Abstract
Individuals differ in susceptibility to mercury neurotoxicity, in part, due to underlying genetic differences. This review aims to evaluate the state-of-the-art of the effect of (1) genetics on mercury toxicokinetics and (2) gene-mercury interactions on neurodevelopment and neurotoxicity. We conducted a PubMed search in September 2014 and retrieved 14 studies on the influence of genetics on mercury toxicokinetics and ten on neurological effects of gene-mercury interactions. Genes frequently studied for their influence on mercury toxicokinetics were mainly related to the metabolism of glutathione, but the results were contradictory for most of the genes. The gene-mercury interactions on child neurodevelopment and adult neurotoxicity reported were too few to draw any definite conclusion. So far, candidate gene approaches have not identified any major gene/s modifying the kinetics or toxicity of mercury, suggesting that these might be polygenic traits. More research is highly warranted to clarify if there are vulnerable subgroups to mercury neurotoxicity in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury is a ubiquitous environmental toxicant that derives both from natural sources and human activity. It can exist under several forms: elemental, inorganic, and organic; where the organic form, in particular methylmercury (MeHg), is the greatest cause of concern due to its irreversible neurotoxic effects early in life [1, 2]. Most MeHg originates in aquatic systems where it is formed from the inorganic form through the action of bacteria present in water and sediments [3]. Consumption of marine species is currently considered the major source of human exposure, and predatory fish such as swordfish and shark have the highest concentrations of MeHg [4, 5]. For inorganic (IHg) or elemental mercury, the main exposure sources are from some occupational activities and dental amalgams [6]. The occupational exposure to IHg has been well documented in gold miners [7], chloralkali workers [8], and dental professionals [9].
MeHg crosses the placenta and blood–brain barrier, and may affect critical neurodevelopmental processes including cell proliferation, migration, differentiation, synaptogenesis, myelination, and apoptosis [10]. Children and fetuses are especially vulnerable, since their brain, immune system, and detoxification mechanisms are being developed [11, 12]. Epidemiological studies have been conducted in fish-eating populations in order to evaluate the consequences of prenatal or early life exposure to MeHg on the cognitive development; however, the results have been conflicting [13–16]. A few cross-sectional studies of low-level MeHg exposure in adults and neurologic effects have been performed, and the results have been contradictory as well [15]. Neurotoxicity associated with exposure to IHg has mainly been studied in highly exposed populations. Long-term exposure to IHg was associated with tremor and abnormal motor function in chloralkali workers [8], as well as decreased peripheral nerve function in dental professionals [9], but only some of the IHg-exposed workers developed neurotoxicity.
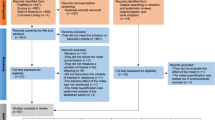
The lack of homogeneity for the effect of MeHg and IHg in different study populations or between individuals could be explained by several biological and non-biological factors affecting both exposure and toxicity to mercury (e.g., for MeHg toxicity, the content of beneficial nutrients from fish such as selenium or fatty acids, or co-exposure to other contaminants probably matters) [17]. A further component is the underlying genetic background that might modify mercury uptake, biotransformation, distribution, and elimination, and in turn, determines the active dose [18] (Fig. 1a).
a Scheme of how genetics can modify both mercury toxicokinetics and mercury-related neurotoxicity. b Mercury (IHg and MeHg) metabolism interacts with the glutathione (GSH) system pathway. Differences in mercury body burden among populations have been related to polymorphisms in genes coding for enzymes involved in glutathione (GSH) synthesis and metabolism: glutamyl-cysteine-ligase (GCL; catalytic subunit: GCLC, modifier subunit: GCLM), GSH synthetase (GS), and glutathione-S-transferase (GST), and also in genes coding for ABC transporters
An expert committee of the U.S. National Research Council (2000) concluded that 3 % of neurodevelopmental disorders may be a direct result of exposure to environmental toxins, and up to 25 % would be the result of the interaction between exposure to environmental toxicants and individual susceptibility genes [19]. Recently, there has been an increase in the identification of genetic variants involved in cognitive disorders [20, 21], which might also play a role in the relationship between mercury exposure and neurodevelopment and neurotoxicity.
The purpose of this review was to evaluate the state-of-the-art of the effect of (1) genetics on mercury (both IHg and MeHg) toxicokinetics and (2) gene-mercury (both IHg and MeHg) interactions on child neurodevelopment and adult neurotoxicity.
