mEq/day). The mean PRALR score difference between Mexican Americans and Non-Hispanic Whites was approximately 7.06 mEq/day. When compared to Non-Hispanic Whites, mean unadjusted NEAPF scores were almost 9 mEq/day higher in Non-Hispanic Blacks.
Tables 4 and 5 show the final multivariate linear regression models examining the associations between race/ethnicity and dietary acid load (as assessed by PRALR (Table 4) and NEAPF (Table 5)). In model 1, we adjusted for age and sex. In model 2, we additionally adjusted for BMI and total energy intake. As shown in Table 4, Mexican Americans and Non-Hispanic Blacks yielded significantly higher PRALR values after adjustment for confounders in both regression models. Mexican American ethnicity increased PRALR values by more than 4.37 mEq/day (p < 0.001) in model 1. After adjustment for BMI and total energy intake, this association was still significant (3.91 mEq/day, p < 0.001). Non-Hispanic Black ethnicity increased PRALR values by more than 3.16 mEq/day in both models.
We found significantly higher NEAPF values in Non-Hispanic Blacks (+ 7.78 in model 1 and 7.25 mEq/day in model 2, respectively) compared to the reference group. Energy intake and obesity were also associated with high DAL scores (Model 2, Tables 4 and 5). Our initial hypothesis was thus partially supported with regard to Mexican Americans and Non-Hispanic Blacks.
Marginsplots were used to graph statistics from the fitted models. Marginal predicted values of PRALR are shown in Fig. 1 by race/ethnicity for each age category (based on model 2, Table 4). In a similar manner, Fig. 2 shows marginal predicted values of NEAPF by race/ethnicity for each age category (based on model 2, Table 5).
We refrained from constructing the same models for formula NEAPR, as the estimation formula is based on anthropometric data. Using model 2 for NEAPR may thus have introduced multicollinearity problems and therefore we decided to adjust only for age, sex, and ethnicity. Results may be obtained from the supplementary Table 1.
Discussion
Using data from the NHANES, the present study sought to analyze potential differences in DAL scores among racial/ethnic groups in the USA. When compared to Non-Hispanic Whites, Non-Hispanic Blacks and Mexican Americans had significantly higher (crude) DAL scores. PRALR was highest in Mexican Americans (20.42 (0.61) mEq/day), followed by Non-Hispanic Blacks (17.47 (0.42) mEq/day). Crude NEAPF was highest in Non-Hispanic Blacks (64.66 (0.43) mEq/day), and almost 9 mEq/day higher compared to Non-Hispanic Whites.
A high DAL has been associated with an elevated cardio-metabolic risk and may unfavorably affect lipid metabolism [49]. Han et al. and Murakami et al. both reported positive associations between higher PRAL-values and the concentrations of total cholesterol and low-density lipoprotein [30, 50]. Higher DAL scores were also associated with higher HbA1c concentrations and elevated triacylglycerol (TAG) concentrations [51]. Moreover, a high acid load burden from diet was associated with a higher obesity prevalence, and has thus been discussed as a potential risk factor for the development of metabolic disorders [52, 53].
The elevated DAL scores in Non-Hispanic Blacks and Mexican Americans reported in our study warrant further discussion—particularly in the potential context of chronic diseases. In 2018, African Americans were 30% more likely to die from heart disease than non-Hispanic Whites [54]. Mexican Americans on the other hand were significantly more likely to suffer from obesity or diabetes than non-Hispanic Whites [55, 56].
The numerous reasons that may contribute to these ethnic/racial disparities include socioeconomic factors, a lack of health-promoting resources in certain areas and among certain minority groups, lifestyle-related factors, genetics, and varying social norms [13, 14, 16]. Whether a high DAL may be one of the many contributors in this multifactorial process has not yet been scientifically discussed. Notably, the present analysis does not allow for any causal attributions but suggests that DAL differences across ethnic/racial groups exist.
The low-grade metabolic acidosis state induced by a high DAL has been associated with the development of metabolic alterations such as insulin resistance, diabetes, and cardiovascular disease [26]. Said conditions are more frequently found in ethnic minorities in the USA. It is not inconceivable that DAL differences could potentially play a role in ethnic/racial disparities in many chronic conditions, and our analysis builds the foundation for future research in this area.
