Abstract
Purpose of Review
The endplates form the interface between the rigid vertebral bodies and compliant intervertebral discs. Proper endplate function involves a balance between conflicting biomechanical and nutritional demands. This review summarizes recent data that highlight the importance of proper endplate function and the relationships between endplate dysfunction, adjacent disc degeneration, and axial low back pain.
Recent Findings
Changes to endplate morphology and composition that impair its permeability associate with disc degeneration. Endplate damage also associates with disc degeneration, and the progression of degeneration may be accelerated and the chronicity of symptoms heightened when damage coincides with evidence of adjacent bone marrow lesions.
Summary
The endplate plays a key role in the development of disc degeneration and low back pain. Clarification of the mechanisms governing endplate degeneration and developments in clinical imaging that enable precise evaluation of endplate function and dysfunction will distinguish the correlative vs. causative nature of endplate damage and motivate new treatments that target pathologic endplate function.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ringwalt C, Gugelmann H, Garrettson M, Dasgupta N, Chung AE, Proescholdbell SK, et al. Differential prescribing of opioid analgesics according to physician specialty for Medicaid patients with chronic noncancer pain diagnoses. Pain Res Manag. 2014;19(4):179–85.
Chou D, Samartzis D, Bellabarba C, Patel A, Luk KD, Kisser JM, et al. Degenerative magnetic resonance imaging changes in patients with chronic low back pain: a systematic review. Spine. 2011;36(21 Suppl):S43–53.
Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52–7.
Lotz JC. Animal models of intervertebral disc degeneration: lessons learned. Spine (Phila Pa 1976). 2004;29(23):2742–50.
Adams MA, Dolan P, Hutton WC. The stages of disc degeneration as revealed by discograms. J Bone Joint Surg (Br). 1986;68(1):36–41.
Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26(23):2543–9.
Roberts S, Menage J, Urban JP. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine. 1989;14(2):166–74.
Edwards WT, Zheng YG, Ferrara LA, Yuan HA. Structural features and thickness of the vertebral cortex in the thoracolumbar spine. Spine. 2001;26(2):218–25.
Fields AJ, Sahli Costabal F, Rodriguez AG, Lotz JC. Seeing double: a comparison of microstructure, biomechanical function and adjacent disc health between double- and single-layer vertebral endplates. Spine. 2012;37(21):E1310–7.
Rodriguez AG, Rodriguez-Soto AE, Burghardt AJ, Berven S, Majumdar S, Lotz JC. Morphology of the human vertebral endplate. J Orthop Res. 2011.
Silva MJ, Wang C, Keaveny TM, Hayes WC. Direct and computed-tomography thickness measurements of the human lumbar vertebral shell and end-plate. Bone. 1994;15(4):409–14.
Zhao FD, Pollintine P, Hole BD, Adams MA, Dolan P. Vertebral fractures usually affect the cranial endplate because it is thinner and supported by less-dense trabecular bone. Bone. 2009;44(2):372–9.
Berg-Johansen B, Han M, Fields AJ, Liebenberg EC, Lim BJ, Larson PE, et al. Cartilage endplate thickness variation measured by ultrashort echo-time MRI is associated with adjacent disc degeneration. Spine (Phila Pa 1976). 2018;43(10):E592–600.
Rodriguez AG, Slichter CK, Acosta FL, Rodriguez-Soto AE, Burghardt AJ, Majumdar S, et al. Human disc nucleus properties and vertebral endplate permeability. Spine. 2011;36(7):512–20.
Fields AJ, Rodriguez D, Gary KN, Liebenberg EC, Lotz JC. Influence of biochemical composition on endplate cartilage tensile properties in the human lumbar spine. J Orthop Res. 2014;32(2):245–52.
Roberts S, Menage J, Duance V, Wotton S, Ayad S. 1991 Volvo Award in basic sciences. Collagen types around the cells of the intervertebral disc and cartilage end plate: an immunolocalization study. Spine. 1991;16(9):1030–8.
• Fields AJ, Han M, Krug R, Lotz JC. Cartilaginous end plates: quantitative MR imaging with very short echo times-orientation dependence and correlation with biochemical composition. Radiology. 2015;274(2):482–9 This study demonstrated that T2* relaxation times of human CEP tissue were highly correlated with glycosaminoglycan content, the ratio of collagen:glycosaminoglycan contents, and water content. Owing to magic angle effects, the accuracy of T2*-based estimates of biochemical composition depended on the orientation of the CEPs; accuracy was greatest at ~ 55°, which approximates the orientation of the L4-S1 CEPs.
