Abstract
Purpose of Review
For this review, we use a One Health approach to examine two globally emerging public health threats related to antifungal drug resistance: triazole-resistant Aspergillus fumigatus infections, which can cause a life-threatening illness in immunocompromised hosts, and antifungal-resistant dermatophytosis, which is an aggressive skin infection caused by dermatophyte molds. We describe the state of current scientific knowledge and outline necessary public health actions to address each issue.
Recent Findings
Recent evidence has identified the agricultural use of triazole fungicides as an important driver of triazole-resistant A. fumigatus infections. Antifungal-resistant dermatophyte infections are likely driven by the inappropriate use of antifungal drugs and antibacterial and corticosteroid creams.
Summary
This review highlights the need for a One Health approach to address emerging antifungal resistant infections, emphasizing judicious antifungal use to preserve available treatments; strengthened laboratory capacity to identify antifungal resistance; and improved human, animal, and environmental surveillance to detect emerging resistance, monitor trends, and evaluate the effectiveness of efforts to decrease spread.
Similar content being viewed by others
Introduction
Fungi are a kingdom of eukaryotic organisms found throughout the environment. Pathogenic fungi cause fungal infections that impose a substantial burden on the health of humans, animals, and plants [1, 2, 3••, 4]. Approximately 1.5–2 million human deaths from fungi occur globally each year [5]. Fungal infections also have a substantial impact on animal species, triggering extinction events and biodiversity loss in wildlife [6]. The estimated annual economic burden of fungal infections in the USA exceeds $7.2 billion in direct costs [7], and 20% of the global annual perennial crop losses are caused by fungal diseases [8].
Antifungal compounds play an essential role in protecting human, animal, and plant health from fungal diseases. In humans and animals, antifungal drugs treat infections such as aspergillosis and histoplasmosis; in plants, antifungal compounds help control a variety of diseases [4]. Unfortunately, the development and approval processes for antifungal drugs are challenging and slow paced. The first antifungals used in the medical field were discovered in the 1950s [8]; triazole agricultural fungicides entered the market in the 1970s, and clinical triazole drugs in the 1980s [9]. Currently, only six classes of drugs are approved to treat fungal infections (just three of which are for invasive fungal disease): polyenes, azoles, echinocandins, allylamines (e.g., terbinafine), the pyrimidine analog flucytosine, and the recently developed triterpenoid, ibrexafungerp [5, 10]. A greater number of antifungal compounds exist to treat plant mycoses compared with the number of compounds licensed to treat human and animal infections [8], highlighting the markedly limited antifungal drug arsenal for human disease.
The emergence of antifungal drug resistance is a major public health concern, spanning the healthcare, veterinary, and agricultural sectors. The One Health public health approach recognizes the interconnectedness of human, animal, plant, and environmental health; this approach increases the likelihood of understanding and successfully addressing the multifactorial causes of fungal diseases and antimicrobial resistance. For this review, we use a One Health lens to examine two emerging public health threats related to antifungal drug resistance: triazole-resistant Aspergillus fumigatus infections and antifungal-resistant dermatophytosis (commonly known as ringworm or tinea). For these public health threats, we describe the state of current scientific knowledge and outline necessary public health actions.
Environmental Origins: the Case of Triazole-Resistant Aspergillus fumigatus
A. fumigatus is a globally distributed saprophytic mold found in soil, compost, and air. An opportunistic pathogen of humans and animals [11, 12, 13], A. fumigatus is the leading cause of invasive aspergillosis (IA), a life-threatening infection in immunocompromised persons responsible for > 14,000 annual hospitalizations in the USA [7]. A. fumigatus also causes bronchopneumonia, sino-nasal aspergillosis, invasive pulmonary aspergillosis, and Aspergillus otitis in animal species such as cats, dogs, birds, and horses [14, 15, 16, 17, 18]. In captive penguins, aspergillosis is the most common cause of death [17]. At-risk persons and animals acquire IA by inhaling fungal spores from the environment [19], though a study in horses suggests other routes of infection, such as mycotic invasion from the gut, are also possible [20]. IA generally affects persons with conditions that weaken the immune system, such as cancer, solid organ or stem cell transplantation, advanced HIV disease, and critical illness; in particular, severe COVID-19 has emerged as an important risk factor for IA [21]. Predisposing factors in animals are similar, with severe immunosuppression associated with fatal infections, and invasive disease causing visceral necrotic and granulomatous inflammation [17, 22]. The global incidence of aspergillosis in humans has been steadily rising, likely because of medical advancements leading to longer lifespans for immunocompromised persons [19], the recent COVID-19 pandemic [23, 24], and greater disease detection [25].
