Abstract
Background
Racial/ethnic disparities in trauma care have been reported. The American Indian/Alaska Native (AI/AN) population faces a twofold to fourfold increase of risk for traumatic injury. We hypothesized that surgical intervention and time to surgery were associated with race/ethnicity, specifically AI/AN compared to other race/ethnicity groups with open pelvic and lower extremity fractures (OPLEFx).
Methods
Non-AI/AN racial/ethnic groups were compared to AI/ANs among adults aged 15 years and older using the National Trauma Data Bank for 2008–2012. OPLEFx were identified via ICD-9-CM. Predictors of surgery and time to surgery were modeled via logistic regression and survival analyses.
Results
AI/AN patients (2.7 %, n = 206) were younger (36 ± 16 versus 41 ± 18 years, p < 0.001) and more likely to have Medicaid and other government insurance. There were no differences in AI/ANs versus non-AI/ANs undergoing surgery (88.4 versus 86.8 %, respectively) or time to surgery (11.7 ± 25.3 versus 12.0 ± 22.5 h, respectively). Injury severity was predictive of surgery in all six models (OR = 0.04 to 0.32). A race-gender interaction increased odds of surgery in the AI/AN versus all other races model (OR = 3.58, 95 % CI 1.18–10.84) and in three of five pairwise models. Median time to surgery varied by race, favoring AI/ANs with least preoperative time.
Conclusion
The AI/AN population experienced no disparities in rate of, or time to, OPLEFx surgery. Race-specific predictors for surgery included gender, probability of death, and multiple fractures. More study is warranted to ameliorate trauma care disparities and achieve reasonably equitable care as demonstrated in AI/ANs with OPLEFx.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Disparities in healthcare among racial/ethnic groups have been well documented across the spectrum of human disease [1–4]. However, in the treatment of traumatic injuries, disparity of care among racial/ethnic groups remains controversial. When smaller racial/ethnic groups are aggregated into a single category or dropped from analysis, the assumption is made that the provision of care and clinical outcomes is uniform among individuals in the category regardless of racial/ethnic distinction and important information is lost.
Investigating the processes and outcomes of healthcare in all racial/ethnic groups is warranted. One such population is the American Indian/Alaska Natives (AI/AN) whose burden of traumatic injury and healthcare outcomes merit study. In 2000, Burhansstipanov and Satter presented an overview of the AI/AN population, noting that the routine collapsing of smaller minority populations into an “other” category or excluding them altogether during analysis can lead to biased conclusions [5]. Policymakers, researchers, and AI/AN tribal planners require such evidence to develop effective programs for the AI/AN population. The Office of Management and Budget (OMB) recommends all federally funded research and service projects follow the racial categories outlined in Directive 15 when reporting study findings [6, 7]. These include American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, and White; for data on ethnicity: “Hispanic or Latino” and “not Hispanic or Latino” [7].
The AI/AN population currently represents approximately 2 % of the US population and is noted to be one of the fastest growing racial minorities [8]. In 2010, approximately 2.6 million people identified their race as AI/AN alone, while 5.2 million identified themselves as AI/AN in combination with one or more other race(s) [8]. The AI/AN minority’s growth rate is currently three times that of the national rate (27 versus 9 %) [8]. Unintentional injuries are the greatest cause of death for AI/AN less than 44 years of age [9]. Among the AI/AN population, unintentional injuries account for 28 % of the years of potential life lost before age 65 [9]. Motor vehicle crash, including pedestrian-motor vehicle trauma, is the leading mechanism of injury for this group, commonly resulting in severe, open pelvic and lower extremity fractures (OPLEFx) [10].