• Wu Y, Cisewski SE, Wegner N, Zhao S, Pellegrini VD Jr, Slate EH, et al. Region and strain-dependent diffusivities of glucose and lactate in healthy human cartilage endplate. J Biomech. 2016;49(13):2756–62 This study reported that the diffusivities of glucose and lactate in the human CEP were significantly correlated with matrix porosity. The highest diffusivities were measured in CEP tissues belonging to the central region adjacent to the NP and under lower compressive strains.
Paietta RC, Burger E, Ferguson VL. Mineralization and collagen orientation throughout aging at the vertebral endplate in the human lumbar spine. J Struct Biol. 2013;184:310–20.
Reiser KM, Bratton C, Yankelevich DR, Knoesen A, Rocha-Mendoza I, Lotz J. Quantitative analysis of structural disorder in intervertebral disks using second harmonic generation imaging: comparison with morphometric analysis. J Biomed Opt. 2007;12(6):064019.
Wade KR, Robertson PA, Broom ND. A fresh look at the nucleus-endplate region: new evidence for significant structural integration. Eur Spine J. 2011;20(8):1225–32.
• Berg-Johansen B, Fields AJ, Liebenberg EC, Li A, Lotz JC. Structure-function relationships at the human spinal disc-vertebra interface. J Orthop Res. 2018;36(1):192–201 This study used excised bone-annulus-bone samples and observed a high proportion of structural failures at the CEP-bone interface in the inner annulus region, which coincided with poor integration between the CEP and bone. After initial failure at the CEP-bone interface, the failure surface propagated to the outer annulus region, where secondary failures were observed within the annulus or bone. Firm anchoring between the annulus and bone in the outer annulus region was believed to explain the shift from interface failure to tissue substance failure. These failure mechanisms may be important for clarifying factors that are predictive of herniation risk.
Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471–90.
Locke RC, Peloquin JM, Lemmon EA, Szostek A, Elliott DM, Killian ML. Strain distribution of intact rat rotator cuff tendon-to-bone attachments and attachments with defects. J Biomech Eng. 2017;139(11):111007.
Bailey JF, Liebenberg E, Degmetich S, Lotz JC. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat. 2011;218(3):263–70.
Crock HV, Yoshizawa H. The blood supply of the lumbar vertebral column. Clin Orthop Relat Res. 1976;115:6–21.
Oki S, Matsuda Y, Itoh T, Shibata T, Okumura H, Desaki J. Scanning electron microscopic observations of the vascular structure of vertebral end-plates in rabbits. J Orthop Res. 1994;12(3):447–9.
Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg (Br). 2007;89(9):1135–9.
Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132(Pt 1):39–56.
Fagan A, Moore R, Vernon Roberts B, Blumbergs P, Fraser R. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine. 2003;28(23):2570–6.
van Dieën JH, Weinans H, Toussaint HM. Fractures of the lumbar vertebral endplate in the etiology of low back pain: a hypothesis on the causative role of spinal compression in aspecific low back pain. Med Hypotheses. 1999;53(3):246–52.
Antonacci MD, Mody DR, Heggeness MH. Innervation of the human vertebral body: a histologic study. J Spinal Disord. 1998;11(6):526–31.
Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014;14(3):513–21.
Ohshima H, Tsuji H, Hirano N, Ishihara H, Katoh Y, Yamada H. Water diffusion pathway, swelling pressure, and biomechanical properties of the intervertebral disc during compression load. Spine. 1989;14(11):1234–44.
Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop Relat Res 1977(129):101–14.
Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120(1):113–30.
Nachemson A, Lewin T, Maroudas A, Freeman MA. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand. 1970;41(6):589–607.
Crock HV, Goldwasser M. Anatomic studies of the circulation in the region of the vertebral end-plate in adult Greyhound dogs. Spine. 1984;9(7):702–6.
Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clin Orthop Relat Res. 1982;(170):296–302.
Katz MM, Hargens AR, Garfin SR. Intervertebral disc nutrition. Diffusion versus convection. Clin Orthop Relat Res. 1986;210:243–5.
Ferguson SJ, Ito K, Nolte LP. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 2004;37(2):213–21.
Gullbrand SE, Peterson J, Ahlborn J, Mastropolo R, Fricker A, Roberts TT, et al. Dynamic loading-induced convective transport enhances intervertebral disc nutrition. Spine. 2015;40(15):1158–64.