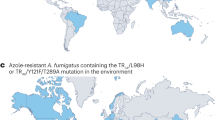
Triazole antifungal drugs for treating IA (i.e., voriconazole, posaconazole, isavuconazole, itraconazole), introduced during the 2000s and 2010s, are the first line treatment for IA [13]. However, triazole-resistant A. fumigatus threatens successful treatment with these lifesaving drugs [26]. A. fumigatus is intrinsically resistant to fluconazole and ketoconazole, further constraining treatment options [27]. Patients with triazole-resistant IA have a mortality rate of approximately 60%, about twice the mortality observed among patients with triazole-susceptible infections [28•]. Triazole-resistant A. fumigatus infections have been documented worldwide; the prevalence of aspergillosis cases involving triazole resistance is 20% in certain European healthcare settings [29]. In the USA, triazole-resistant A. fumigatus has been infrequently reported. However, low case numbers likely reflect a lack of adequate antifungal susceptibility testing capacity and disease surveillance rather than a true absence of disease [30, 31]. Although data are limited, triazole-resistant A. fumigatus has been isolated from animals, including birds and a bottlenose dolphin [32, 33, 34, 35].
A growing body of evidence has identified the agricultural use of triazole fungicides as an important driver of triazole-resistant infections in humans [36••, 37, 38]. Triazole fungicides are applied in various agricultural settings to treat fungal infections, prevent crop loss, and improve agricultural yield [39]. Although A. fumigatus itself is not a plant pathogen, it is present throughout agricultural settings and can develop resistance to medically important triazole drugs when the fungus is incidentally exposed to triazole fungicides. A. fumigatus strains that develop resistance in this manner harbor unique CYP51A gene mutations such as TR34/L98H that can confer pan-triazole-resistant infections in patients [30]. A. fumigatus clinical isolates with triazole-resistant genotypes have been found to have near-identical genotypes as those of environmental isolates that became resistant due to fungicide exposure, confirming that humans can become infected with A. fumigatus strains that originally developed resistance from fungicides used in the environment [36••]. A. fumigatus can also develop triazole resistance within patients who have had repeated exposure to antifungal drug therapy for chronic aspergillosis. Of note, triazole use in US hospitals has generally been in decline [40]. In contrast, US triazole fungicide use quadrupled in the decade from 2006 to 2016 [41].
The global emergence of triazole-resistant A. fumigatus in the setting of increasing use of triazole fungicides poses an alarming public health concern. Emphasis on antifungal stewardship is urgently needed in the human medicine, veterinary, and agricultural sectors to preserve the availability of current antifungal compounds. The judicious use of triazole fungicides is not only an important concern from the human and animal health perspective, but also critical to prevent the emergence of fungicide resistant plant pathogens [42]. In addition to actions and policies that promote antifungal stewardship, improved clinical and environmental surveillance, paired with increased clinical capacity to detect antifungal resistant A. fumigatus, are needed to identify emerging pockets of resistance, monitor trends, and evaluate the impact of interventions aimed at curbing the spread of resistance. Additional research, using a One Health approach, is also needed to evaluate strategies to reduce the impact of triazole fungicide use on promotion of triazole-resistant A. fumigatus in the environment and ultimately in animals and humans.
Easy Access: the Bane and Boon of Creams and Terbinafine
Dermatophytosis, commonly known as ringworm or tinea, is a contagious fungal infection of the skin, hair, and nails, affecting an estimated 20–25% of the global population [43]. Transmission of dermatophyte infections can occur by fomites, by direct contact between humans, or by spread among humans and animals [44]. In veterinary medicine, dermatophytosis is a common superficial fungal infection, contributing to adverse economic outcomes in production animals [45, 46, 47]. Though not generally considered life threatening, dermatophytosis can cause intense discomfort, severe immune reactions, and secondary bacterial infections in certain patient populations, both human and animal [48, 49, 50].