Disparities in chronic diseases between various segments of the population, such as racial and ethnic groups, have increasingly become a major focus of public health research [57,58]. Individuals with a greater number of chronic conditions often present with poorer overall dietary intakes [59]. Non-Hispanic Whites were shown to have a higher diet quality (as assessed by the Alternative Healthy Eating Index 2010) compared to Hispanics and African Americans [60]. Recent trends in diet quality by ethnicity/race among United States adults have been summarized in detail by Tao et al. [61]. Yet, studies investigating DAL among racial and ethnic groups are very scarce and only available for very specific populations (e.g. patients with renal disease) [19]. A comparative discussion with other studies is thus difficult, yet DAL is worthy of further investigations for its contribution to chronic disease outcomes across race/ethnic groups.
Essentially in line with the findings by Crews et al. [19], our results suggest higher DAL scores in Non-Hispanic Blacks than Non-Hispanic Whites. Yet, a numerical DAL score comparison between both studies may not expedient, because, unlike us, Crews et al. investigated patients with end-stage renal disease [19]. Of note, Crews et al. suggested that among US adults with chronic kidney disease, the association of DAL with progression to end-stage renal disease is stronger among Non-Hispanic Blacks than Non-Hispanic Whites.
Notably, the cross-sectional nature of our study is an intrinsic limitation and does not allow for such causal attributions. Our study did not include disease-specific endpoints but focused on descriptive recordings of DAL scores in ethnic/racial minorities.
Future prospective studies are thus warranted to gain a better understanding of DAL in ethnic/racial minorities. Such studies should preferably include food group analyses and investigate barriers to and opportunities for DAL-reduction strategies in the respective groups.
The present analysis draws upon a number of strengths. It is based on a nationally representative and large dataset from the National Health and Nutrition Examination Survey. The large sample size and the consideration of relevant confounders (e.g. special diets) are an additional asset. The topic is innovative and has not been analyzed before. Meanwhile, this analysis has several weaknesses worth mentioning, including the intrinsic limitations of a cross-sectional study, the inherent potential for various biases, and the lack of a food group analysis. Designed to investigate potential differences in DAL across ethnic/racial groups in the USA, this analysis does not allow for any causal interferences. Thus, it may not answer the question whether a high DAL actually contributes to ethnical/racial disparities in the USA. Although our statistical regression models took into account a number of potential confounders, we did not adjust for physical activity in order to avoid a substantial reduction in sample size. Finally, we excluded individuals on a special diet to reduce the likelihood of selection bias and to allow for a more homogenous comparison across ethnic groups. The various special diets captured in the NHANES yield different DAL scores [37]; by excluding individuals on special diets, we aimed to present a comparison of the ethnic groups on a standard Western diet. Yet, we acknowledge that in the more recent NHANES cycles, slightly more Non-Hispanic White adults were on a special diet than Non-Hispanic Black or Non-Hispanic Asian Adults [62].
Conclusions
To the best of our knowledge, we present the first analysis to systematically investigate DAL scores across ethnic/racial minorities in the USA. Using data from the NHANES, we found substantial and significant DAL differences across the involved ethnic groups. Mexican Americans and Non-Hispanic Blacks had the highest DAL scores, with notable differences to Non-Hispanic Whites. Our analysis builds the foundation for future research in this area and raises many interesting research questions, including (but not limited to) as to whether these DAL differences could potentially play a role in population health inequity in the USA. Future prospective studies are urgently warranted to gain additional insights into this unexplored area of nutritional epidemiology.
Data Availability
Data is publicly available online (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NEAP :
-
Net endogenous acid production
- NCHS :
-
National Center for Health Statistics
- NHANES :
-
National Health and Nutrition Examination Surveys
- PRAL :
-
Potential renal acid load
References
Plough AL. Building a culture of health: a critical role for public health services and systems research. Am J Public Health. 2015;105(Suppl 2):S150-152.
Anderson NW, Zimmerman FJ. Trends in health equity among children in the United States, 1997–2018. Matern Child Health J. 2021;25(12):1939–59.
Zimmerman FJ, Anderson NW. Trends in health equity in the United States by race/ethnicity, sex, and income, 1993–2017. JAMA Netw Open. 2019;2(6):e196386.