For patients with OPLEFx, evidence suggests that minimized time to primary and definitive operative management is critical to optimize outcomes, specifically fewer infections and improved survival [11–14]. In contrast to exsanguinating hemorrhage where small delays of care can be rapidly fatal, a complex of orthopedic injuries requiring urgent intervention was selected because there is a temporal margin of safety where variation in timing of care can take place. Thus, the time from emergency department arrival until operative intervention is a measure of care at the very start of hospitalization for patients with OPLEFx. In prior work, we observed access to rehabilitative care at the conclusion of hospitalization for AI/ANs with spinal cord injury occurred at a rate equitable to or greater than other races when other important factors are taken into account [15]. The medical literature is sparse regarding racial differences in surgical treatment for OPLEFx. We sought to identify such differences in this growing segment of the US population using rates of operative treatment of OPLEFx and elapsed time from hospital arrival to surgical procedure as finite measures of one aspect of healthcare provision for AI/ANs relative to other race/ethnicity groups at the beginning of the trauma care process. We hypothesized that surgical intervention would be equitable and that time to surgery for OPLEFx would be equal or shorter for AI/ANs relative to other race/ethnicity groups.
Methods
Data Source
After approval from the Chandler Regional Medical Center Institutional Review Board, we conducted a cohort study using the National Trauma Data Bank (NTDB) for years 2008–2012. The NTDB is the largest, voluntarily submitted, national trauma registry [16]. Patient and hospital identifiers are removed. Currently, the NTDB contains data on over five million cases from over 900 trauma centers [16]. Injuries, comorbidities, and procedures were identified by their respective International Classification of Diseases, Ninth Revision, Clinical Modification [17] (ICD-9) codes. See Table 4 in Appendix for the diagnostic codes used to define these injuries.
Cohort Selection
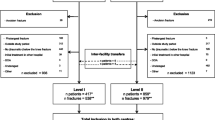
We included patients aged 15 years and older who had open fractures of the pelvis, femur, tibia/fibula, and tarsal bones. Age 15 was selected because this age differentiates the adult from pediatric population in terms of trauma center capability and accreditation. Moreover, patients younger than 15 represent a heterogeneous population due to the progressive stages of bone development. OPLEFx were selected because they collectively represent an urgent need for orthopedic intervention. Patients were included if their OPLEFx injury was their most severe; defined as the injury contributing the greatest probability of death using the trauma mortality prediction model (TMPM) [18]. The TMPM estimates each patient’s probability of death based only on their anatomic injuries. The TMPM has been validated as a superior predictor of mortality over the injury severity score [19]. We included all racial/ethnic categories represented in the NTDB registry: White, Black, Hispanic/Latino, Asian, American Indian or Alaska Native, and other race. The race/ethnic categories were entered in the NTDB by patient self-identification. Of note, although Hispanic/Latino is an ethnic distinction, it is treated as a racial/ethnic category equal to White, Black, etc., and includes all patients who self-identify Hispanic in their ethnicity response. This was done to keep the racial/ethnic groups adequately sized to allow for stable effect estimates. The patients were excluded if their race/ethnicity was unknown, were admitted to a hospital that did not treat AI/AN patients during the study period, were admitted to the hospital for less than 24 h, were injured by a penetrating mechanism, or were not admitted from the emergency department.
Comorbidities were enumerated using the Elixhauser comorbidity score [20]. Whether a patient’s OPLEFx was managed operatively and the elapsed hours from admission until surgical procedure were our outcomes of interest. Surgical procedures were grouped into seven categories of similarity and an “other procedures” for smaller subgroups for comparison between AI/AN and non-AI/AN groups. Similarly, OPLEFx injuries were grouped by bone fractured.
Statistical Analysis
Frequencies with percentages and means with standard deviations were used to describe the overall cohort of patients. Independent sample t tests were used to compare mean differences for continuous versus parametric variables. Chi-square tests were used to compare distributions for ordinal or dichotomous variables. Partial eta-squared values are reported to demonstrate effect size. A series of hierarchical multivariate logistic regression (HMVLR) models clustered on hospital facility were used to predict surgical versus non-surgical cases. Covariates in the model included the following: age, gender, insurance (payment), injury severity, comorbidities, single versus multiple fractures, and level of hospital trauma center level.
