Abstract
Purpose of Review
Identify barriers to epinephrine autoinjector (EAI) use at the healthcare system, health care provider (HCP), and patient and caregiver levels.
Recent Findings
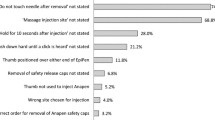
EAI prescription, carriage, and use for the treatment of anaphylaxis remain suboptimal. The under-diagnosis of anaphylaxis contributes to suboptimal EAI prescription rates. The high costs of EAIs and concerns about their efficacy, safety, side effects, or convenience may prevent some patients from filling prescriptions or carrying EAIs or administering EAIs. Gaps in EAI training, knowledge, and skills persist among not only patients and caregivers but also HCPs.
Summary
Multiple stakeholders, including health policy-makers, HCPs, patients, and caregivers, have roles to play in promoting epinephrine access and the proper use of EAIs to treat anaphylaxis. HCPs require education to develop EAI-related knowledge and skills. They should engage at-risk patients in a shared decision-making process to evaluate their need for EAI prescription, develop a comprehensive anaphylaxis management plan, and identify and address any barriers to accessing, carrying, and using EAIs. They should regularly review proper techniques for storing, carrying, and using EAIs with patients and caregivers. Interprofessional collaboration is also essential to increase awareness, education, and availability of EAIs in the wider community.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145:1082–123. Presents the American Academy of Allergy, Asthma & Immunology and the American College of Allergy, Asthma and Immunology’s Joint Task Force on Practice Parameter (JTFPP)’s systematic review findings and GRADE recommendations for the evaluation, prevention, and management of anaphylaxis.
Feng C, Kim J-H. Beyond avoidance: the psychosocial impact of food allergies. Clin Rev Allergy Immunol. 2019;57:74–82.
Miles LM, Ratnarajah K, Gabrielli S, Abrams EM, Protudjer JLP, Bégin P, et al. Community use of epinephrine for the treatment of anaphylaxis: a review and meta-analysis. J Allergy Clin Immunol Pract. 2021;9:2321–33.
Sicherer SH, Simons FER, Mahr TA, Abramson SL, Dinakar C, Fleisher TA, et al. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017;139. https://doi.org/10.1542/PEDS.2016-4006.
•• Tanno LK, Demoly P. Action plan to ensure global availability of adrenaline autoinjectors. J Investig Allergol Clin Immunol. 2020;30:77–85. Reports on the current status of the global availability of EAIs and presents the Joint Allergy Academies’ action plan to ensure global availability of EAIs.
Tanno LK, Simons FER, Sanchez-Borges M, Cardona V, Moon HB, Calderon MA, et al. Applying prevention concepts to anaphylaxis: a call for worldwide availability of adrenaline auto-injectors. Clin Exp Allergy. 2017;47:1108–14.
Ansotegui IJ, Sánchez-Borges M, Cardona V. Current trends in prevalence and mortality of anaphylaxis. Curr Treat Options Allergy. 2016;3:205–11.
Russell WS, Farrar JR, Nowak R, Hays DP, Schmitz N, Wood J, et al. Evaluating the management of anaphylaxis in US emergency departments: guidelines vs. practice. World J Emerg Med. 2013;4:98.
Sclar DA, Lieberman PL. Anaphylaxis: underdiagnosed, underreported, and undertreated. Am J Med. 2014;127:S1–5.
Alvarez-Perea A, Tanno LK, Baeza ML. How to manage anaphylaxis in primary care. Clin Transl Allergy. 2017;7:45.
Arroabarren E, Lasa EM, Olaciregui I, Sarasqueta C, Muñoz JA, Pérez-Yarza EG. Improving anaphylaxis management in a pediatric emergency department. Pediatr Allergy Immunol. 2011;22:708–14.
Rueter K, Ta B, Bear N, Lucas M, Prescott S. Physician training programs significantly improve diagnosis in cases coded as anaphylaxis over time: a major factor compounding time-trend data? J Allergy Clin Immunol In Pract. 2017;5:858–60.
Pepper AN, Westermann-Clark E, Lockey RF. The high cost of epinephrine autoinjectors and possible alternatives. J Allergy Clin Immunol Pract. 2017;5:665-668.e1.
Warren CM, Zaslavsky JM, Kan K, Spergel JM, Gupta RS. Epinephrine auto-injector carriage and use practices among US children, adolescents, and adults. Ann Allergy Asthma Immunol. 2018;121:479–91.
Pepper AN, Westermann-Clark E, Lockey RF. The high cost of epinephrine autoinjectors and possible alternatives. J Allergy Clin Immunol Pract. 2017;5:665-668.e1.
Parish HG, Morton JR, Brown JC. A systematic review of epinephrine stability and sterility with storage in a syringe. Allergy Asthma Clin Immunol. 2019;15:7.
Moss RB, Daniels K, Moll T, Carlo DJ. Human factors study in untrained adolescents comparing a recently approved single-dose epinephrine prefilled syringe with an approved autoinjector. Ann Allergy Asthma Immunol. 2018;120:540–1.