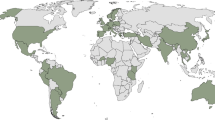
Antifungal drugs provide critical relief for humans and animals with dermatophytosis, but the emergence of infections resistant to terbinafine (the primary treatment for many types of dermatophyte infections) and other antifungal drugs is a growing public health threat. One of the first reported cases of an infection with a terbinafine-resistant Trichophyton rubrum, a species of dermatophyte, occurred in 2003 in a US patient with tinea unguium (dermatophytosis of the nail) [51]. Since then, the global incidence of antifungal resistant dermatophytosis has risen at an alarming pace, affecting both animals and humans [52, 53, 54]. In India, cases of resistant dermatophytosis have reached epidemic proportions [55••]. Trichophyton indotineae (also referred to as Trichophyton mentagrophytes type VIII), a dermatophyte frequently exhibiting resistance to terbinafine and triazoles, is the most commonly isolated dermatophyte, with 76% of isolates from northern Indian regions exhibiting terbinafine resistance [55••]. Infections from this organism can be devastating, persisting for years [55••] and spreading easily among household members [49]. In Europe, reports of difficult-to-treat T. indotineae infections are increasing [53, 54, 56•]. Resistant dermatophyte strains have been identified across the globe [53, 56•, 57], including in the USA and Canada, although the extent of the problem is currently unclear because diagnostic testing, particularly antifungal susceptibility testing for dermatophytes, is rarely performed [58, 60, 61].
The drivers of emerging dermatophyte resistance are still being investigated, but inappropriate use of antifungal drugs (both oral and topical) and powerful corticosteroid creams in human medicine is likely important contributors. Over-the-counter (OTC) antifungal drugs are widely available, potentially allowing patients to self-diagnose and overuse OTC treatments; a recent Indian study found that 81% of dermatophytosis patients reported at-home pharmaceutical treatment before seeking care from a health professional [55••]. Patients reported self-prescribed use of OTC drugs, including oral antifungals and topical creams containing varying combinations of steroids, antifungals, or antibiotics, a practice that can promote antifungal resistance [55••]. However, self-treatment is unlikely to be the sole contributor to dermatophyte resistance. Inaccurate diagnoses and low rates of diagnostic testing performed by clinicians can lead to unnecessary antifungal treatments, which, along with patient noncompliance to treatment guidelines, might contribute to antifungal resistance. Given that up to 50% of antifungal compounds in human medicine might be inappropriately prescribed [62], there is an urgent need for improved antifungal stewardship practices. Likewise, in veterinary medicine, antifungal treatments are often chosen based on financial and specific patient considerations rather than antifungal susceptibility testing results. With recommendations that all cats or dogs presenting with dermatophytosis (most commonly caused by Microsporum canis) receive treatment, the lack of susceptibility testing and zoonotic potential of M. canis is concerning [48]. These considerations underscore the need for antifungal stewardship in both human and animal medicines.
Corticosteroid creams, some of which are highly potent, are easily accessible as OTC drugs but are often not used appropriately. While high-potency OTC corticosteroid creams can help relieve symptoms, these medicines do not treat the underlying fungal infection and can actually exacerbate infections [63, 64]. The resulting localized immune suppression can lead to severe recalcitrant infections and abnormal clinical presentations [64, 65]. Combination corticosteroid-antifungal creams further complicate treatment. When symptom relief from use of these creams occurs, patients might prematurely discontinue use, exposing dermatophytes to inadequate antifungal drug (e.g., terbinafine) concentrations and potentially promoting the development of resistance [64].
Several key actions are needed to address the emergence of resistant dermatophytosis. Educational efforts and policies should focus on improving the appropriate diagnostic testing and treatment of dermatophytosis in humans and animals, with an emphasis on judicious antifungal use to preserve available treatment options. Increased clinician awareness of resistant dermatophytosis and access to antifungal susceptibility testing will be important to curbing the spread of resistance. Patients, too, should be educated on the need for proper adherence to prescribed antifungal therapies and the importance of seeking a clinical diagnosis rather than relying on the empiric use of potentially harmful over-the-counter remedies. Finally, additional research is needed to further characterize the epidemiology of antifungal resistant dermatophyte infections, with a focus on quantifying the overall burden of disease and identifying potential drivers of infection. This research is needed to inform policies aimed at improving antifungal stewardship and curbing the spread of antifungal resistant dermatophyte infections.