Petersen R. Racial and ethnic disparities in adult obesity in the United States: CDC’s tracking to inform state and local action. Prev Chronic Dis [Internet]. 2019 [cited 2022 Jul 10];16. Available from: https://www.cdc.gov/pcd/issues/2019/18_0579.htm
National Academies of Sciences E, Division H and M, Practice B on PH and PH, States C on CBS to PHE in the U, Baciu A, Negussie Y, et al. The state of health disparities in the United States [Internet]. Communities in action: pathways to health equity. National Academies Press (US); 2017 [cited 2022 Jul 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425844/
Brown AF, Liang LJ, Vassar SD, Escarce JJ, Merkin SS, Cheng E, et al. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. 2018;168(8):541–9.
Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA, Cheskin LJ. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020;49(3):810–23.
Age-Adjusted total CVD mortality rates by Race/Ethnicity [Internet]. www.heart.org. [cited 2022 Jul 10]. Available from: https://www.heart.org/en/about-us/2024-health-equity-impact-goal/age-adjusted-total-cvd-mortality-rates-by-race-ethnicity
Diaz CL, Shah NS, Lloyd-Jones DM, Khan SS. State of the nation’s cardiovascular health and targeting health equity in the United States: a narrative review. JAMA Cardiol. 2021;6(8):963–70.
Benjamins MR, Silva A, Saiyed NS, De Maio FG. Comparison of all-cause mortality rates and inequities between black and white populations across the 30 most populous US cities. JAMA Netw Open. 2021;4(1):e2032086.
MacDorman MF, Thoma M, Declcerq E, Howell EA. Racial and ethnic disparities in maternal mortality in the United States using enhanced vital records, 2016–2017. Am J Public Health. 2021;111(9):1673–81.
Zhang H, Rodriguez-Monguio R. Racial disparities in the risk of developing obesity-related diseases: a cross-sectional study. Ethn Dis. 2012;22(3):308–16.
Krueger PM, Reither EN. Mind the gap: race/ethnic and socioeconomic disparities in obesity. Curr Diab Rep. 2015;15(11):95.
Byrd AS, Toth AT, Stanford FC. Racial disparities in obesity treatment. Curr Obes Rep. 2018;7(2):130–8.
NHANES - About the National Health and Nutrition Examination Survey [Internet]. 2020 [cited 2022 Jul 6]. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
Pool LR, Carnethon MR, Goff DC, Gordon-Larsen P, Robinson WR, Kershaw KN. Longitudinal associations of neighborhood-level racial residential segregation with obesity among blacks. Epidemiology. 2018;29(2):207–14.
Wang L, Du M, Cudhea F, Griecci C, Michaud DS, Mozaffarian D, et al. Disparities in health and economic burdens of cancer attributable to suboptimal diet in the United States, 2015–2018. Am J Public Health. 2021;111(11):2008–18.
Kris-Etherton PM, Petersen KS, Velarde G, Barnard ND, Miller M, Ros E, et al. Barriers, opportunities, and challenges in addressing disparities in diet-related cardiovascular disease in the United States. J Am Heart Assoc. 2020;9(7):e014433.
Crews DC, Banerjee T, Wesson DE, Morgenstern H, Saran R, Burrows NR, et al. Race/ethnicity, dietary acid load, and risk of end-stage renal disease among US adults with chronic kidney disease. AJN. 2018;47(3):174–81.
Kiefte-de Jong JC, Li Y, Chen M, Curhan GC, Mattei J, Malik VS, et al. Diet-dependent acid load and type 2 diabetes: pooled results from three prospective cohort studies. Diabetologia. 2017;60(2):270–9.
Banerjee T, Crews DC, Wesson DE, Tilea A, Saran R, Rios Burrows N, et al. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol. 2014;15(1):137.
Robey IF. Examining the relationship between diet-induced acidosis and cancer. Nutr Metab (Lond). 2012;1(9):72.
Schwalfenberg GK. The alkaline diet: is there evidence that an alkaline pH diet benefits health? J Environ Public Health. 2012;2012:727630.
Gannon RHT, Millward DJ, Brown JE, Macdonald HM, Lovell DP, Frassetto LA, et al. Estimates of daily net endogenous acid production in the elderly UK population: analysis of the National Diet and Nutrition Survey (NDNS) of British adults aged 65 years and over. Br J Nutr. 2008;100(3):615–23.