Methods
Sources of Information
We used the electronic data source PubMed (National Library of Medicine, Bethesda, MD, USA: http://www.ncbi.nlm.nih.gov/pubmed) to conduct two different bibliographic searches of published human studies on gene-mercury interactions for (1) toxicokinetics of mercury in child and adult populations and (2) neurological effects in child and adult populations.
Search Strategy
We selected relevant articles with an end date of 31st of August 2014. We used the following key terms or combinations of them, for the literature search:
-
1.
Gene-mercury interactions for mercury toxicokinetics: “gene*”,“*mercury”
-
2.
Gene-mercury interactions for neurodevelopment: “gene*”, “*mercury”, “neurodevelopment”, “cognitive”, “behaviour”, “brain”, “nervous system”, “neurotoxicity”.
Selection Criteria and Identification of Relevant Articles
Epidemiological studies of pre- and/or postnatal measures of genes and mercury interaction in association with (1) mercury toxicokinetics or/and (2) adverse effects on child neurodevelopment or/and adult neurotoxicity were selected and reviewed. The articles included these following criteria: (1) original article; (2) observational epidemiological study; (3) assessment of the exposure to IHg or MeHg in humans; (4) evaluation of neurodevelopment during childhood or/and neurotoxicity during adulthood; and (d) languages such as English, French, Spanish, Portuguese, or Italian. In addition to the search in PubMed, we searched in the references of the selected articles.
We referred to the associations or interactions as statistically significant when p values were less than 0.05. The complete name of all genes, gene families, and their potential role in mercury toxicokinetics and neurotoxicity can be found in Tables 1 and 2.
Results
Literature Review
Twenty-three articles met the inclusion criteria. Among them, 14 studied the influence of gene interactions on mercury toxicokinetics (seven on MeHg, two on IHg, and five on both), four evaluated the effect on neurodevelopment among children, and six evaluated the neurotoxicity among adults. There was one article that studied both the mercury toxicokinetics and neurotoxicity. The characteristics of the reviewed studies, including exposure levels, and main results are summarized in Tables 3 and 4. All selected studies were written in English.
Genes or Gene Families Examined in the Studies
Most articles studied the role of genes related to the small tripeptide glutathione (GSH), as the main mechanism of mercury elimination (both for inorganic and organic Hg) is through conjugation with [22] (Fig. 1b, Tables 1 and 2). Other systems of relevance for MeHg and IHg elimination and in turn toxicity were metallothionein (MTs) and genes coding for transporters (ABC, OATs, and LATs). Further, some genes with key function in the nervous system were evaluated for mercury neurotoxicity.
Genetic Effects on Mercury Toxicokinetics
Fourteen studies evaluated the effect of the genetic background on mercury concentrations in different human populations (Table 3). We grouped the manuscripts based on study population (newborn/ adults and general population/highly exposed population). Urine is the main excretion pathway for IHg [23], and total mercury concentrations (THg) in urine are common measure for exposure to IHg. Almost all MeHg ingested from fish is absorbed into the bloodstream, and in blood, 90 % of MeHg is bound to erythrocytes; the main excretion routes are through bile and hair [24]. Hence, blood/erythrocytes and hair THg are common biomarkers of exposure to MeHg.
Methylmercury Toxicokinetics in Children
Two studies were conducted among newborns (Table 3). The association between maternal fish consumption in Mediterranean countries and cord blood THg was analysed in relation to the genotype of four genes encoding members of the superfamily of ABC transporters [25•]. ABC transporters are found in the blood–brain barrier, placenta, liver, gut, and kidney and they could potentially participate in the cellular export of GSH-conjugated mercury complexes in humans [26, 27] (Tables 1 and 2). Significant differences in THg were found between carriers of different genotypes of ABCB1 and ABCC1, and also significant interactions were found for maternal fish intake and ABCB1, ABCC1, and ABCC2. For a doubling in fish intake of the mothers, children with the rs2032582 GG genotype accumulated 35 % more THg than children with TT. In a second study, the association between blood THg in Korean mothers and newborns and deletions in the glutathione S-transferases GSTM1 and GSTT1 were evaluated, but no significant genetic differences were found [28].
Mercury Toxicokinetics in Adults
Twelve studies evaluated the influence of different polymorphisms on mercury concentrations in adult populations (Table 3). Among them, five studies were conducted on individuals from the general population and seven on individuals highly exposed to mercury (five with occupational exposure to IHg and two where individuals with high fish consumption were selected, i.e., individuals mainly exposed to MeHg).