Ayotte DC, Ito K, Tepic S. Direction-dependent resistance to flow in the endplate of the intervertebral disc: an ex vivo study. J Orthop Res. 2001;19(6):1073–7.
Brinckmann P, Frobin W, Hierholzer E, Horst M. Deformation of the vertebral end-plate under axial loading of the spine. Spine. 1983;8(8):851–6.
Rolander SD, Blair WE. Deformation and fracture of the lumbar vertebral end plate. Orthop Clin North Am. 1975;6(1):75–81.
Yoganandan N, Maiman DJ, Pintar F, Ray G, Myklebust JB, Sances A Jr, et al. Microtrauma in the lumbar spine: a cause of low back pain. Neurosurgery. 1988;23(2):162–8.
Hulme PA, Boyd SK, Ferguson SJ. Regional variation in vertebral bone morphology and its contribution to vertebral fracture strength. Bone. 2007;41(6):946–57.
Langrana NA, Kale SP, Edwards WT, Lee CK, Kopacz KJ. Measurement and analyses of the effects of adjacent end plate curvatures on vertebral stresses. Spine J. 2006;6(3):267–78.
Dudli S, Enns-Bray W, Pauchard Y, Rommeler A, Fields AJ, Ferguson SJ, et al. Larger vertebral endplate concavities cause higher failure load and work at failure under high-rate impact loading of rabbit spinal explants. J Mech Behav Biomed Mater. 2018;80:104–10.
O’Connell GD, Johannessen W, Vresilovic EJ, Elliott DM. Human internal disc strains in axial compression measured noninvasively using magnetic resonance imaging. Spine. 2007;32(25):2860–8.
Fields AJ, Lee GL, Keaveny TM. Mechanisms of initial endplate failure in the human vertebral body. J Biomech. 2010;43:3126–31.
• DeLucca JF, Cortes DH, Jacobs NT, Vresilovic EJ, Duncan RL, Elliott DM. Human cartilage endplate permeability varies with degeneration and intervertebral disc site. J Biomech. 2016;49(4):550–7 This study is important since it observed 50–60% reductions in human CEP permeability with disc degeneration. The authors also reported that collagen fiber reinforcement of the CEP tissue may play important roles in its biomechanical and transport properties.
Veres SP, Robertson PA, Broom ND. ISSLS prize winner: how loading rate influences disc failure mechanics: a microstructural assessment of internal disruption. Spine. 2010;35(21):1897–908.
Rodrigues SA, Wade KR, Thambyah A, Broom ND. Micromechanics of annulus-end plate integration in the intervertebral disc. Spine J. 2012;12(2):143–50.
Tanaka M, Nakahara S, Inoue H. A pathologic study of discs in the elderly. Separation between the cartilaginous endplate and the vertebral body. Spine. 1993;18(11):1456–62.
Rajasekaran S, Bajaj N, Tubaki V, Kanna RM, Shetty AP. ISSLS prize winner: the anatomy of failure in lumbar disc herniation: an in vivo, multimodal, prospective study of 181 subjects. Spine. 2013;38(17):1491–500.
Vernon-Roberts B, Moore RJ, Fraser RD. The natural history of age-related disc degeneration: the pathology and sequelae of tears. Spine. 2007;32(25):2797–804.
Aoki J, Yamamoto I, Kitamura N, Sone T, Itoh H, Torizuka K, et al. End plate of the discovertebral joint: degenerative change in the elderly adult. Radiology. 1987;164(2):411–4.
Bernick S, Cailliet R. Vertebral end-plate changes with aging of human vertebrae. Spine. 1982;7(2):97–102.
Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27(23):2631–44.
Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine. 2005;30(2):167–73.
•• Grant MP, Epure LM, Bokhari R, Roughley P, Antoniou J, Mwale F. Human cartilaginous endplate degeneration is induced by calcium and the extracellular calcium-sensing receptor in the intervertebral disc. Eur Cell Mater. 2016;32:137–51 This study showed that calcium content was significantly higher in CEP tissues adjacent to more severely degenerated discs. Importantly, increasing the concentration of calcium caused reductions in the secretion and accumulation of collagens and proteoglycan in cultured human CEP cells. Calcium supplementation reduced glucose diffusion and induced disc degeneration in a bovine organ culture model.
Bishop PB, Pearce RH. The proteoglycans of the cartilaginous end-plate of the human intervertebral disc change after maturity. J Orthop Res. 1993;11(3):324–31.