Wesson DE. The continuum of acid stress. Clin J Am Soc Nephrol. 2021;16(8):1292–9.
Osuna-Padilla IA, Leal-Escobar G, Garza-García CA, Rodríguez-Castellanos FE. Dietary Acid Load: mechanisms and evidence of its health repercussions. Nefrologia (Engl Ed). 2019;39(4):343–54.
Arisawa K, Katsuura-Kamano S, Uemura H, Van Tien N, Hishida A, Tamura T, et al. Association of dietary acid load with the prevalence of metabolic syndrome among participants in baseline survey of the Japan Multi-Institutional Collaborative Cohort Study. Nutrients. 2020;12(6):1605.
Iwase H, Tanaka M, Kobayashi Y, Wada S, Kuwahata M, Kido Y, et al. Lower vegetable protein intake and higher dietary acid load associated with lower carbohydrate intake are risk factors for metabolic syndrome in patients with type 2 diabetes: post-hoc analysis of a cross-sectional study. J Diabetes Investig. 2015;6(4):465–72.
Daneshzad E, Haghighatdoost F, Azadbakht L. Dietary acid load and cardiometabolic risk factors: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2019;22(15):2823–34.
Han E, Kim G, Hong N, Lee Y ho, Kim DW, Shin HJ, et al Association between dietary acid load and the risk of cardiovascular disease: nationwide surveys (KNHANES 2008–2011). Cardiovasc Diabetol. 2016;26:15(1):122.
NHANES - Participants - About [Internet]. 2021 [cited 2022 Jul 6]. Available from: https://www.cdc.gov/nchs/nhanes/participant/participant-about.htm
NHANES – Survey Brochure - About [Internet]. 2021 [cited 2022 Jul 6]. https://wwwn.cdc.gov/nchs/data/nhanes/survey_contents.pdf
NHANES - NCHS Research Ethics Review Board Approval [Internet]. 2021 [cited 2022 Jul 6]. Available from: https://www.cdc.gov/nchs/nhanes/irba98.htm
NHANES Survey Methods and Analytic Guidelines [Internet]. [cited 2022 Jul 6]. Available from: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx
Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey MEC In-Person Dietary Interviewers Procedure Manual. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention 2008. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_dietarymec.pdf. Accessed on 5 Feb 2022.
NHANES - Measuring Guides [Internet]. 2019 [cited 2022 Nov 6]. Available from: https://www.cdc.gov/nchs/nhanes/measuring_guides_dri/measuringguides.htm
Storz MA, Müller A, Ronco AL. Nutrient intake and dietary acid load of special diets in the NHANES: a descriptive analysis (2009–2018). Int J Environ Res Public Health. 2022;19(9):5748.
Storz MA, Ronco AL. Reduced dietary acid load in U.S. vegetarian adults: results from the National Health and Nutrition Examination Survey. Food Sci Nutr. 2022;10(6):2091–100.
Müller A, Zimmermann-Klemd AM, Lederer AK, Hannibal L, Kowarschik S, Huber R, et al. A vegan diet is associated with a significant reduction in dietary acid load: post hoc analysis of a randomized controlled trial in healthy individuals. Int J Environ Res Public Health. 2021;18(19):9998.
Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95(7):791–7.
Frassetto LA, Todd KM, Morris RC Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68(3):576–83.
Parmenter BH, Dymock M, Banerjee T, Sebastian A, Slater GJ, Frassetto LA. Performance of predictive equations and biochemical measures quantifying net endogenous acid production and the potential renal acid load. Kidney Int Rep. 2020;5(10):1738–45.
Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59(6):1356–61.
Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–87.
Willett W. Nutritional Epidemiology. 3rd ed. New York, NY, USA: Oxford University Press; 2013.
Parker JD, Talih M, Malec DJ, Beresovsky V, Carroll M, Gonzalez JF, et al. National Center for Health Statistics data presentation standards for proportions. Vital Health Stat. 2017;2(175):1–22.
Ward BW. kg_nchs: A command for Korn-Graubard confidence intervals and National Center for Health Statistics’ Data Presentation Standards for Proportions. Stata J. 2019;19(3):510–22.