General Population
-
a.
Inorganic mercury
Two studies analyzed urinary THg [29, 30] in medicine students in Austria and evaluated mercury toxicokinetics as a function of GSH- and MT-related genes. MTs are detoxification proteins that bind certain metals including mercury [31]. No significant differences in THg were found in relation to the different SNPs evaluated.
-
b.
Methylmercury
THg was also analysed in hair and blood samples in the two studies described above on students from Austria [29, 30] and in erythrocyte samples in two studies conducted on sub-groups from the same population in northern Sweden [32, 33]. In the fifth study, speciation for MeHg was also performed in erythrocyte samples from the same Swedish population [34].
Here, THg and MeHg in blood and hair were analysed as a function of the promoter SNPs GCLC rs17883901 and GCLM rs41303970 and three of them (the three Swedish studies) observed higher THg and MeHg in erythrocytes among carriers of the variant allele (T in both genes) [32–34]. Also, two non-synonymous SNPs in GSTP1 (rs1695 and rs1138272) were frequently studied. In Austria and Sweden [34, 30], it was found that carriers of variant alleles for both GSTP1 variants had higher blood THg and MeHg in erythrocytes, respectively. Additionally, rs1695 showed a synergistic effect on hair THg with both GCLC rs17883901 and GCLM rs41303970, compared to individual analysis of each SNP [30]. Conversely, in a Swedish population selected for higher fish intake [32], an effect in the opposite direction was found; individuals with the variant allele Val had lower THg in erythrocytes, and the same authors [33] did not find any significant effect related to this gene.
The influences of the deletion alleles of GSTM1 and GSTT1 were also analyzed. Two studies from the same population in Austria [29, 30] found that individuals with homozygous deletions for both genes had higher blood THg, but in a Swedish population [34] there was no significant effect of these genotypes on THg in erythrocytes.
Populations Highly Exposed to Mercury
-
a.
Inorganic mercury
Five studies evaluated genetic interactions on mercury toxicokinetics among individuals occupationally exposed to IHg [35, 36•, 37–39] (Table 3). Polymorphisms in transporter genes, such OATs, LATs, and ABCs, were studied in relation to urinary THg in gold miners and controls from Africa and Asia [36•]. Significant associations were found as a function of ABCC2, OAT3, and LAT1 genotypes. Urinary THg were higher among ABCC2 rs1885301 and rs717620 A-allele carriers and ABCC2 rs2273697 G-allele carriers. The LAT1 rs33916661 GG genotype was associated with higher THg in all populations, and OAT1 rs4149170 and OAT3 rs4149182 were associated with THg mainly in the Tanzanian study groups. Urinary THg as a function of GCLC, GCLM and GST’s polymorphisms were evaluated in gold miners in Ecuador [38]. Here, GCLM rs41303970 T-allele carriers had statistically significant higher THg, but authors did not find significant differences as a function of GCLC rs17883901, GSTA1-52, GSTM1 or GSTT1 deletions, GSTP1 rs1695, or GSTP1 rs1138272 genotypes. In Ecuadorian gold miners, the presence of T allele in GCLM rs41303970 was related to increased blood THg [35].
Two studies evaluated the influence of polymorphisms in MTs, GCLM, GCLC, GSTs, and SEPP1 on urinary THg among American dental professionals [37, 39]. Subjects with the MT1M rs2270837 AA genotype or the MT2A rs10636 CC genotype had lower urinary THg than did those with the MT1M or MT2A GG genotypes. Further, in the same study population [37], it was found that the deletion genotype of GSTT1 and the allele T of SEPP1 rs7579 were associated with lower urinary THg.
-
b.
Methylmercury
Two studies conducted in a high fish-eating population from the Brazilian Amazonas showed that subjects with homozygous deletion of GSTM1 had higher blood and hair THg than subjects with the GSTM1 genotype, and further, that individuals with GCLM rs41303970 TT genotype had lower THg [40•, 41].
The influence of some GSH- and MTs-related genes was studied in relation to THg in hair of dental professionals [37, 39]. Subjects with MT1A rs8052394 GA and GG genotypes or the MT1M rs9936741 TT genotype had lower hair THg than did subjects with MT1A AA or MT1M TC and CC genotypes, respectively [39]. Furthermore, it was found that Val-alleles of GSTP1 rs1695 and rs1138272 were associated with decreased hair THg [37].
