Abstract
Purpose of Review
The discovery of insulin was a true landmark in biomedical research and provided a framework for the understanding of many pivotal mechanisms in cell biology throughout the twentieth century, as insulin for instance was the first major protein to have its amino acid sequence and 3D structure resolved. The elucidation of the processes that regulate and mediate insulin secretion has also contributed with crucial mechanistic insights on the pathways that lead to both type 1 and type 2 diabetes mellitus. More than 100 years after the discovery of this hormone, an overview of the present knowledge on insulin output from β-cells should be timely to the general researcher interested in the mechanisms that couple glucose stimulation to insulin secretion.
Recent Findings
Although the mechanisms underlying insulin secretion have been exhaustively studied and understood in animal models, the last decades have shown that important differences can be identified compared to human β-cells. Additionally, despite both reactive oxygen species as well as the immune system have been initially implicated in β-cell dysfunction and the progression to diabetes, increasing evidence indicates that both can also have physiological effects for proper insulin secretion.
Summary
Given this background, this brief review focused on discussing various means by which glucose elicits insulin secretion by the β-cells, particularly on the modulatory role of redox balance and inflammation on β-cell function and/or demise, also drawing attention to the specific mechanisms connecting glucose stimulation to insulin secretion in humans.




Similar content being viewed by others
Data and Material Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- ATF6:
-
Activating transcription factor 6
- BK:
-
Big potassium, large conductance calcium-activated potassium channel
- C3a:
-
Complement component 3 fragment a
- ER:
-
Endoplasmic reticulum
- GLP-1:
-
Glucagon-like peptide 1
- GLUT:
-
Glucose transporter
- GSIS:
-
Glucose-stimulated insulin secretion
- HVA:
-
High-voltage-activated calcium channel
- IAPP:
-
Islet amyloid polypeptide
- IFN-γ:
-
Interferon-γ
- IKK:
-
Inhibitor of NF-κB kinase
- IL-1β:
-
Interleukin-1β
- IL-1R1:
-
IL-1β receptor 1
- IRE1α:
-
Inositol-requiring enzyme 1 α
- JNK:
-
Jun N-terminal kinase
- KATP :
-
ATP-sensitive potassium channel
- Kir6.2:
-
Inward rectifier potassium channel 6.2 isoform
- LVA:
-
Low voltage-activated calcium channel
- MIF:
-
Macrophage migration inhibitory factor
- Nav:
-
Voltage-dependent sodium channel alpha subunit
- NF-κB:
-
Nuclear factor kappa B
- NOD:
-
Non-obese diabetic
- PDI:
-
Protein disulfide isomerase
- PERK:
-
Protein kinase RNA-like ER kinase
- PKR:
-
Protein kinase R
- ROS:
-
Reactive oxygen species
- SUR1:
-
Sulphonylurea receptor 1
- T1D:
-
Type 1 diabetes mellitus
- T2D:
-
Type 2 diabetes mellitus
- TNF:
-
Tumor necrosis factor
- UPR:
-
Unfolded protein response
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Leiva-Hidalgo A, Leiva-Pérez A. The Nobel Prize of Physiology or Medicine, 1923: controversies on the discovery of the antidiabetic hormone. Acta Diabetol. 2023;60(9):1241–56. https://doi.org/10.1007/s00592-023-02098-9.
Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960;39(7):1157–75. https://doi.org/10.1172/JCI104130.
Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16(1):35–9. https://doi.org/10.2337/diab.16.1.35.
Malaisse WJ, Sener A, Herchuelz A, Hutton JC. Insulin release: the fuel hypothesis. Metabolism. 1979;28(4):373–86. https://doi.org/10.1016/0026-0495(79)90111-2.
Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311(5983):271–3. https://doi.org/10.1038/311271a0.
Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312(5993):446–8. https://doi.org/10.1038/312446a0.
Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751–60. https://doi.org/10.2337/diabetes.49.11.1751.
•• Ashcroft FM. KATP channels and the metabolic regulation of insulin secretion in health and disease: the 2022 Banting Medal for Scientific Achievement Award Lecture. Diabetes. 2023;72(6):693–702. https://doi.org/10.2337/dbi22-0030. A timely and updated review on the history of KATP research in β-cells by one of its discoverers.
Ohara-Imaizumi M, Fujiwara T, Nakamichi Y, Okamura T, Akimoto Y, Kawai J, et al. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J Cell Biol. 2007;177(4):695–705. https://doi.org/10.1083/jcb.200608132.
Eliasson L, Abdulkader F, Braun M, Galvanovskis J, Hoppa MB, Rorsman P. Novel aspects of the molecular mechanisms controlling insulin secretion. J Physiol. 2008;586(14):3313–24. https://doi.org/10.1113/jphysiol.2008.155317.
Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes. 2002;51(Suppl 1):S117–21. https://doi.org/10.2337/diabetes.51.2007.s117.
Gandasi NR, Yin P, Omar-Hmeadi M, Laakso EO, Vikman P, Barg S. Glucose-dependent granule docking limits insulin secretion and is decreased in human type 2 diabetes. Cell Metab. 2018;27(2):470-478.e4. https://doi.org/10.1016/j.cmet.2017.12.017.
Pedersen MG, Tagliavini A, Henquin JC. Calcium signaling and secretory granule pool dynamics underlie biphasic insulin secretion and its amplification by glucose: experiments and modeling. Am J Physiol Endocrinol Metab. 2019;316(3):E475–86. https://doi.org/10.1152/ajpendo.00380.2018.
• Ye Y, Barghouth M, Dou H, Luan C, Wang Y, Karagiannopoulos A, et al. A critical role of the mechanosensor PIEZO1 in glucose-induced insulin secretion in pancreatic β-cells. Nat Commun. 2022;13(1):4237. https://doi.org/10.1038/s41467-022-31103-y. This study indicates that the mechanosensor channel PIEZO1 can induce a small depolarizing current in β-cells in stimulatory glucose concentrations that are due to β-cell swelling by the intracellular accumulation of glucose metabolites. This may thus constitute an additional triggering pathway for glucose-stimulated insulin secretion.
De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96(5):2489–95. https://doi.org/10.1172/JCI118308.
McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, Gloyn AL. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol Genet Metab. 2011;104(4):648–53. https://doi.org/10.1016/j.ymgme.2011.08.026.
•• Jo S, Beetch M, Gustafson E, Wong A, Oribamise E, Chung G, et al. Sex differences in pancreatic β-cell physiology and glucose homeostasis in C57BL/6J mice. J Endocr Soc. 2023;7(9):bvad099. https://doi.org/10.1210/jendso/bvad099. An elegant study demonstrating that there are striking differences between female and male islets in intracellular calcium handling and glucose-induced insulin secretion in what is probably most used background mouse model in biomedical research at present.
•• Tudurí E, Soriano S, Almagro L, García-Heredia A, Rafacho A, Alonso-Magdalena P, et al. The effects of aging on male mouse pancreatic β-cell function involve multiple events in the regulation of secretion: influence of insulin sensitivity. J Gerontol A Biol Sci Med Sci. 2022;77(3):405–15. https://doi.org/10.1093/gerona/glab276. Another elegant study that investigated the changes in the efficiency of the insulin secretion mechanism with aging - from the electrical activity through calcium handling to insulin secretion 0 demonstrating that these are correlated with decreased insulin sensitivity.
DeFronzo RA. Glucose Intolerance and Aging: Evidence for Tissue Insensitivity to Insulin. Diabetes. 1979;28(12):1095–101. https://doi.org/10.2337/diab.28.12.1095.
• Lewandowski SL, Cardone RL, Foster HR, Ho T, Potapenko E, Poudel C, et al. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab. 2020;32(5):736-750.e5. https://doi.org/10.1016/j.cmet.2020.10.007. Article demonstrating that the localization of the glycolytic enzyme pyruvate kinase to the plasma membrane creates local levels of ATP that can underlie oscillatory metabolic and calcium signaling in glucose-stimulated insulin secretion.
Merrins MJ, Corkey BE, Kibbey RG, Prentki M. Metabolic cycles and signals for insulin secretion. Cell Metab. 2022;34(7):947–68. https://doi.org/10.1016/j.cmet.2022.06.003.
Paggio A, Checchetto V, Campo A, Menabò R, Di Marco G, Di Lisa F, et al. Identification of an ATP-sensitive potassium channel in mitochondria. Nature. 2019;572(7771):609–13. https://doi.org/10.1038/s41586-019-1498-3.
Prentki M, Matschinsky FM, Madiraju SRM. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162–85. https://doi.org/10.1016/j.cmet.2013.05.018.
Prentki M, Corkey BE, Madiraju SRM. Lipid-associated metabolic signalling networks in pancreatic beta cell function. Diabetologia. 2020;63(1):10–20. https://doi.org/10.1007/s00125-019-04976-w.
Newsholme P, Cruzat V, Arfuso F, Keane K. Nutrient regulation of insulin secretion and action. J Endocrinol. 2014;221(3):R105–20. https://doi.org/10.1530/JOE-13-0616.
Zúñiga-Hertz JP, Rebelato E, Kassan A, Khalifa AM, Ali SS, Patel HH, et al. Distinct pathways of cholesterol biosynthesis impact on insulin secretion. J Endocrinol. 2015;224(1):75–84. https://doi.org/10.1530/JOE-14-0348.
