Abstract
Introduction
Previous observational studies have found an increased risk of frailty in patients with stroke. However, evidence of a causal relationship between stroke and frailty is scarce. The aim of this study was to investigate the potential causal relationship between stroke and frailty index (FI).
Methods
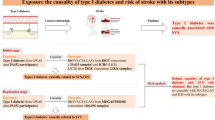
Pooled data on stroke and debility were obtained from genome-wide association studies (GWAS).The MEGASTROKE Consortium provided data on stroke (N = 40,585), ischemic stroke (IS,N = 34,217), large-vessel atherosclerotic stroke (LAS,N = 4373), and cardioembolic stroke (CES,N = 7 193).Summary statistics for the FI were obtained from the most recent GWAS meta-analysis of UK BioBank participants and Swedish TwinGene participants of European ancestry (N = 175,226).Two-sample Mendelian randomization (MR) analyses were performed by inverse variance weighting (IVW), weighted median, MR-Egger regression, Simple mode, and Weighted mode, and heterogeneity and horizontal multiplicity of results were assessed using Cochran’s Q test and MR-Egger regression intercept term test.
Results
The results of the current MR study showed a significant correlation between stroke gene prediction and FI (odds ratio 1.104, 95% confidence interval 1.064 − 1.144, P < 0.001). In terms of stroke subtypes, IS (odds ratio 1.081, 95% confidence interval 1.044 − 1.120, P < 0.001) and LAS (odds ratio 1.037, 95% confidence interval 1.012 − 1.062, P = 0.005). There was no causal relationship between gene-predicted CES and FI. Horizontal multidimensionality was not found in the intercept test for MR Egger regression (P > 0.05), nor in the heterogeneity test (P > 0.05).
Conclusions
This study provides evidence for a causal relationship between stroke and FI and offers new insights into the genetic study of FI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a cerebrovascular lesion caused by sudden cerebrovascular injury with high morbidity, disability, mortality and recurrence rates [1]. Stroke is the second leading cause of death worldwide, with an annual death rate of about 5.5 million [2]. Studies have shown that the incidence of stroke increases dramatically with age, with about three-quarters of all strokes occurring in people over the age of 65 [3]. The United Nations Population Prospects predicts that the number of people aged 60 years and older will reach 2.1 billion globally by 2050 and 3.2 billion by 2100 [4]. Elderly stroke patients suffer from long-term sequelae such as incapacitation, emotional deficits, and cognitive disorders [5], and because of the long duration of illness, high medical costs, and poor adherence to treatment, elderly stroke patients impose a heavy burden on society, families, and patients [6].
Frailty, characterized by age-related multisystem dysfunction, is a major public health problem in older adults [7, 8]. Studies show that frailty is common in strokes, with at least a quarter of stroke victims being physically frail [9]. Understanding the potential association between frailty and senescence-related diseases and the underlying mechanisms may facilitate the individualized management and early interventions of frail patients.FI reflects the accumulation of physiological deficits in various systems of the body, and assesses frailty by calculating the number of deficits in the health variables, taking into account the effects of physical, psychosocial, and social factors on the human body [10]. Studies have shown that stroke frailty can be assessed by physicians or researchers in the early stages of stroke by means of a scale survey, and studies have confirmed the applicability of the FI in stroke patients [11].
Randomized controlled trials (RCTs) are the gold standard of clinical evidence and are widely used to infer causality, mainly by eliminating confounding bias through randomized grouping. However, randomized controlled trials require a great deal of time, money, and human resources; therefore, RCT studies are difficult to perform in many medical studies. Mendelian randomization (MR) is a genetic epidemiology study design method that allows for the exploration of causal relationships between exposures and outcomes through the use of genetic variation as an instrumental variable (IV) [12]. Due to the ability to overcome the effects of potential confounding and reverse causation, MR methods have been increasingly used in observational studies in recent years [13]. MR research has been facilitated by the discovery in biology of a large number of genetic variants that are strongly associated with specific traits, and by the public release of hundreds of thousands of pooled data on the association of exposures and diseases with genetic variants from many large-sample genome-wide association studies (GWAS),which have allowed researchers to estimate genetic associations in large-sample data.The method of MR, with its use of genetic variables, allows for the avoidance of reverse causality and minimizes the interference of environmental factors in a manner similar to that of RCTs [14].
In this study, we aimed to investigate the causal relationship between stroke and frailty index using MR methods.
Materials and methods
Mendelian randomization assumptions
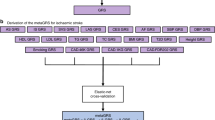
The MR method is an instrumental variables analysis that uses genetic variants as proxies for exposure. As in Fig. 1, the MR analysis relies on 3 important assumptions: (1) instrumental variables are closely related to exposure factors; (2) instrumental variables are independent of confounding factors; and (3) instrumental variables affect outcome only through exposure and not through other means [15].
Data sources and SNP selection for stroke
The exposure factor for this study was defined as stroke, and data were obtained from the MEGASTROKE consortium, including 446,696 individuals of European ancestry (406,111 non-stroke cases and 40,585 stroke cases); the total number of ischemic stroke cases was 34,217, including 4373 large-vessel atherosclerotic stroke (LAS) cases, 5386 small-vessel stroke cases, and 7193 cardiac stroke cases.
Data sources and SNP selection for frailty index
Frailty is commonly defined using the Frailty Index (FI) [16]. In this MR study, frailty was measured according to the FI, which was calculated based on the accumulation of 44–49 self-reported health deficits over the life course.Summary statistics for the FI were obtained from the most recent GWAS meta-analysis of UK BioBank participants and Swedish TwinGene participants of European ancestry (N = 175,226).