As shown in Table 3, the AI/AN group is compared to all non-AI/AN. Subsequent models compared the AI/AN group to each of the individual racial groups. Of note, there were only 30 patients identified as Native Hawaiian or Other Pacific Islander. Estimates using this small group would be unstable so they were omitted from regression analyses. For the models, the variable payer was coded such that a response of uninsured was utilized as the reference group and compared to the privately insured (i.e., insurance provided by one’s employer), government insurance (e.g., Medicaid, Medicare, Veterans Benefits Administration, Indian Health Services), and payment (other) cohorts. Predicted probabilities from models were used to calculate the area under the receiver operating characteristic curve. The continuous variable of probability of death was logarithmically transformed to account for skew and kurtosis. Model discrimination was evaluated using the area under the receiver operating characteristic curve (AUC). Kaplan-Meier survival curves with the log rank test were used to compare time to surgery between cohorts. To achieve a combined type I error rate of 0.05 for the 15 comparisons, we used the following formula which adjusted our alpha to 0.003: 1 – (1 – α) [15]. This adjustment did not change our post hoc results as stated in the last paragraph of the results section. SPSS version 22, IBM Corporation, Armonk, NY and STATA version 14.0, Stata Corporation, College Station, TX were used for statistical analysis. p Values of less than 0.05 were considered statistically significant.
Results
Our cohort consisted of 7667 patients between the ages of 15 and 97 years who were admitted to a trauma center for OPLEFx injuries between 2008 and 2012. The patients were 70.21 % male (n = 5383) and predominantly White (66.30 %) with mean age of 40.90 years ± 17.82. Collectively, there were 9291 fractures in our cohort treated with 14,122 orthopedic procedures. There were no significant differences in fracture types or numbers of fractures between AI/AN and non-AI/AN patients. However, two procedures differed between these groups. Open reduction of fractures was performed more often in AI/AN group (76.37 versus 66.67 % in non-AI/AN, p = 0.006), whereas application of an external fixator was performed more often in the Non-AI/AN group (21.27 versus 14.84 % in AI/AN, p = 0.036). The average length of hospital stay was 7.55 days ± 7.92 with an average intensive care unit (ICU) stay of 5.46 days ± 6.66 and a mean time on ventilator of 5.68 days ± 7.92, for the 1334 and 601 patients admitted to the ICU and ventilated, respectively. The mean TMPM was 0.03 ± 0.05 with 92.47 % of patients demonstrating a Glasgow Coma Scale (GCS) score of 15 upon admission. The majority of patients were treated surgically (86.81 %). Nearly one half of patients were privately insured (47.87 %) followed by uninsured (18.36 %), Medicaid (12.99 %), and then Medicare (11.75 %). The most frequent patient comorbidities were hypertension requiring medication (15.52 %), smoking (13.21 %), alcoholism (7.54 %), diabetes (6.85 %), obesity (5.11 %), and respiratory disease (5.06 %).
The cohort was 2.69 % (n = 206) AI/AN. Proportionately, there were fewer AI/AN males compared to non-AI/AN males (62.14 versus 70.43 %, p = 0.010), respectively. The AI/AN patients were significantly younger than the non-AI/AN cohort (35.64 years ± 15.59 versus 41.05 ± 17.86, p < 0.001), had proportionately more comorbidities (60.68 versus 46.09 %, p < 0.001), and were more likely to have alcohol in their system (32.52 versus 14.05 %, p < 0.001). Cohort characteristics are shown in Table 1.
Univariate comparisons of patient and treatment characteristics between patients undergoing surgery versus not undergoing surgery are shown in Table 2. Patients undergoing surgery had a lower probability of death (0.02 ± 0.04 versus 0.06 ± 0.07, p < 0.001) and fewer ventilated days (5.31 days ± 7.55 versus 7.46 days ± 9.34, p = 0.012). Surgical patients were more likely to have multiple fractures compared to non-surgical patients (19.29 versus 8.70 %, p < 0.001) and more likely to have at least one comorbidity in comparison to non-surgical cases (47.31 versus 41.05 %, p < 0.001).