Jones B, Bloom SR, Buenaventura T, Tomas A, Rutter GA. Control of insulin secretion by GLP-1. Peptides. 2018;100:75–84. https://doi.org/10.1016/j.peptides.2017.12.013.
Antunes CM, Salgado AP, Rosário LM, Santos RM. Differential patterns of glucose-induced electrical activity and intracellular calcium responses in single mouse and rat pancreatic islets. Diabetes. 2000;49(12):2028–38. https://doi.org/10.2337/diabetes.49.12.2028.
Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, et al. Voltage-gated ion channels in human pancreatic β-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57(6):1618–28. https://doi.org/10.2337/db07-0991.
Rorsman P, Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol Rev. 2018;98(1):117–214. https://doi.org/10.1152/physrev.00008.2017.
Scharfmann, R. Pechberty S, Hazhouz Y, von Bülow M, Bricout-Neveu E, Grenier-Godard M, Guezet F, et al. Development of a conditionally immortalized human pancreatic beta cell line. J Clin Invest. 2014;124(5):2087–98. https://doi.org/10.1172/JCI72674.
Hastoy B, Godazgar M, Clark A, Nylander V, Spiliotis I, van de Bunt M, et al. Electrophysiological properties of human beta-cell lines EndoC-βH1 and -βH2 conform with human beta-cells. Sci Rep. 2018;8(1):16994. https://doi.org/10.1038/s41598-018-34743-7.
Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–79. https://doi.org/10.1146/annurev-physiol-030212-183754.
Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–9. https://doi.org/10.1016/j.cell.2015.10.001.
Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46(11):1733–42. https://doi.org/10.2337/diab.46.11.1733.
Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in ‘t Veld P, Renström E, et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54(7):2132–42. https://doi.org/10.2337/diabetes.54.7.2132.
Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56(7):1783–91. https://doi.org/10.2337/db06-1601.
Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem. 1999;274(39):27905–13. https://doi.org/10.1074/jbc.274.39.27905.
Nakazaki M, Kakei M, Koriyama N, Tanaka H. Involvement of ATP-sensitive K+ channels in free radical-mediated inhibition of insulin secretion in rat pancreatic β-cells. Diabetes. 1995;44(8):878–83. https://doi.org/10.2337/diab.44.8.878.
Rebelato E, Abdulkader F, Curi R, Carpinelli AR. Low doses of hydrogen peroxide impair glucose-stimulated insulin secretion via inhibition of glucose metabolism and intracellular calcium oscillations. Metabolism. 2010;59(3):409–13. https://doi.org/10.1016/j.metabol.2009.08.010.
Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, et al. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58(3):673–81. https://doi.org/10.2337/db07-1056.
Rebelato E, Abdulkader F, Curi R, Carpinelli AR. Control of the intracellular redox state by glucose participates in the insulin secretion mechanism. PLoS ONE. 2011;6(8):e24507. https://doi.org/10.1371/journal.pone.0024507.
Deglasse JP, Roma LP, Pastor-Flores D, Gilon P, Dick TP, Jonas JC. Glucose acutely reduces cytosolic and mitochondrial H2O2 in rat pancreatic beta cells. Antioxid Redox Signal. 2019;30(3):297–313. https://doi.org/10.1089/ars.2017.7287.
Verspohl EJ, Händel M, Ammon HP. Pentosephosphate shunt activity of rat pancreatic islets: its dependence on glucose concentration. Endocrinology. 1979;105(5):1269–74. https://doi.org/10.1210/endo-105-5-1269.
Oliveira HR, Verlengia R, Carvalho CRO, Britto LRG, Curi R, Carpinelli AR. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003;52(6):1457–63. https://doi.org/10.2337/diabetes.52.6.1457.
Rebelato E, Mares-Guia TR, Graciano MFR, Labriola L, Britto LRG, Garay-Malpartida HM, et al. Expression of NADPH oxidase in human pancreatic islets. Life Sci. 2012;91(7–8):244–9. https://doi.org/10.1016/j.lfs.2012.07.004.
Morgan D, Rebelato E, Abdulkader F, Graciano MFR, Oliveira-Emilio HR, Hirata AE, et al. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology. 2009;150(5):2197–201. https://doi.org/10.1210/en.2008-1149.
• Plecitá-Hlavatá L, Jabůrek M, Holendová B, Tauber J, Pavluch V, Berková Z, et al. Glucose-stimulated insulin secretion fundamentally requires H2O2 signaling by NADPH oxidase 4. Diabetes. 2020;69(7):1341–54. https://doi.org/10.2337/db19-1130. Demonstration of the importance of the ROS-producing activity of a specific isoform of NADPH oxidase to the induction of insulin by glucose. This suggests that the control of local subcellular ROS levels are important factor for GSIS.