Ratanaprug C, Srisuwatchari W, Jirapongsananuruk O, Visitsunthorn N, Pacharn P. Carrying rates of epinephrine devices in children with food-induced anaphylaxis. Asia Pac Allergy. 2019;9. https://doi.org/10.5415/APALLERGY.2019.9.E12.
Sánchez J. Anaphylaxis. How often patients carry epinephrine in real life? https://pubmed.ncbi.nlm.nih.gov/24912909/.
Curtis C, Stukus D, Scherzer R. Epinephrine preparedness in pediatric patients with food allergy: an ideal time for change. Ann Allergy Asthma Immunol 2014;112(6):560–562
Money AG, Barnett J, Kuljis J, Lucas J. Patient perceptions of epinephrine auto-injectors: exploring barriers to use. Scand J Caring Sci. 2013;27:335–44.
•• Waserman S, Cruickshank H, Hildebrand KJ, Mack D, Bantock L, Bingemann T et al. Prevention and management of allergic reactions to food in child care centers and schools: practice guidelines. https://doi.org/10.1016/j.jaci.2021.01.034. Presents systematic review findings and GRADE recommendations for the management of anaphylaxis in child care centers and schools, including recommendations related to the stocking and use of EAIs.
Schellpfeffer NR, Leo HL, Ambrose M, Hashikawa AN. Food allergy trends and epinephrine autoinjector presence in summer camps. J Allergy Clin Immunol Pract. 2017;5:358–62.
Waserman S, Avilla E, Harada L, Allen M, Isaranuwatchai W, Perdrizet J et al. To stock or not to stock? Implementation of epinephrine autoinjectors in food establishments. J Allergy Clin Immunol: In Pract. 2019;7:678–680.e5.
Kao LM, Wang J, Kagan O, Russell A, Mustafa SS, Houdek D, et al. School nurse perspectives on school policies for food allergy and anaphylaxis. Ann Allergy Asthma Immunol. 2018;120:304–9.
Denny SA, Merryweather A, Kline JM, Stanley R. Stock epinephrine in schools: a survey of implementation, use, and barriers. J Allergy Clin Immunol Pract. 2020;8:380–2.
Love MA, Breeden M, Dack K, Milner A, Rorie AC, Gierer SA, et al. A law is not enough: geographical disparities in stock epinephrine access in Kansas. J Allergy Clin Immunol. 2016;137:AB56.
Morris P, Baker D, Belot C, Edwards A. Preparedness for students and staff with anaphylaxis. J Sch Health. 2011;81:471–6.
Yeh C-Y, White KD, Konvinse KC, Pilkinton MA, Redwood A, Trubiano JA, et al. Identifying barriers to implementation of stock epinephrine bills: the Texas experience. J Allergy Clin Immunol. 2018;141:AB88.
Campbell RL, Bellolio MF, Knutson BD, Bellamkonda VR, Fedko MG, Nestler DM, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3:76–80.
Wood JP, Traub SJ, Lipinski C. Safety of epinephrine for anaphylaxis in the emergency setting. World J Emerg Med. 2013;4:245.
Brown JC, Tuuri RE, Akhter S, Guerra LD, Goodman IS, Myers SR, et al. Lacerations and embedded needles caused by epinephrine autoinjector use in children. Ann Emerg Med. 2016;67:307-315.e8.
Anshien M, Rose SR, Wills BK. Unintentional epinephrine auto-injector injuries: a national poison center observational study. Am J Ther. 2019;26:e110–4.
Walsh K, Baker BG, Iyer S. Adrenaline auto-injector injuries to digits; a systematic review and recommendations for emergency management. Surgeon. 2020;18:305–10.
Goldman RD, Long KC, Brown JC. Hooked epinephrine auto-injector devices in children: four case reports with three different proposed mechanisms. Allergy Asthma Clin Immunol. 2020;16:19.
Bonds RS, Asawa A, Ghazi AI. Misuse of medical devices: a persistent problem in self-management of asthma and allergic disease. Ann Allergy Asthma Immunol. 2015;114:74-76.e2.
Ridolo E, Montagni M, Bonzano L, Savi E, Peveri S, Costantino MT, et al. How far from correct is the use of adrenaline auto-injectors? A survey in Italian patients. Intern Emerg Med. 2015;10:937–41.
Segal N, Garty B-Z, Hoffer V, Levy Y. Effect of instruction on the ability to use a self-administered epinephrine injector. Isr Med Assoc J. 2012;14:14–7.
Schoeben L-S, Mohr N, Bubak C, Schmieder A, Schaarschmidt M-L. Effects of a 90-min educational intervention for patients with insect venom allergy: a prospective controlled pilot study. Allergy Asthma Clin Immunol. 2021;17:22.
Brockow K, Schallmayer S, Beyer K, Biedermann T, Fischer J, Gebert N, et al. Effects of a structured educational intervention on knowledge and emergency management in patients at risk for anaphylaxis. Allergy. 2015;70:227–35.
Sicherer SH, Vargas PA, Groetch ME, Christie L, Carlisle SK, Noone S, et al. Development and validation of educational materials for food allergy. J Pediatr. 2012;160:651–6.