Although there was not a statistically significant difference in the proportion of AI/ANs versus non-AI/ANs undergoing surgery, p = 0.509; the distribution of the proportion of patients across all races undergoing surgery versus not undergoing surgery was significantly different, p < 0.001. The distribution of payment was also significantly different for surgical versus non-surgical patients, p = 0.012.
Our sample of Native Hawaiian/Pacific Islanders was too small for a regression model; however, we felt it important to describe this cohort. There were 30 Native Hawaiian/Pacific Islanders (76.7 % male, n = 23) with an average age of 33.40 years ± 12.78 and mean TMPM of 0.02 ± 0.02. Five patients (16.67 %) were hospitalized in the ICU with a mean stay of 2.20 days ± 1.10. All 30 Native Hawaiian/Pacific Islanders underwent surgery, 5 (16.7 %) had multiple fractures, 10 (33.3 %) experienced a complication, and 15 (50 %) had at least 1 comorbidity. Mean time to surgery for this cohort was 8.93 h ± 7.66 with a median time to surgery of 6.5 h.
Regression Models
A series of HMVLR models were used to understand how patient characteristics predicted surgery for each racial group compared to AI/ANs. The AUC for the six models ranged from 0.72 (95 % CI 0.70–0.72) to 0.88 (95 % CI 0.79–0.97) suggesting that our predictors were good discriminators between patients who did versus did not have surgery.
Race was the predictor of primary importance in our analyses. Race did not emerge as a main effect predicting surgical status in any of the six models; however, in four models, a gender by race interaction emerged such that the odds of undergoing surgery were higher for AI/AN males. In the AI/AN versus non-AI/AN model, the odds of undergoing surgery was increased by 3.58 times (95 % CI 1.18–10.84) for AI/AN males. The odds of surgery were 4.53 (95 % CI 1.27–16.18) for AI/AN males compared to Hispanic. In the AI/AN versus African American or Black model, the AI/AN males’ odds of surgery were 6.59 (95 % CI 1.95–22.23) times greater. Finally, the odds of surgery for AI/AN males was 13.42 (95 % CI 2.15–83.91) greater than the odds of surgery for patients belonging to the other races group. The race by gender interaction failed to emerge as a predictor in the AI/AN versus White model; however, the p value was only slightly above the standard threshold at p = 0.057. The only model for which the race by gender interaction was not significant was the AI/AN versus Asian model.
Covariates played significant, albeit different, roles across models. Payment was entered into our models as a categorical variable with private insurance as the reference group. Five significant comparisons across three of our six models emerged as predictors of surgery, all with odds ratios less than one suggesting that patients with Medicaid, Medicare, uninsured, other government, and other payments were less likely to undergo surgery (with all other predictors held constant), in a model. The log transformation of probability of death was a significant covariate in all six models. Odds ratios of less than one were suggestive that odds of undergoing surgery decreased as injury severity increased. Patients with multiple fractures were at increased odds of undergoing surgery in all models with the exception of the AI/AN versus Asian model, with odds ratios ranging from 3.23 (95 % CI 2.26–4.62) in the AI/AN versus White model to 11.46 (95 % CI 1.39–94.36) in the AI/AN versus other races model. Age, GCS score and trauma level were non-significant. Models are shown in Table 3.
Survival functions were graphed to demonstrate the significant race by gender interactions in our HMVLR models. The median time to surgery was significantly lower for AI/AN versus non-AI/AN males, 4.0 h (95 % CI 3.38–4.62) and 6.0 h (95 % CI 5.83–6.18), respectively, p = 0.011. Statistics related to the survival analysis, including patients at risk, are illustrated in the Kaplan-Meier curves in Fig. 1. Figure 2 depicts the analysis with all races and both males and females. The six racial groups yielded 15 pairwise comparisons, of which 10 were significant (p < 0.05). The log rank test suggested time to surgery was significantly different by race, p < 0.001.