Effect of Gene-Mercury Interactions on Neurodevelopment and Neurotoxicity
Effect of Gene-Mercury Interactions on Neurodevelopment in Children
Four studies evaluated the effect of gene-mercury interactions on neurodevelopment among populations <18 years (Table 4).
Three of them were birth cohort studies and evaluated prenatal exposure to MeHg by THg in cord blood/tissue samples [42•, 43, 44]. Two of these studies [43, 44] were conducted on the same population in Taiwan and evaluated the interaction between THg in cord blood and the APOE gene on neurodevelopment in 2-year-old children. The authors found that children carrying the ε4 allele showed impaired scores for the whole test, as well as for parts related to cognition, and social domain, the interaction between APOE and THg concentrations was significant for these neurodevelopmental domains [43]. In a further study, mercury-related impairment in different behavioral domains (total problems, internalizing, externalizing, emotionally reactive, anxious/depressed, and aggressive behavior) was observed only among children carrying the ε4 allele [44].
Another birth cohort study (ALSPAC cohort) from UK evaluated the interaction between prenatal exposure to THg in cord tissue and 247 SNPs within 66 genes in 8-year-old children [42•]. Children with AA genotype of TF rs3811647 or BDNF rs2049046 showed negative and statistically significant associations between THg and performance scores. The p values for the interaction genotype*THg were marginally significant for both genes. Children with PON1 rs662 TT genotype showed a positive and statistically significant association between THg and total IQ scores.
Children in the Casa Pia Children’s Amalgam Clinical Trial study in Portugal were genotyped for 27 SNPs in 13 genes, and postnatal exposure to IHg was measured as THg in urine samples taken annually from the children between 2 and 7 years of age [45]. The relationship between THg and neurodevelopmental domains as a function of different genotypes was evaluated in 2- and 7-year-old boys and girls. Several statistically significant gene-mercury interactions were found on different domains. Boys’ variant alleles carriers for CPOX rs1729995, MTM rs2270837, MT2A rs10636, TDO2 rs3755907, COMT rs4680, COMT rs4633, COMT rs6269, GRIN2A rs727605, GRIN2B rs7301328, BDNF rs6265, GSTT1 deletion polymorphism, SLC6A4 insertion/deletion, KIBRA rs17070145, or APOE ε4 obtained mercury-related impaired scores in different neurodevelopmental domains. The associations between THg and the tests scores were positive in girls’ variant allele carriers for TDO2 rs3755907, COMT rs4818, COMT rs6269, GRIN2B rs7301328, and APOE ε4. For more details see Table 4.
Effect of Gene-Mercury Interactions on Neurotoxicity in Adults
Six studies evaluated gene-mercury interactions for neurotoxicity in adults with occupational exposure to IHg. In gold miners from Ecuador, the interaction of urinary THg with GST-related genes was evaluated in relation to tremor and performance in coordination tests but no significant associations were found [38]. The other five studies were conducted on the same population of American dental professionals, and the interaction of urinary THg with SLC6A4, COMT, CPOX4, and BDNF was evaluated with several neurological outcomes [46–50, 15]. Some significant additive interactions were observed; carriers of SLC6A4 variant allele showed a negative effect on cognitive flexibility, manual coordination, attention, working memory, manual coordination, and some mood states [48]. Some mood states were also associated with IHg and COMT rs4680 [50], and detrimental effects on visuomotor domain were observed among CPOX4 rs1131857 variant allele carriers [47]. The variant allele in BDNF rs6265 was associated with impaired coordination and some mood states [46, 49].
Discussion
The amount of literature about genetic influences on mercury (both IHg and MeHg) toxicokinetics is still limited and focused mainly on adult populations (general, occupationally exposed, or high fish consumers). Fetuses and children are the population sub-groups that are more vulnerable to the neurotoxic effects of MeHg, and the bibliography on them is really sparse.
Due to the central role of the GSH molecule in mercury metabolism, the GSH-related genes were the most frequently studied, both for Mehg and IHg, but the results were, apart from one gene (GCLM), contradictory, the same alleles were associated to higher and lower mercury concentrations in different populations. Information regarding the effect of gene-mercury interactions on neurodevelopment and neurotoxicity is too scarce to draw a definite conclusion, but one gene (APOE) with consistent results in different studies on children was identified. Neurotoxicity associated with IHg-gene interactions in adults has been assessed in few cross-sectional studies on occupationally exposed populations, and BDNF might be a candidate gene to follow-up on in future studies.