Antoniou J, Goudsouzian NM, Heathfield TF, Winterbottom N, Steffen T, Poole AR, et al. The human lumbar endplate. Evidence of changes in biosynthesis and denaturation of the extracellular matrix with growth, maturation, aging, and degeneration. Spine. 1996;21(10):1153–61.
Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13(8):695–701.
Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8(2):101–19.
Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17(6):829–35.
Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12(4):341–9.
Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29(23):2700–9.
Shirazi-Adl A, Taheri M, Urban JP. Analysis of cell viability in intervertebral disc: effect of endplate permeability on cell population. J Biomech. 2010;43(7):1330–6.
Kauppila LI. Atherosclerosis and disc degeneration/low-back pain—a systematic review. Eur J Vasc Endovasc Surg. 2009;37(6):661–70.
Tokuda O, Okada M, Fujita T, Matsunaga N. Correlation between diffusion in lumbar intervertebral disks and lumbar artery status: evaluation with fresh blood imaging technique. J Magn Reson Imaging. 2007;25(1):185–91.
Grunhagen T, Shirazi-Adl A, Fairbank JC, Urban JP. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42(4):465–77 vii.
Holm S, Nachemson A. Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Ups J Med Sci. 1988;93(1):91–9.
Rajasekaran S, Venkatadass K, Naresh Babu J, Ganesh K, Shetty AP. Pharmacological enhancement of disc diffusion and differentiation of healthy, ageing and degenerated discs: results from in-vivo serial post-contrast MRI studies in 365 human lumbar discs. Eur Spine J. 2008;17(5):626–43.
Turgut M, Uysal A, Uslu S, Tavus N, Yurtseven ME. The effects of calcium channel antagonist nimodipine on end-plate vascularity of the degenerated intervertebral disc in rats. J Clin Neurosci. 2003;10(2):219–23.
Montazel JL, Divine M, Lepage E, Kobeiter H, Breil S, Rahmouni A. Normal spinal bone marrow in adults: dynamic gadolinium-enhanced MR imaging. Radiology. 2003;229(3):703–9.
Whitby LEH, Britton CJC. Disorders of the blood: diagnosis, pathology, treatment and technique. New York: Grune & Stratton; 1963.
Drescher W, Li H, Qvesel D, Jensen SD, Flo C, Hansen ES, et al. Vertebral blood flow and bone mineral density during long-term corticosteroid treatment: an experimental study in immature pigs. Spine. 2000;25(23):3021–5.
Wei F, Zhong R, Pan X, Khaleel M, Hammoud A, Zhou Z, Liu S, Sun H, Zhao Y, Zou X, Jiang B, Zhuang W, Chen N, Chen Y Computed tomography guided subendplate injection of pingyangmycin for a novel rabbit model of slowly progressive disc degeneration. Spine J 2015.
Wei F, Zhong R, Wang L, Zhou Z, Pan X, Cui S, et al. Pingyangmycin-induced in vivo lumbar disc degeneration model of rhesus monkeys. Spine (Phila Pa 1976). 2015;40(4):E199–210.
Wang Y, Videman T, Battie MC. Lumbar vertebral endplate lesions: prevalence, classification, and association with age. Spine (Phila Pa 1976). 2012;37(17):1432–9.
Berg-Johansen B, Jain D, Liebenberg EC, Fields AJ, Link TM, O’Neill CW et al. Tidemark avulsions are a predominant form of endplate irregularity. Spine (Phila Pa 1976). 2018.
•• Rade M, Maatta JH, Freidin MB, Airaksinen O, Karppinen J, Williams FMK. Vertebral endplate defect as initiating factor in intervertebral disc degeneration: strong association between endplate defect and disc degeneration in the general population. Spine (Phila Pa 1976). 2018;43(6):412–9 This analysis of MR images from the TwinsUK cohort showed that total endplate damage score assigned to each disc was strongly and independently associated with degeneration severity. The probability of having disc degeneration was significantly increased for individuals with the highest damage scores. These findings are important since they suggest that endplate damage has a causative role in disc degeneration.
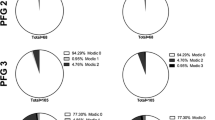
•• Feng Z, Liu Y, Yang G, Battie MC, Wang Y. Lumbar vertebral endplate defects on magnetic resonance images: classification, distribution patterns, and associations with Modic changes and disc degeneration. Spine (Phila Pa 1976). 2017. This study found that the presence and size of endplate defects were associated with increased severity of disc degeneration, including lower disc signal intensity, shorter disc height, and increased disc bulging. Importantly, endplate defects and MCs had similar distribution patterns, and the presence and size of endplate defects were associated with the presence of MCs (OR = 4.29, p < 0.001). These findings suggest that endplate defects may play an etiologic role in bone marrow lesions seen on MRI, i.e., Modic changes.