Discussion
Inequalities in health status between AI/ANs and Europeans and non-AI/AN Americans have been recognized for five centuries [21]. Our results demonstrate surgical intervention and time to initial surgery for OPLEFx vary by race. However, this study demonstrates the AI/AN patients experience equitable rates of operative intervention for OPFLEx injuries, hospital mortality, and discharge to rehabilitative facilities compared to non-AI/AN groups, and AI/AN males received surgery 2 hours earlier on average. Moreover, our findings underscore the importance of the OMB recommendation that all racial/ethnic groups be considered as distinct entities rather than collapsing these distinctions into an aggregate “other” racial category. Yet it is common in this literature to do so and obscure important findings among the injured.
For example, Shafi et al. compared non-Hispanic White, Black, and Hispanic groups and found that ethnic minorities were less likely to be discharged to rehabilitation after severe blunt traumatic brain injuries [22]. Following this, Shafi et al. found that ethnic minorities fared worse long-term functional outcomes after traumatic brain injury compared to non-Hispanic Whites [23]. Conversely, Osler et al. found no difference in mortality among Whites, Blacks, and Hispanics younger than 65 years old [24]. Similarly, Branch et al. found no difference in the timing of definitive fixation for open femoral fractures among Whites and Blacks with all other racial categories included under the category of “other race” [25]. Despite divergent findings, these four studies share similar methods of racial categorization, thus leaving gaps in our understanding of healthcare equality among smaller race/ethnic groups. Additionally, investigators have identified that misclassification of the AI/AN people limits accurate injury and mortality rate estimates [26]. For example, Sugarman et al. found a 69 % difference between the number of patients listed as AI/AN in the Oregon Injury Registry and the number of those listed as injured in the Indian Health Service records [27]. However, solutions have been proposed such as geocoding and surname analysis or linkage between state trauma registries and an AI/AN registry [28, 29].
Concordant with other investigators, we found that AI/ANs were a larger proportion of patients testing positive for alcohol on board in the emergency departments [26, 30]. In fact, the AI/AN group testing positive for alcohol was more than twice that of the non-AI/AN group. The association of injury, death, and alcohol in the AI/AN population has been extensively studied and found to be significant [10, 26]. Fortunately, successful interventions aimed at decreasing the injury morbidity and mortality associated with alcohol in the AI/AN population have been described [10]. Testing positive for alcohol in the ED bore no association with whether or not a patient underwent surgery for OPLEFx.
We observed a significant interaction between AI/AN and male sex favoring surgery for OPLEFx except in the comparisons of AI/AN to White and Asian groups. The interaction of AI/AN and male sex has been observed in studies of healthcare disparities for AI/ANs in other areas of concern. For example, high rates of risk taking behavior, including binge drinking, have been observed in AI/AN males compared to AI/AN females [31]. Finally, it is noteworthy that no differences were observed in overall mortality and discharge to rehabilitation, long-term care, or skilled nursing facilities. This is in agreement with previous work investigating the equality or disparity of trauma care for AI/ANs [15].
This study has several limitations. First, the NTDB data do not represent a population-based sample of injured patients. Additionally, AI/ANs have been shown to be misclassified as such in several reports. Recently, Liebler et al. found that “…less than one third of ever–American Indian people in the data had the same race/Hispanic response in 2000 and 2010” [32]. As such, the AI/AN group in this study may be underestimated. However, we were adequately powered to accept our hypotheses, and the lack of misclassification would likely strengthen our findings. Next, the retrospective clinical registry data in the NTDB were not gathered specifically to measure healthcare disparities among racial/ethnic groups. As such, these data may omit important contributors to the clinical decision making processes regarding timing of surgical intervention. Finally, a small group (2.63 %) of the cohort remained identified as other race due to the taxonomy of the NTDB. We emphasize one caveat regarding our conclusions. Our finding of well-met needs of surgical treatment and the time to surgery for OPLEFx injuries among AI/ANs should not be extrapolated beyond the endpoints of this study as an omnibus conclusion that racial/ethnic disparities in healthcare do not exist for AI/ANs or other minority racial/ethnic groups. Nonetheless, the strength of this study is the large, adequately powered cohort from over 900 trauma centers across the USA, allowing investigation of disparities of care for AI/ANs with OPLEFx compared to other race/ethnic groups.