If we address the studies in more detail, GCLM rs41303970 seems so far to be the most promising genetic polymorphism for mercury toxicokinetics among the GSH-related genes studied. In two studies, the T allele for GCLM rs41303970 was associated with higher erythrocytes THg in general population from Sweden [32, 33], and higher urinary THg in gold miners from Ecuador [35, 38]. However, individuals from a high fishing eating population from Brazil with TT genotype had lower hair and plasma THg concentrations [40•]. For the other GSH-related genes, the results are more conflicting. The variant alleles of GSTP1 rs1695 and rs1138272 were associated with higher THg and MeHg in general populations from Austria [30] and Sweden [34], and with lower THg in Sweden [32] and dental professionals from USA [37]. The T allele of GCLC rs17883901 was associated with increased THg in Sweden [34] and Brazil [41], and the GSTM1 deletion genotype was associated with increased THg in general population from Austria [29, 30] and Brazil [40•, 41]. However, the number of studies without a statistically significant association is sizeable for both GCLC and GSTM1 genes (n = 6 and 5, respectively). GSH system is developed for protection against many different endogenous and xenobiotic substances, and polymorphisms resulting in less efficient proteins are probably compensated by other proteins in the same pathway.
The systems coding for MTs and transporter proteins in relationship to mercury concentrations have been less frequently studied, but some significant results were observed. Wang et al. (2012) studied mercury concentrations as a function of some MTs polymorphisms among dental professionals [39], and they found some statistical significant associations regarding the MTI and MT2 isoforms in relation to hair and urinary THg. Significant differences in THg as a function of MT4 rs11643815 have been observed in students from Austria [30]. Among potential mercury-transporting proteins, ABCB1 rs2032582, ABCC1 rs11075290, and ABCC2 rs2273697 were associated with THg accumulation in the fetus from maternal fish intake [25•], and ABCC2 rs1885301, rs717620, and rs2273697 were related to IHg metabolism in a gold mining population [36•]. Considering that these studies were performed on rather large populations, ABCC2 rs2273697 could be a candidate SNP to follow in other populations exposed to MeHg or IHg. Moreover, the evidence for an important role of ABCC1 in MeHg accumulation and neurotoxicity in fruit flies suggests that this is a transporter to be further studied in relation to MeHg exposure [51].
On the basis of available literature, we can postulate several reasons for the discrepancy between studies. First, differences could depend on the compound of mercury analyzed (IHg or MeHg), the matrix where mercury was analyzed (blood, hair, or urine is related to different kinetics of mercury in the human body) and mercury concentrations (different genetic response depending on dose). Secondly, sample size is an important factor that must be considered carefully in the assessment of interactions. Thirdly, the xenobiotic defence (GSH related, MTs, and transporters) encoding genes are highly polymorphic and other functional SNPs in linkage disequilibrium with the SNPs and with different allele frequencies in different populations analysed might explain some inconsistencies between studies. Still, there were very few attempts to consider the effects of haplotypes of gene-gene interactions in the studies evaluated, mainly due to low power of the studies. Fourthly, publication bias may be another factor since studies with statistically significant findings are more likely to be published than are studies with null results.
In the few studies on the effect of gene-mercury interaction on neurodevelopment, one polymorphism in APOE appeared to modify the mercury toxicity. APOE gene plays an important role in lipid-transported proteins and it is known to be a crucial mediating factor in neuronal repair. Three APOE alleles have been identified: ε2 has two cysteine amino-acids in its structure, ε3 has one cysteine and one arginine, and ε4 has two arginine amino-acids and no cysteine. Cysteine, with its sulfhydryl (−SH) bonds, is potentially able to bind to, and remove metals from nervous tissues, whereas arginine, lacking the -SH bonds, would be unable to do this and more toxicity might be expected [52]. Two studies conducted on the same study population from Taiwan found that children who were allele ε4 carriers obtained the worst scores in neuropsychological tests [43, 44]. Another study conducted in Portugal observed a statistically significant interaction between urinary THg, APOE, and sex; boys’ carriers of APOE allele ε4 obtained impaired scores in learning and memory and girls obtained improved scores in attention and motor domains [45]. In fact, these authors found other statistically significant gene-mercury-sex interactions, which, overall, were negative among boys and positive among girls. This fact suggests a sexual different role in the influence of gene-mercury interactions on neuropsychological development, but this should be confirmed in further studies.