Conclusion
The global emergence of triazole-resistant A. fumigatus and antifungal resistant dermatophytosis represents two urgent public health threats, each requiring a One Health approach. The scope of emerging antifungal resistance and its potential impact on society extends beyond the two issues discussed in this report. Incidence is increasing of infections caused by drug resistant molds (e.g., lomentosporiosis, scedosporiosis) [66] and other fungi, including yeasts such as Candida auris [67] and the fungus Sporothrix brasilienses, which can be transmitted from cats to humans [68]. In summary, a cross-sector (human medicine, veterinary medicine, agriculture) emphasis is needed on antifungal stewardship, clinician, industry and public awareness, and increased laboratory capacity to detect and monitor antifungal drug resistance in humans, animals, and the environment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Denning DW, Bromley MJ. Infectious disease How to bolster the antifungal pipeline. Science. 2015;347(6229):1414–6.
Álvarez-Pérez S, García ME, Anega B, Blanco JL. Antifungal resistance in animal medicine: current state and future challenges. In: Gupta A, Pratap Singh N, editors. Fungal diseases in animals: from infections to prevention. Cham: Springer International Publishing; 2021. p. 163–79.
Fisher MC, Alastruey-Izquierdo A, Berman J, et al. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 2022: 1–15. Comprehensive review article highlighting the public health importance of antifungal resistance and outlining the research and risk reduction strategies needed to address this problem.
Jain A, Sarsaiya S, Wu Q, Lu Y, Shi J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered. 2019;10(1):409–24.
Mota Fernandes C, Dasilva D, Haranahalli K, et al. The future of antifungal drug therapy: novel compounds and targets. Antimicrob Agent chemother 2021; 65(2).
Fisher MC, Henk DA, Briggs CJ, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393):186–94.
Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infectious Dis: Official Pub Infectious Diseases Soc Am. 2019;68(11):1791–7.
Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360(6390):739–42.
Chow NA, Muñoz JF, Gade L, et al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 2020; 11(2).
Ghannoum M, Arendrup MC, Chaturvedi VP, et al. Ibrexafungerp: a novel oral triterpenoid antifungal in development for the treatment of Candida auris infections. Antibiotics (Basel) 2020; 9(9).
Melo AM, Stevens DA, Tell LA, Veríssimo C, Sabino R, Xavier MO. Aspergillosis, avian species and the one health perspective: the possible importance of birds in azole resistance. Microorganisms 2020; 8(12).
Sugui JA, Kwon-Chung KJ, Juvvadi PR, Latgé JP, Steinbach WJ. Aspergillus fumigatus and related species. Cold Spring Harb Perspect Med. 2014;5(2):a019786.
Patterson TF, Thompson GR III, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–60.
Adamama-Moraitou KK, Pardali D, Day MJ, et al. Aspergillus fumigatus Bronchopneumonia in a Hellenic Shepherd Dog. J Am Anim Hosp Assoc. 2011;47(2):e13–8.
Day MJ. Canine sino-nasal aspergillosis: parallels with human disease. Medical mycology. 2009;47(Supplement_1):S315–23.
Goodale EC, Outerbridge CA, White SD. Aspergillus otitis in small animals – a retrospective study of 17 cases. Vet Dermatol. 2016;27(1):3-e2.
Stidworthy MF, Denk D. Chapter 27 - Sphenisciformes, Gaviiformes, Podicipediformes, Procellariiformes, and Pelecaniformes. In: Terio KA, McAloose D, editors. Leger JS. Pathology of Wildlife and Zoo Animals: Academic Press; 2018. p. 653–86.
Sellon DC, Kohn C. Chapter 52 - Aspergillosis. In: Sellon DC, Long MT, editors. Equine infectious diseases (Second Edition). St. Louis: W.B. Saunders; 2014. p. 421- 33.e4.
Cadena J, Thompson GR 3rd, Patterson TF. Aspergillosis: epidemiology, diagnosis, and treatment. Infect Dis Clin North Am. 2021;35(2):415–34.
Slocombe RF, Slauson DO. Invasive pulmonary aspergillosis of horses: an association with acute enteritis. Vet Pathol. 1988;25(4):277–81.
Baddley JW, Thompson GR III, Chen SCA, et al. Coronavirus disease 2019–associated invasive fungal infection. Open Forum Infectious Diseases. 2021;8(12):510.
Redig P. Fungal Diseases. In: Samour J. Avian medicine (Third Edition): Mosby, 2016:434–521.
Gold JAW, Ahmad FB, Cisewski JA, et al. Increased deaths from fungal infections during the COVID-19 pandemic—National Vital Statistics System, United States, January 2020–December 2021. Clinical Infectious Diseases 2022.