Patrawala M, Shih J. P353 epinephrine autoinjector education: a quality improvement project. Ann Allergy Asthma Immunol. 2019;123:S54–5.
Samstein M, Li T, Cassara M, Jongco A. Adoption of 2016 EpiPen administration instructions by pediatric emergency department staff. J Allergy Clin Immunol. 2020;145:AB3.
Sirin SK, Asilsoy S, Tezcan D, Al S, Atay O, Kangalli O, et al. Is there an optimal training interval to improve the correct use of adrenaline auto-injectors? Int Arch Allergy Immunol. 2020;181:136–40.
Southall K, Euberto Mendes Reyes J, Hazi A, Andre M, Virkud Y, Shreffler W, et al. Epinephrine auto-injector parental survey and skills demonstration. J Allergy Clin Immunol. 2020;145:AB232.
Soller L, Teoh T, Baerg I, Wong T, Hildebrand KJ, Cook VE, et al. Extended analysis of parent and child confidence in recognizing anaphylaxis and using the epinephrine autoinjector during oral food challenges. J Allergy Clin Immunol: In Pract. 2019;7:693–5.
Soller L, Teoh T, Baerg I, Wong T, Chan ES. One-year sustained impact of supervised epinephrine autoinjector administration during food challenge on parent confidence. Ann Allergy Asthma Immunol. 2020;125:705–7.
Shemesh E, D’Urso C, Knight C, Rubes M, Picerno KM, Posillico AM, et al. Food-allergic adolescents at risk for anaphylaxis: a randomized controlled study of supervised injection to improve comfort with epinephrine self-injection. J Allergy Clin Immunol Pract. 2017;5:391-397.e4.
Mahoney B, Walklet E, Bradley E, O’Hickey S. Improving adrenaline autoinjector adherence: a psychologically informed training for healthcare professionals. Immun Inflamm Dis. 2019;7:214–28.
Grouhi M, Alshehri M, Hummel D, Roifman CM. Anaphylaxis and epinephrine auto-injector training: who will teach the teachers? J Allergy Clin Immunol. 1999;104:190–3.
Posner LS, Camargo CA. Update on the usage and safety of epinephrine auto-injectors, 2017. Drug Healthc Patient Saf. 2017;9:9.
el Turki A, Smith H, Llewellyn C, Jones CJ. A systematic review of patients’, parents’ and healthcare professionals’ adrenaline auto-injector administration techniques. Emerg Med J. 2017;34:402–15.
Baççioǧlu A, Yilmazel UE. Level of knowledge about anaphylaxis among health care providers. Tuberkuloz ve toraks. 2013;61:140–6.
Salter SM, Loh R, Sanfilippo FM, Clifford RM. Demonstration of epinephrine autoinjectors (EpiPen and Anapen) by pharmacists in a randomised, simulated patient assessment: acceptable, but room for improvement. Allergy Asthma Clin Immunol. 2014;10:1–10.
Arga M, Bakirtas A, Catal F, Derinoz O, Harmanci K, Razi CH, et al. Training of trainers on epinephrine autoinjector use. Pediatr Allergy Immunol. 2011;22:590–3.
Chow TG, Bonnet E, Roman H, Bird JA. Efficacy of video-based training to improve epinephrine autoinjector use competency. J Allergy Clin Immunol. 2019;143:AB152.
Yuenyongviwat A, Wirodwanich T, Jessadapakorn W, Sangsupawanich P. Utility of an educational video on epinephrine prefilled syringe usage for anaphylaxis: a randomized control trial. Asia Pac Allergy. 2020;10:e32–e32.
Salter SM, Delfante B, de Klerk S, Sanfilippo FM, Clifford RM. Pharmacists’ response to anaphylaxis in the community (PRAC): a randomised, simulated patient study of pharmacist practice. BMJ Open. 2014;4: e005648.
Aguilera A, O’Neill M, Slaven J, Vitalpur G. Improving knowledge of epinephrine auto-injector use and peanut guidelines at an academic medical center. J Allergy Clin Immunol. 2020;145:AB168.
Kaur N, McCrossin T, Gunasekera H. Improving anaphylaxis management by health care professional education and practical skills training in a regional centre. J Paediatr Child Health. 2017;53:1029–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nishi Parikh declares she has no conflict of interest.
Heather Cruickshank received payment or Honoria from Healthline Media.
Susan Waserman received grants or contacts from Aimmune and ALK, payment or Honoria from AbbVie, GSK, AZ, Sanofi, Pediapharm, Medexus, Novartis, CSL Behring, Bausch and Lomb, Covis, Takeda, Valeo, and Pfizer, participates on a Data Safety Monitoring Board/Advisory Board for Siolta, and HAL-ragweed Immunotherapy, and holds a leadership or fiduciary role in Asthma Canada, Food Allergy Canada, Canadian Allergy Asthma Immunology Foundation, and Canadian Heritage Angioedema Network.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Anaphylaxis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parikh, N., Cruickshank, H. & Waserman, S. Underuse of Epinephrine Autoinjectors in Anaphylaxis: Who Is to Blame?. Curr Treat Options Allergy 9, 323–334 (2022). https://doi.org/10.1007/s40521-022-00325-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-022-00325-2