Conclusion
During the years studied, reasonably equitable care was achieved for OPLEFx for the often overlooked AI/AN people compared to other racial/ethnic groups. As such, these findings argue against broad conclusions that racial disparities exist in trauma care, at least with regard to the treatment of AI/AN patients for OPLEFx. Research is needed to transform other areas of healthcare, such that racial disparities are ameliorated as demonstrated in the operative care of patients with OPLEFx.
References
Krieger N, Waterman PD, Chen JT, Soobader MJ, Subramanian S. Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: geocoding and choice of area-based socioeconomic measures—the public health disparities geocoding project (US). Public Health Rep. 2003;118(3):240.
Livingston EH, Fairlie RW. Little effect of insurance status or socioeconomic condition on disparities in minority appendicitis perforation rates. Arch Surg. 2012;147(1):11–7. doi:10.1001/archsurg.2011.746.
Mullins CD, Cooke Jr JL, Wang J, Shaya FT, Hsu DV, Brooks S. Disparities in prevalence rates for lung, colorectal, breast, and prostate cancers in Medicaid. J Natl Med Assoc. 2004;96(6):809–16.
Pappas G, Queen S, Hadden W, Fisher G. The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. N Engl J Med. 1993;329(2):103–9.
Burhansstipanov L, Satter DE. Office of Management and Budget racial categories and implications for American Indians and Alaska Natives. Am J Public Health. 2000;90(11):1720.
Office of Management and Budget. Standards and guidelines for federal statistics: race and ethnic standards for federal statistics and administrative reporting. Washington, D.C: Federal Register; 1977. p. Exhibit F.
Office of Management and Budget. Revisions to the standards for the classification of federal data on race and ethnicity. Fed Regist. 1997;62(210):58782–90.
Norris T, Vines PL, Hoeffel EM. The American Indian and Alaska Native population: 2010 Census Brief. C2010BR-10. Available at www.census.gov/prod/cen2010/briefs/c2010br-10.pdf.
Piland N, Berger L. The economic burden of injuries involving American Indians and Alaska Natives: a critical need for prevention. IHS Prim Care Provid. 2007;32(9):269–73.
Pollack KM, Frattaroli S, Young JL, Dana-Sacco G, Gielen AC. Motor vehicle deaths among American Indian and Alaska Native populations. Epidemiol Rev. 2012;34(1):73–88.
Bednar DA, Parikh J. Effect of time delay from injury to primary management on the incidence of deep infection after open fractures of the lower extremities caused by blunt trauma in adults. J Orthop Trauma. 1993;7(6):532–5.
Harley BJ, Beaupre LA, Jones CA, Dulai SK, Weber DW. The effect of time to definitive treatment on the rate of nonunion and infection in open fractures. J Orthop Trauma. 2002;16(7):484–90.
Khatod M, Botte MJ, Hoyt DB, Meyer RS, Smith JM, Akeson WH. Outcomes in open tibia fractures: relationship between delay in treatment and infection. J Trauma Acute Care Surg. 2003;55(5):949–54.
Morshed S, Miclau T, Bembom O, Cohen M, Knudson MM, Colford JM. Delayed internal fixation of femoral shaft fracture reduces mortality among patients with multisystem trauma. J Bone Joint Surg. 2009;91(1):3–13. doi:10.2106/jbjs.h.00338.
Cook AD, Ward JG, Chapple KM, Akinbiyi H, Garrett M, Moore III FOD. Race and rehabilitation following spinal cord injury: equality of access for American Indians/Alaska Natives compared to other racial groups. Inj Epidemiol. 2015;2(1):1–10.