Wang Y, Videman T, Battie MC. ISSLS prize winner: lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine. 2012;37(17):1490–6.
Lotz JC, Fields AJ, Liebenberg EC. The role of the vertebral end plate in low back pain. Global Spine J. 2013;3(3):153–64.
Brinckmann P, Horst M. The influence of vertebral body fracture, intradiscal injection, and partial discectomy on the radial bulge and height of human lumbar discs. Spine. 1985;10(2):138–45.
Ranu HS. Multipoint determination of pressure-volume curves in human intervertebral discs. Ann Rheum Dis. 1993;52(2):142–6.
Dolan P, Luo J, Pollintine P, Landham PR, Stefanakis M, Adams MA. Intervertebral disc decompression following endplate damage: implications for disc degeneration depend on spinal level and age. Spine (Phila Pa 1976). 2013;38(17):1473–81.
Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25(13):1625–36.
Hsieh AH, Lotz JC. Prolonged spinal loading induces matrix metalloproteinase-2 activation in intervertebral discs. Spine (Phila Pa 1976). 2003;28(16):1781–8.
Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37(3):329–37.
Handa T, Ishihara H, Ohshima H, Osada R, Tsuji H, Obata K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22(10):1085–91.
Ishihara H, McNally DS, Urban JP, Hall AC. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol. 1996;80(3):839–46.
Chan SC, Ferguson SJ, Wuertz K, Gantenbein-Ritter B. Biological response of the intervertebral disc to repetitive short-term cyclic torsion. Spine (Phila Pa 1976). 2011;36(24):2021–30.
Kasra M, Merryman WD, Loveless KN, Goel VK, Martin JD, Buckwalter JA. Frequency response of pig intervertebral disc cells subjected to dynamic hydrostatic pressure. J Orthop Res. 2006;24(10):1967–73.
Holm S, Baranto A, Kaigle Holm A, Ekstrom L, Sward L, Hansson T, et al. Reactive changes in the adolescent porcine spine with disc degeneration due to endplate injury. Vet Comp Orthop Traumatol. 2007;20(1):12–7.
Holm S, Holm AK, Ekstrom L, Karladani A, Hansson T. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004;17(1):64–71.
Dudli S, Haschtmann D, Ferguson SJ. Fracture of the vertebral endplates, but not equienergetic impact load, promotes disc degeneration in vitro. J Orthop Res. 2012;30(5):809–16.
Rajasekaran S, Babu JN, Arun R, Armstrong BR, Shetty AP, Murugan S. ISSLS prize winner: a study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine. 2004;29(23):2654–67.
Bae WC, Statum S, Zhang Z, Yamaguchi T, Wolfson T, Gamst AC, et al. Morphology of the cartilaginous endplates in human intervertebral disks with ultrashort echo time MR imaging. Radiology. 2013;266(2):564–74.
Moon SM, Yoder JH, Wright AC, Smith LJ, Vresilovic EJ, Elliott DM. Evaluation of intervertebral disc cartilaginous endplate structure using magnetic resonance imaging. Eur Spine J. 2013;22(8):1820–8.
Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–9.
de Roos A, Kressel H, Spritzer C, Dalinka M. MR imaging of marrow changes adjacent to end plates in degenerative lumbar disk disease. AJR Am J Roentgenol. 1987;149(3):531–4.
Kerttula L, Luoma K, Vehmas T, Gronblad M, Kaapa E. Modic type I change may predict rapid progressive, deforming disc degeneration: a prospective 1-year follow-up study. Eur Spine J. 2012;21(6):1135–42.
Crock HV. Internal disc disruption. A challenge to disc prolapse fifty years on. Spine. 1986;11(6):650–3.
Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723–34.
Dudli S, Liebenberg E, Magnitsky S, Lu B, Lauricella M, Lotz JC. Modic type 1 change is an autoimmune response that requires a proinflammatory milieu provided by the ‘Modic disc’. Spine J. 2018;18(5):831–44.