Armstrong-James D, Youngs J, Bicanic T, et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur Respir J 2020; 56(4).
Gold JAW, Revis A, Thomas S, et al. Clinical characteristics, healthcare utilization, and outcomes among patients in a pilot surveillance system for invasive mold disease—Georgia, United States, 2017–2019. Open forum infectious diseases 2022.
Verweij PE, Chowdhary A, Melchers WJG, Meis JF. Azole Resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2015;62(3):362–8.
Leonardelli F, Macedo D, Dudiuk C, Cabeza MS, Gamarra S, Garcia-Effron G. Aspergillus fumigatus intrinsic fluconazole resistance is due to the naturally occurring T301I substitution in Cyp51Ap. Antimicrob Agents Chemother. 2016;60(9):5420–6.
Lestrade PP, Bentvelsen RG, Schauwvlieghe AFAD, et al. Voriconazole resistance and mortality in invasive aspergillosis: a multicenter retrospective cohort study. Clinical Infectious Diseases. 2018;68(9):1463–71. Multicenter retrospective cohort study demonstrating higher mortality in invasive aspergillosis patients with azole-resistant infections versus those with azole-sensitive infections.
Rybak JM, Fortwendel JR, Rogers PD. Emerging threat of triazole-resistant Aspergillus fumigatus. J Antimicrob Chemother. 2019;74(4):835–42.
Beer KD, Farnon EC, Jain S, et al. Multidrug-resistant Aspergillus fumigatus carrying mutations linked to environmental fungicide exposure - three states, 2010–2017. MMWR Morb Mortal Wkly Rep. 2018;67(38):1064–7.
Bradley K, Le-Mahajan A, Morris B, Peritz T, Chiller T, Forsberg K, et al. Fatal fungicide-associated triazole-resistant Aspergillus fumigatus infection, Pennsylvania, USA. Emerg Infect Dis. 2022;28(9):1904–5.
Barber AE, Scheufen S, Walther G, Kurzai O, Schmidt V. Low rate of azole resistance in cases of avian aspergillosis in Germany. Med Mycol. 2020;58(8):1187–90.
Beernaert LA, Pasmans F, Waeyenberghe LV, et al. Avian Aspergillus fumigatus Strains resistant to both Itraconazole and Voriconazole. Antimicrob Agents Chemother. 2009;53(5):2199–201.
Bunskoek PE, Seyedmousavi S, Gans SJM, et al. Successful treatment of azole-resistant invasive aspergillosis in a bottlenose dolphin with high-dose posaconazole. Med Mycol Case Rep. 2017;16:16–9.
Ziołkowska G, Tokarzewski S, Nowakiewicz A. Drug resistance of Aspergillus fumigatus strains isolated from flocks of domestic geese in Poland. Poult Sci. 2014;93(5):1106–12.
Rhodes J, Abdolrasouli A, Dunne K, et al. Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat Microbiol. 2022;7(5):663–74. Recent study confirming that humans can acquire drug-resistant A. fumigatus infections from the environment.
Kang SE, Sumabat LG, Melie T, Mangum B, Momany M, Brewer MT. Evidence for the agricultural origin of resistance to multiple antimicrobials in Aspergillus fumigatus, a fungal pathogen of humans. G3 Genes|Genomes|Genetics 2021.
Gonzalez-Jimenez I, Garcia-Rubio R, Monzon S, Lucio J, Cuesta I, Mellado E. Multiresistance to nonazole fungicides in Aspergillus fumigatus TR(34)/L98H azole-resistant isolates. Antimicrob Agents Chemother. 2021;65(9):e0064221.
Jørgensen LN, Heick TM. Azole Use in agriculture, horticulture, and wood preservation - is it indispensable? Front Cell Infect Microbiol. 2021;11:730297.
Vallabhaneni S, Baggs J, Tsay S, Srinivasan AR, Jernigan JA, Jackson BR. Trends in antifungal use in US hospitals, 2006–12. J Antimicrob Chemother. 2018;73(10):2867–75.
Toda M, Beer KD, Kuivila KM, Chiller TM, Jackson BR. Trends in agricultural triazole fungicide use in the United States, 1992–2016 and possible implications for antifungal-resistant fungi in human disease. Environ Health Perspect. 2021;129(5):55001.
Price CL, Parker JE, Warrilow AG, Kelly DE, Kelly SL. Azole fungicides - understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag Sci. 2015;71(8):1054–8.
Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15.
Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;67(1):101–8.