Surgeons ACo. National Trauma Data Bank Annual Report 2014. American College of Surgeons, Chicago. 2014. https://www.facs.org/quality%20programs/trauma/ntdb/docpub. Accessed November 10, 2015.
World Health Organization. International Classification of Diseases (ICD). 2013. http://www.who.int/classifications/icd/en/. Accessed November 24, 2013.
Glance LG, Osler TM, Mukamel DB, Meredith W, Wagner J, Dick AW. TMPM-ICD9: a trauma mortality prediction model based on ICD-9-CM codes. Ann Surg. 2009;249(6):1032–9. doi:10.1097/SLA.0b013e3181a38f28.
Cook A, Weddle J, Baker S, Hosmer D, Glance L, Friedman L, et al. A comparison of the injury severity score and the trauma mortality prediction model. J Trauma Acute Care Surg. 2014;76(1):47–52. doi:10.1097/TA.0b013e3182ab0d5d. discussion −3.
Elixhauser A, Steiner C, Harris D, Coffey R. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Jones DS. The persistence of American Indian health disparities. Am J Public Health. 2006;96(12):2122.
Shafi S, de la Plata CM, Diaz-Arrastia R, Bransky A, Frankel H, Elliott AC, et al. Ethnic disparities exist in trauma care. J Trauma Acute Care Surg. 2007;63(5):1138–42.
Shafi S, Marquez de la Plata C, Diaz-Arrastia R, Shipman K, Carlile M, Frankel H, et al. Racial disparities in long-term functional outcome after traumatic brain injury. J Trauma. 2007;63(6):1263–8. doi:10.1097/TA.0b013e31815b8f00. discussion 8–70.
Osler T, Glance LG, Li W, Buzas JS, Wetzel ML, Hosmer DW. Trauma care does not discriminate: the association of race and health insurance with mortality following traumatic injury. J Trauma Acute Care Surg. 2015;78(5):1026–33.
Branch NN, Obirieze A, Wilson RH. Assessment of racial and sex disparities in open femoral fractures. Am J Surg. 2015;209(4):666–74.
Centers for Disease Control and Prevention. Injury mortality among American Indian and Alaska Native children and youth—United States, 1989–1998. MMWR Morb Mortal Wkly Rep. 2003;52(30):697–701.
Sugarman JR, Soderberg R, Gordon JE, Rivara FP. Racial misclassification of American Indians: its effect on injury rates in Oregon, 1989 through 1990. Am J Public Health. 1993;83(5):681–4.
Fiscella K, Fremont AM. Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res. 2006;41(4p1):1482–500.
Hoopes MJ, Dankovchik J, Weiser T, Cheng T, Bigback K, Knaster ES, et al. Uncovering a missing demographic in trauma registries: epidemiology of trauma among American Indians and Alaska Natives in Washington State. Inj Prev. 2015. injuryprev-2014-041419.
Grossman DC, Sugarman JR, Fox C, Moran J. Motor-vehicle crash-injury risk factors among American Indians. Accid Anal Prev. 1997;29(3):313–9.
Rhoades ER. The health status of American Indian and Alaska Native males. Am J Public Health. 2003;93(5):774–8.
Liebler CA, Bhaskar R, Porter SR. Joining, leaving, and staying in the American Indian/Alaska Native race category between 2000 and 2010. Demography. 2016;53:1–34.
Acknowledgments
The authors thank Annette Taylor, RN, research coordinator for her invaluable administrative support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This is an unfunded study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Cook, A., Chapple, K., Motzkin, N. et al. No Disparity for American Indians in Surgery for Pelvis/Lower Extremity Fractures: a Cohort Study of the National Trauma Data Bank (NTDB). J. Racial and Ethnic Health Disparities 4, 725–734 (2017). https://doi.org/10.1007/s40615-016-0276-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-016-0276-2