•• Dudli S, Sing DC, Hu SS, Berven SH, Burch S, Deviren V et al. ISSLS PRIZE IN BASIC SCIENCE 2017: intervertebral disc/bone marrow cross-talk with Modic changes. Eur Spine J 2017. This study analyzed relative gene expression profiles of matched marrow and disc samples from levels with and without Modic changes. The results showed fibrogenic and pro-inflammatory cross-talk between MC bone marrow and adjacent discs, which provide insight into the pain generator at MC levels and inform novel therapeutic targets for treatment of MC-associated LBP.
Perilli E, Parkinson IH, Truong LH, Chong KC, Fazzalari NL, Osti OL. Modic (endplate) changes in the lumbar spine: bone micro-architecture and remodelling. Eur Spine J. 2015;24(9):1926–34.
Dudli S, Miller S, Demir-Deviren S, Lotz JC. Inflammatory response of disc cells against Propionibacterium acnes depends on the presence of lumbar Modic changes. Eur Spine J. 2018;27(5):1013–20.
Chen Z, Zheng Y, Yuan Y, Jiao Y, Xiao J, Zhou Z, et al. Modic changes and disc degeneration caused by inoculation of Propionibacterium acnes inside intervertebral discs of rabbits: a pilot study. Biomed Res Int. 2016;2016:9612437.
Patel KB, Poplawski MM, Pawha PS, Naidich TP, Tanenbaum LN. Diffusion-weighted MRI “claw sign” improves differentiation of infectious from degenerative modic type 1 signal changes of the spine. AJNR Am J Neuroradiol. 2014;35(8):1647–52.
Mok FP, Samartzis D, Karppinen J, Fong DY, Luk KD, Cheung KM. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J. 2016;16(1):32–41.
Thompson KJ, Dagher AP, Eckel TS, Clark M, Reinig JW. Modic changes on MR images as studied with provocative diskography: clinical relevance—a retrospective study of 2457 disks. Radiology. 2009;250(3):849–55.
Jensen TS, Bendix T, Sorensen JS, Manniche C, Korsholm L, Kjaer P. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet Disord. 2009;10:81.
Jensen TS, Karppinen J, Sorensen JS, Niinimaki J, Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17(11):1407–22.
Kaapa E, Luoma K, Pitkaniemi J, Kerttula L, Gronblad M. Correlation of size and type of modic types 1 and 2 lesions with clinical symptoms: a descriptive study in a subgroup of patients with chronic low back pain on the basis of a university hospital patient sample. Spine (Phila Pa 1976). 2012;37(2):134–9.
Kuisma M, Karppinen J, Niinimaki J, Ojala R, Haapea M, Heliovaara M, et al. Modic changes in endplates of lumbar vertebral bodies: prevalence and association with low back and sciatic pain among middle-aged male workers. Spine (Phila Pa 1976). 2007;32(10):1116–22.
Jarvinen J, Karppinen J, Niinimaki J, Haapea M, Gronblad M, Luoma K, et al. Association between changes in lumbar Modic changes and low back symptoms over a two-year period. BMC Musculoskelet Disord. 2015;16:98.
Brown MF, Hukkanen MV, McCarthy ID, Redfern DR, Batten JJ, Crock HV, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg (Br). 1997;79(1):147–53.
Ohtori S, Inoue G, Ito T, Koshi T, Ozawa T, Doya H, et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and Modic type 1 or type 2 changes on MRI. Spine. 2006;31(9):1026–31.
Luoma K, Vehmas T, Kerttula L, Gronblad M, Rinne E. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur Spine J. 2016;25(9):2873–81.
Farshad-Amacker NA, Hughes A, Herzog RJ, Seifert B, Farshad M. The intervertebral disc, the endplates and the vertebral bone marrow as a unit in the process of degeneration. Eur Radiol. 2017;27(6):2507–20.
Funding
This work was supported by the National Institutes of Health (AJF: AR070198; JCL: R01 AR063705).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Aaron J. Fields reports a grant from the National Institutes of Health (NIH/NIAMS R01 AR070198). Alexander Ballatori and Ellen C. Liebenberg each declare no potential conflict of interest. Jeffrey C. Lotz is co-founder and has shares in Relievant Mesystems.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Intervertebral Disk Degeneration and Regeneration
Rights and permissions
About this article
Cite this article
Fields, A.J., Ballatori, A., Liebenberg, E.C. et al. Contribution of the Endplates to Disc Degeneration. Curr Mol Bio Rep 4, 151–160 (2018). https://doi.org/10.1007/s40610-018-0105-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-018-0105-y