Another interesting gene for future studies is BDNF. BDNF is a protein that regulates neuronal growth and differentiation in the nervous system. A polymorphism in BDNF gene (rs6265) which substitutes methionine (Met) to valine (Val) at amino acid position 66 has been identified and associated with the processing and secretion of BDNF protein, Met carriers had reduced hippocampal activity in comparison with Val homozygotes [53]. Statistically significant interactions were observed regarding polymorphisms in BDNF in both children in the UK [42•] and in Portugal [45]. THg was associated with impaired performance scores among BDNF rs2049046 AA genotype carriers and also with impaired learning and memory domains in the Portugese children with the variant allele for BDNF rs6265. Further, BDNF rs6265 Met allele was found to have a role in the vulnerability to neurological damage among dental professionals exposed to IHg [46, 49].
Regarding gene-mercury interactions on child neurodevelopment and on adult neurotoxicity, there are some limitations that should be taken into account. First, there could be heterogeneity in the evaluation of the phenotypes. Children are evaluated at different ages when vast psychological changes occur and using a great variability of neuropsychological tests, which make the comparison between studies difficult and the performance of a meta-analysis unfeasible. Also, the number of polymorphisms evaluated in some of the studies is sizeable, thus the probability of spurious associations is high. Other limitations, previously commented for studies on mercury toxicokinetics, are the limited sample size and publication bias.
Conclusions
The number of studies about the genetics influence on mercury toxicokinetics is still limited and most of them were focused mainly on adult populations. Moreover, there are very scarce evidences for the effect of gene-mercury interactions on child neuropsychological development and adult neurotoxicity to draw any definite conclusion and further studies are highly warranted. Differences in the study population (age for neurodevelopment testing), mercury exposure assessment (concentrations, biological matrices, timing of exposure, and compounds of mercury), small sample sizes, and the neuropsychological tests used for the evaluation make the comparison between studies difficult and could be the cause of the contradictory results observed. More investigations about this topic are required. Additionally, environmental epidemiologic studies should be properly designed to study the effect of genetic interactions without bias. Birth cohort studies with a prospective follow-up of children and detailed information about socio-demographic characteristics, exposure assessment, and neurodevelopment are encouraged.
The review identified a few candidate genes in the literature that could be important for genetic susceptibility to mercury. However, so far, the candidate gene approaches have not identified any major gene/s strongly modifying the kinetics or neurotoxicity of mercury, suggesting that these might be polygenic traits or that the major gene/s have not yet been identified. The explorative genome-wide analysis could be a suitable method in order to identify genetic variants important for mercury kinetics and neurotoxicity.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Driscoll CT, Mason RP, Chan HM, et al. Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol. 2013;47(10):4967–83.
World Health Organization (WHO). Children's exposure to mercury compounds. 2010. http://www.who.int/phe/news/Mercury-flyer.pdf.
Parks JM, Johs A, Podar M, et al. The genetic basis for bacterial mercury methylation. Science. 2013;339(6125):1332–5.
Institute of Medicine (IOM). Seafood Choices, Balancing Benefits and Risks. Committee on Nutrient Relationships in Seafood: Selections to Balance Benefits and Risks. Washington, D.C: The National Academies Press; 2006.
Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296(15):1885–99.
Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36(8):609–62.
Bose-O'Reilly S, Drasch G, Beinhoff C, et al. Health assessment of artisanal gold miners in Indonesia. Sci Total Environ. 2010;408(4):713–25.
Frumkin H, Letz R, Williams PL, et al. Health effects of long-term mercury exposure among chloralkali plant workers. Am J Ind Med. 2001;39(1):1–18.
Anglen J, Stayner L, Gruninger S. 0414 Cumulative mercury exposure and peripheral nerve function in a sample of U.S. dental professionals. Occup Environ Med. 2014;71 Suppl 1:A116.
Rice D, Barone Jr S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 Suppl 3:511–33.
Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–8.
Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children's health. Environ Health Perspect. 2000;108 Suppl 3:451–5.
Davidson P, Myers G, Cox C, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment. JAMA. 1998;280(8):701–7.
Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19(6):417–28.
Karagas MR, Choi AL, Oken E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120(6):799–806.