Begum J, Kumar R. Prevalence of dermatophytosis in animals and antifungal susceptibility testing of isolated Trichophyton and Microsporum species. Trop Anim Health Prod. 2020;53(1):3.
Bond R. Superficial veterinary mycoses. Clin Dermatol. 2010;28(2):226–36.
Frymus T, Gruffydd-Jones T, Pennisi MG, et al. Dermatophytosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2013;15(7):598–604.
Miller WH, Griffin CE, Campbell KL. Muller and Kirk’s small animal dermatology. Chapter 5: Fungal and Algal Skin Diseases: Elsevier Health Sciences, 2012.
Dogra S, Narang T. Emerging atypical and unusual presentations of dermatophytosis in India. Clinical Dermatology Review. 2017;1(3):12–8.
Urban K, Chu S, Scheufele C, et al. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: a cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int. 2021;2:22–7.
Mukherjee PK, Leidich SD, Isham N, Leitner I, Ryder NS, Ghannoum MA. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother. 2003;47(1):82–6.
Hsiao Y-H, Chen C, Han HS, Kano R. The first report of terbinafine resistance Microsporum canis from a cat. J Vet Med Sci. 2018;80(6):898–900.
Nenoff P, Verma SB, Ebert A, et al. Spread of terbinafine-resistant Trichophyton mentagrophytes type VIII (India) in Germany–“The Tip of the Iceberg?” Journal of Fungi. 2020;6(4):207.
Dellière S, Joannard B, Benderdouche M, et al. Emergence of difficult-to-treat Tinea Corporis caused by Trichophyton mentagrophytes complex isolates, Paris. France Emerging Infectious Diseases. 2022;28(1):224–8.
Ebert A, Monod M, Salamin K, et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: a multicentre study. Mycoses. 2020;63(7):717–28. Large, multicenter study in India demonstrating high prevalence of antifungal resistant dermatophyte infections throughout the nation.
Jabet A, Brun S, Normand AC, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae France. Emerging Infectious Diseases. 2022;28(1):229–33. Article highlighting the global spread of infections caused by T. indotineae.
Hiruma J, Kitagawa H, Noguchi H, et al. Terbinafine-resistant strain of Trichophyton interdigitale strain isolated from a tinea pedis patient. J Dermatol. 2019;46(4):351–3.
Gu D, Hatch M, Ghannoum M, Elewski BE. Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat. JAAD Case Rep. 2020;6(11):1153–5.
Edriss MT, Parker JJ, Pritchett EN. Response to Gu et al’s “Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat”. JAAD Case Reports 2022.
Posso-De Los Rios CJ, Tadros E, Summerbell RC, Scott JA. Terbinafine resistant trichophyton indotineae isolated in patients with superficial dermatophyte infection in Canadian patients. J Cutan Med Surg 2022: 12034754221077891.
Gold JAW, Wu K, Jackson BR, Benedict K. Opportunities to improve guideline adherence for the diagnosis and treatment of onychomycosis: analysis of commercial insurance claims data, United States [published online ahead of print, 2022 Jul 7]. J Am Acad Dermatol. 2022;S0190–9622(22)02252–6.
Johnson MD, Lewis RE, Dodds Ashley ES, et al. Core recommendations for antifungal stewardship: a statement of the Mycoses Study Group Education and Research Consortium. Journal Infectious Diseases. 2020;222(Supplement_3):S175–98.
Verma SB. Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18(7):718–9.
Bishnoi A, Vinay K, Dogra S. Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18(3):250–1.
Verma SB, Vasani R. Male genital dermatophytosis - clinical features and the effects of the misuse of topical steroids and steroid combinations - an alarming problem in India. Mycoses. 2016;59(10):606–14.
Hoenigl M, Salmanton-García J, Walsh TJ, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infectious Diseases. 2021;21(8):e246-e57.67.
Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10):e1008921.
Gremião ID, Miranda LH, Reis EG, Rodrigues AM, Pereira SA. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog. 2017;13(1):e1006077.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Mycology
Rights and permissions
About this article
Cite this article
Langfeldt, A., Gold, J.A.W. & Chiller, T. Emerging Fungal Infections: from the Fields to the Clinic, Resistant Aspergillus fumigatus and Dermatophyte Species: a One Health Perspective on an Urgent Public Health Problem. Curr Clin Micro Rpt 9, 46–51 (2022). https://doi.org/10.1007/s40588-022-00181-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-022-00181-3