Heeringa SG, West BT, Berglund PA. Applied survey data analysis [Internet]. Chapman and Hall/CRC; 2017 [cited 2022 Jul 6]. Available from: https://www.taylorfrancis.com/books/mono/, https://doi.org/10.1201/9781315153278/applied-survey-data-analysis-steven-heeringa-brady-west-patricia-berglund
Ostrowska J, Janiszewska J, Szostak-Węgierek D. Dietary acid load and cardiometabolic risk factors—a narrative review. Nutr. 2020;12(11):3419.
Murakami K, Sasaki S, Takahashi Y, Uenishi K, Japan dietetic students’ study for nutrition and biomarkers group. Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br J Nutr. 2008;100(3):642–51.
Haghighatdoost F, Najafabadi MM, Bellissimo N, Azadbakht L. Association of dietary acid load with cardiovascular disease risk factors in patients with diabetic nephropathy. Nutr. 2015;31(5):697–702.
Abbasalizad Farhangi M, Nikniaz L, Nikniaz Z. Higher dietary acid load potentially increases serum triglyceride and obesity prevalence in adults: an updated systematic review and meta-analysis. PLoS One. 2019;14(5):e0216547.
Bahadoran Z, Mirmiran P, Khosravi H, Azizi F. Associations between dietary acid-base load and cardiometabolic risk factors in adults: the Tehran Lipid and Glucose Study. Endocrinol Metab (Seoul). 2015;30(2):201–7.
Heart Disease and African Americans - The Office of Minority Health [Internet]. [cited 2022 Jul 10]. Available from: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=19
CDC. ¡A la Buena Salud! – To Good Health! [Internet]. Centers for disease control and prevention. 2015 [cited 2022 Jul 10]. Available from: https://www.cdc.gov/vitalsigns/hispanic-health/index.html
Fernandez ML. Lifestyle factors and genetic variants associated to health disparities in the Hispanic population. Nutr. 2021;13(7):2189.
Satia JA. Diet-related disparities: understanding the problem and accelerating solution. J Am Diet Assoc. 2009;109(4):610–5.
Pérez-Escamilla R. Dietary quality among Latinos: is acculturation making us sick? J Am Diet Assoc. 2009;109(6):988–91.
Fanelli SM, Jonnalagadda SS, Pisegna JL, Kelly OJ, Krok-Schoen JL, Taylor CA. Poorer diet quality observed among US adults with a greater number of clinical chronic disease risk factors. J Prim Care Community Health. 2020;1(11):2150132720945898.
Rodriguez LA, Jin Y, Talegawkar SA, de Otto MCO, Kandula NR, Herrington DM, et al. Differences in diet quality among multiple US racial/ethnic groups from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study and the Multi-Ethnic Study of Atherosclerosis (MESA). J Nutr. 2020;150(6):1509–15.
Tao MH, Liu JL, Nguyen USDT. Trends in diet quality by race/ethnicity among adults in the United States for 2011–2018. Nutr. 2022;14(19):4178.
Stierman B, Ansai N, Mishra S, Hales CM. Special diets among adults: United States, 2015–2018. NCHS Data Brief. 2020;289:1–8.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MAS is the sole author of this contribution. MAS performed the analysis and interpreted the data. MAS MAS is the guarantor of this study, designed the idea, and validated data. Writing original draft: MAS. Writing—review and editing: MAS. Illustration: MAS. All authors reviewed and approved the final manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The present study is negligible risk research that involves existing collections of non-identifiable data about human beings. It is a de-identified secondary analysis of freely available data. Research was performed in accordance with the Declaration of Helsinki and approved by the NCHS Research Ethics Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm). NHANES was approved by the National Centre for Health Statistics research ethics review board and informed consent was obtained for all participants.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Open Access
Open access publishing enabled by Project DEAL.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work has not been published before; it is not under consideration for publication anywhere else. This work has been approved by all co-authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Storz, M.A. Health Inequities in the USA: a Role for Dietary Acid Load? Results from the National Health and Nutrition Examination Surveys. J. Racial and Ethnic Health Disparities 10, 2851–2860 (2023). https://doi.org/10.1007/s40615-022-01462-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-022-01462-9