Llop S, Guxens M, Murcia M, et al. Prenatal Exposure to Mercury and Infant Neurodevelopment in a Multicenter Cohort in Spain: Study of Potential Modifiers. Am J Epidemiol. 2012;175(5):451–65.
Castoldi AF, Johansson C, Onishchenko N, et al. Human developmental neurotoxicity of methylmercury: impact of variables and risk modifiers. Regul Toxicol Pharmacol. 2008;51(2):201–14.
Gundacker C, Gencik M, Hengstschlager M. The relevance of the individual genetic background for the toxicokinetics of two significant neurodevelopmental toxicants: mercury and lead. Mutat Res. 2010;705(2):130–40.
National Research Council. Scientific frontiers in developmental toxicology and risk assessment. 2nd ed; 2000
Harris SE, Fox H, Wright AF, et al. A genetic association analysis of cognitive ability and cognitive ageing using 325 markers for 109 genes associated with oxidative stress or cognition. BMC Genet. 2007;8:43.
Ropers HH. Genetics of early onset cognitive impairment. Annu Rev Genomics Hum Genet. 2010;11:161–87.
Ballatori N. Transport of toxic metals by molecular mimicry. Environ Health Perspect. 2002;110 Suppl 5:689–94.
Mason HJ, Hindell P, Williams NR. Biological monitoring and exposure to mercury. Occup Med (Lond). 2001;51(1):2–11.
Clarkson TW, Vyas JB, Ballatori N. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50(10):757–64.
Llop S, Engstrom K, Ballester F, et al. Polymorphisms in ABC transporter genes and concentrations of mercury in newborns—evidence from two Mediterranean birth cohorts. PLoS One. 2014;9(5):e97172. This study provides evidence of the influence of ABC transporters polymorphisms on mercury transfer across the placenta in three Mediterranean birth cohorts.
Miura K, Clarkson TW. Reduced methylmercury accumulation in a methylmercury-resistant rat pheochromocytoma PC12 cell line. Toxicol Appl Pharmacol. 1993;118(1):39–45.
Toyama T, Shinkai Y, Yasutake A, et al. Isothiocyanates reduce mercury accumulation via an Nrf2-dependent mechanism during exposure of mice to methylmercury. Environ Health Perspect. 2011;119(8):1117–22.
Lee BE, Hong YC, Park H, et al. Interaction between GSTM1/GSTT1 polymorphism and blood mercury on birth weight. Environ Health Perspect. 2010;118(3):437–43.
Gundacker C, Komarnicki G, Jagiello P, et al. Glutathione-S-transferase polymorphism, metallothionein expression, and mercury levels among students in Austria. Sci Total Environ. 2007;385(1–3):37–47.
Gundacker C, Wittmann KJ, Kukuckova M, et al. Genetic background of lead and mercury metabolism in a group of medical students in Austria. Environ Res. 2009;109(6):786–96.
Bourdineaud JP, Laclau M, Maury-Brachet R, et al. Effects of Methylmercury Contained in a Diet Mimicking the Wayana Amerindians Contamination through Fish Consumption: Mercury Accumulation, Metallothionein Induction, Gene Expression Variations, and Role of the Chemokine CCL2. Int J Mol Sci. 2012;13(6):7710–38.
Engstrom K, Stromberg U, Lundh T, et al. Genetic variation in glutathione-related genes and body burden of methylmercury. Environ Health Perspect. 2008;116(6):734–9.
Engstrom KS, Wennberg M, Stromberg U, et al. Evaluation of the impact of genetic polymorphisms in glutathione-related genes on the association between methylmercury or n-3 polyunsaturated long chain fatty acids and risk of myocardial infarction: a case–control study. Environ Health. 2011;10:33.
Custodio HM, Broberg K, Wennberg M, et al. Polymorphisms in glutathione-related genes affect methylmercury retention. Arch Environ Health. 2004;59(11):588–95.
Custodio HM, Harari R, Gerhardsson L, et al. Genetic influences on the retention of inorganic mercury. Arch Environ Occup Health. 2005;60(1):17–23.
Engstrom K, Ameer S, Bernaudat L, et al. Polymorphisms in genes encoding potential mercury transporters and urine mercury concentrations in populations exposed to mercury vapor from gold mining. Environ Health Perspect. 2013;121(1):85–91. This study reports the influence of certain polymorphism on inorganic mercury exposure among occupationally exposed population.