Vilas-Boas EA, Almeida DC, Roma LP, Ortis F, Carpinelli AR. Cell failure in type 2 diabetes: oxidative stress linked to NADPH oxidase and ER stress. Cells. 2021;10(12):3328. https://doi.org/10.3390/cells10123328.
Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5(4):219–26. https://doi.org/10.1038/nrendo.2009.21.
Mallone R, Eizirik DL. Presumption of innocence for beta cells: why are they vulnerable autoimmune targets in type 1 diabetes? Diabetologia. 2020;63(10):1999–2006. https://doi.org/10.1007/s00125-020-05176-7.
Donath MY, Dalmas É, Sauter NS, Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 2013;17(6):860–72. https://doi.org/10.1016/j.cmet.2013.05.001.
Citro A, Campo F, Dugnani E, Piemonti L. Innate immunity mediated inflammation and beta cell function: neighbors or enemies? Front Endocrinol. 2021;11:606332. https://doi.org/10.3389/fendo.2020.606332.
Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16(7):349–62. https://doi.org/10.1038/s41574-020-0355-7.
Stojanovic I, Saksida T, Stosic-Grujicic S. Beta cell function: the role of macrophage migration inhibitory factor. Immunol Res. 2012;52(1–2):81–8. https://doi.org/10.1007/s12026-012-8281-y.
Orliaguet L, Dalmas E, Drareni K, Venteclef N, Alzaid F. Mechanisms of macrophage polarization in insulin signaling and sensitivity. Front Endocrinol. 2020;11:62. https://doi.org/10.3389/fendo.2020.00062.
Eguchi K, Manabe I. Macrophages and islet inflammation in type 2 diabetes. Diabetes Obes Metab. 2013;15(Suppl 3):152–8. https://doi.org/10.1111/dom.12168.
Dalmas E. Innate immune priming of insulin secretion. Curr Opin Immunol. 2019;56:44–9. https://doi.org/10.1016/j.coi.2018.10.005.
Meyerovich K, Ortis F, Allagnat F, Cardozo AK. Endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. J Mol Endocrinol. 2016;57(1):R1–17. https://doi.org/10.1530/JME-15-0306.
He W, Yuan T, Maedler K. Macrophage-associated pro-inflammatory state in human islets from obese individuals. Nutr Diabetes. 2019;9(1):36. https://doi.org/10.1038/s41387-019-0103-z.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–40. https://doi.org/10.1038/ni.1831.
Vasiljević J, Torkko JM, Knoch KP, Solimena M. The making of insulin in health and disease. Diabetologia. 2020;63(10):1981–9. https://doi.org/10.1007/s00125-020-05192-7.
Ghiasi SM, Krogh N, Tyrberg B, Mandrup-Poulsen T. The no-go and nonsense-mediated RNA decay pathways are regulated by inflammatory cytokines in insulin-producing cells and human islets and determine β-cell insulin biosynthesis and survival. Diabetes. 2018;67(10):2019–37. https://doi.org/10.2337/db18-0073.
Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J Mol Endocrinol. 2016;56(2):R33-54. https://doi.org/10.1530/JME-15-0232.
Lo JC, Ljubicic S, Leibiger S, Kern M, Leibiger IB, Moede T, et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158(1):41–53. https://doi.org/10.1016/j.cell.2014.06.005.
Dror E, Dalmas E, Meier DT, Wueest S, Thévenet J, Thienel C, et al. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol. 2017;18(3):283–92. https://doi.org/10.1038/ni.3659.
Chang YW, Hung LC, Chen YC, Wang WH, Lin CY, Tzeng HH, et al. Insulin reduces inflammation by regulating the activation of the NLRP3 inflammasome. Front Immunol. 2021;11:587229. https://doi.org/10.3389/fimmu.2020.587229.
Dandona P, Ghanim H, Chaudhuri A, Mohanty P. Macronutrient intake, insulin secretion, oxidative stress & inflammation: clinico-pathological implications. Indian J Med Res. 2016;144(5):645–9. https://doi.org/10.4103/ijmr.IJMR_1807_16.
Acknowledgements
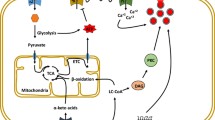
Thanks are due to Prof. Patrik Rorsman, Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford, UK, for permission to use data in Fig. 1 obtained in his laboratory by FA.
Author information
Authors and Affiliations
Contributions
F.O., E.R., A.R.C. and F.A. wrote, reviewed, edited and approved this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
The animal procedures leading to the results described presented in Fig. 1 were carried out according to national and institutional guidelines of the University of Oxford, UK.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ortis, F., Rebelato, E., Carpinelli, A.R. et al. Insulin Secretion and the β-Cell 102 Years After the Discovery of the Hormone. Curr Mol Bio Rep (2024). https://doi.org/10.1007/s40610-024-00158-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s40610-024-00158-9