Goodrich JM, Wang Y, Gillespie B, et al. Glutathione enzyme and selenoprotein polymorphisms associate with mercury biomarker levels in Michigan dental professionals. Toxicol Appl Pharmacol. 2011;257(2):301–8.
Harari R, Harari F, Gerhardsson L, et al. Exposure and toxic effects of elemental mercury in gold-mining activities in Ecuador. Toxicol Lett. 2012;213(1):75–82.
Wang Y, Goodrich JM, Gillespie B, et al. An investigation of modifying effects of metallothionein single-nucleotide polymorphisms on the association between mercury exposure and biomarker levels. Environ Health Perspect. 2012;120(4):530–4.
Barcelos GR, Grotto D, de Marco KC, et al. Polymorphisms in glutathione-related genes modify mercury concentrations and antioxidant status in subjects environmentally exposed to methylmercury. Sci Total Environ. 2013;463–464:319–25. This study reports the influence of GSH related genes polymorphism on MeHg in a population with high exposure from fish intake.
de Oliveira AA, de Souza MF, Lengert A, et al. Genetic polymorphisms in glutathione (GSH-) related genes affect the plasmatic Hg/whole blood Hg partitioning and the distribution between inorganic and methylmercury levels in plasma collected from a fish-eating population. Biomed Res Int. 2014;2014:940952.
Julvez J, Smith GD, Golding J, et al. Prenatal methylmercury exposure and genetic predisposition to cognitive deficit at age 8 years. Epidemiology. 2013;24(5):643–50. This study provides evidence about the effect of gene-MeHg interactions on children neurodevelopment in a large birth cohort study.
Ng S, Lin CC, Hwang YH, et al. Mercury, APOE, and children's neurodevelopment. Neurotoxicology. 2013;37:85–92.
Ng S, Lin CC, Jeng SF, et al. Mercury, APOE, and child behavior. Chemosphere. 2014;120C:123–30.
Woods JS, Heyer NJ, Russo JE, et al. Genetic polymorphisms affecting susceptibility to mercury neurotoxicity in children: Summary findings from the Casa Pia Children's Amalgam Clinical Trial. Neurotoxicology. 2014;44:288–302.
Echeverria D, Woods JS, Heyer NJ, et al. Chronic low-level mercury exposure, BDNF polymorphism, and associations with cognitive and motor function. Neurotoxicol Teratol. 2005;27(6):781–96.
Echeverria D, Woods JS, Heyer NJ, et al. The association between a genetic polymorphism of coproporphyrinogen oxidase, dental mercury exposure and neurobehavioral response in humans. Neurotoxicol Teratol. 2006;28(1):39–48.
Echeverria D, Woods JS, Heyer NJ, et al. The association between serotonin transporter gene promotor polymorphism (5-HTTLPR) and elemental mercury exposure on mood and behavior in humans. J Toxicol Environ Health A. 2010;73(15):1003–20.
Heyer NJ, Echeverria D, Bittner Jr AC, et al. Chronic low-level mercury exposure, BDNF polymorphism, and associations with self-reported symptoms and mood. Toxicol Sci. 2004;81(2):354–63.
Heyer NJ, Echeverria D, Martin MD, et al. Catechol O-methyltransferase (COMT) VAL158MET functional polymorphism, dental mercury exposure, and self-reported symptoms and mood. J Toxicol Environ Health A. 2009;72(9):599–609.
Prince L, Korbas M, Davidson P, et al. Target organ specific activity of drosophila MRP (ABCC1) moderates developmental toxicity of methylmercury. Toxicol Sci. 2014;140(2):425–35.
Siest G, Pillot T, Regis-Bailly A, et al. Apolipoprotein E: an important gene and protein to follow in laboratory medicine. Clin Chem. 1995;41(8 Pt 1):1068–86.
Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23(17):6690–4.
Acknowledgments
This research has been funded by the Swedish Research Council Formas and the Spanish FIS FEDER (13/1944).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Sabrina Llop, Ferran Ballester, and Karin Broberg declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Early Life Environmental Health
Rights and permissions
About this article
Cite this article
Llop, S., Ballester, F. & Broberg, K. Effect of Gene-Mercury Interactions on Mercury Toxicokinetics and Neurotoxicity. Curr Envir Health Rpt 2, 179–194 (2015). https://doi.org/10.1007/s40572-015-0047-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-015-0047-y