Genetic instrumental variable selection
To meet the above hypotheses, the specific instrumental variables screening criteria were: (1) the association of single nucleotide polymorphism(SNPs) with single amino acids was genome-wide significant (P < 5 × 10−8); (2) the linkage disequilibrium (LD) between SNPs was calculated using the European population genome of 1000 individuals as the reference template, and SNPs with r2 < 0.001 and physical genetic distance > 10,000 kb were screened. (3) remove SNPs with minor allele frequencies < 0.01;(4) exclude SNPs with F values < 10 to avoid weak instrumental bias,and the following formula was used to calculate the F statistic [17]: F statistic = R2(N–2)/(1–R2). R2 = 2 × EAF × (1–EAF) × β2.The F values were all > 10, which indicated that our IVs were not biased by weak instrument. (5) apply MR Steiger [18] to assess the causal direction of each SNP on exposure and outcome, and exclude SNPs with reverse causality.
Statistical analysis
Two-sample Mendelian randomization analysis
We performed two-sample MR analyses using the inverse-variance-weighted method as the primary approach.Four other MR methods based on different model assumptions were also used for the analysis: weighted median method [19], MR-Egger regression [20], Weighted mode and Simple mode. According to the MR-Egger regression method, intercepts different from the origin can be used to assess potential pleiotropic effects [21]. These various MR methods can test the stability and reliability of association under different assumptions.
Sensitivity and power analysis
In this study, MR-Egger intercept was used to detect horizontal multiplicity, and if the intercept term in MR-Egger intercept analysis was statistically significant compared to 0, it indicated that the study had horizontal multiplicity [22]; Cochran Q test was applied to determine the heterogeneity of SNPs [23], and if the Cochran Q statistic test is statistically significant and proves that the analysis results have significant heterogeneity, then focus on the results of the random effects IVW method; using the Leave-one-out sensitivity test for sensitivity analysis [24], each SNP is eliminated in turn, and the remaining The MR results are robust if the remaining SNPs are not significantly different from the total results.The above methods were implemented using the TwoSampleMR package in the R 4. 2. 3 software with a test level of α= 0. 05.
Results
Instrumental variable
According to the screening criteria of the instrumental variables in this study, all strokes, ischemic strokes, atherosclerotic strokes of large arteries, and cerebral embolic strokes were finally screened for 17 SNPstroke, 18 SNPIS, 4 SNPLAS, and 4 SNPCES, respectively. Table 1 shows 18 SNPs significantly associated with ischemic stroke.The MR-Egger regression intercepts were bstroke = 0.0179 (P = 0.8888), bIS = 0.1234 (P = 0.2288), bLAS = − 0.0121 (P = 0.8912), and bCES = 0.0306 (P = 0.3773), respectively. That is, there was no genetic pleiotropy between the screened SNPs and the outcome frailty index, and thus the Mendelian randomization method was a valid method for causal inference in this study.
Mendelian randomization analysis
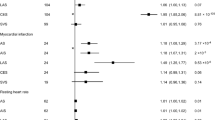
The results of the ivw method showed a significant relationship between stroke (OR = 1.104, 95% CI 1.064–1.144, p < 0.001), IS (OR = 1.081, 95% CI 1.044–1.120, p < 0.001), LAS (OR = 1.037, 95% CI 1.012–1.062, p = 0.005), and FI There was a causal relationship between. In Weighted median method analysis, stroke (OR = 1.085, 95% CI 1.037–1.135, p < 0.001), IS (OR = 1.081, 95% CI 1.034–1.129, p < 0.001), LAS (OR = 1.035, 95% CI 1.005–1.066, and p = 0.026) and FI were also causally related. However, there was no evidence to support a causal relationship between CES and FI. MR estimates and efficacy analyses for stroke and FI are shown in Table 2, and scatter plots of MR analyses for the 5 methods are shown in Fig. 2.
In this study Cochran’s Q test was used to assess the heterogeneity of the results which was done by both IVW and MR-Egger’s analyses.The results of CochranQ test for IVW method showed Qstroke = 22.193, QIS = 26.370, QLAS = 1.813, QCES = 1.208.MR-Egger’s CochranQ test showed Qstroke = 21.590, QIS = 26.016, QLAS = 1.421, QCES = 1.084.Regarding the results of the horizontal multivariate test, it was assessed using MR Egger’s intercept term. The p-value of heterogeneity and horizontal polytropy was greater than 0.05, so there was no heterogeneity and horizontal polytropy in this study, so the results had a weak risk of bias and high reliability. In addition, we performed Leave-one-out analysis, and the results showed that after removing each SNP in turn, the b-values of the remaining SNPs were (0.089–0.105), (0.694–0.841), (0.012–0.150), and (0.012–0.027), respectively, with p < 0.01. The b-values were all > 0, and the directions were all the same, indicating that removing either SNP had little effect on the results, and a positive causal association between stroke and frailty index was still observed. Sensitivity analyses and forest plots of the association between gene-predicted stroke and FI are shown in Fig. 3.
Discussion
In this study, based on large-scale GWAS pooled data, a two-sample MR method was used to analyze the causal association between stroke, IS, LAS, CES and FI. The results showed that stroke, IS, LAS, and CES were risk factors for FI. Further sensitivity analyses showed the consistency and reliability of these results.
Our finding that stroke may cause frailty provides evidence for early observational studies.Previous studies have reported an association between stroke and frailty [25]. The research results show that 12.8% of ischemic stroke patients and 10.3% of hemorrhagic stroke patients are already in a weakened state before stroke, and the degree of weakness worsens after stroke [26]. Stroke may accelerate the occurrence and development of physical weakness. In a large-scale assessment of debility, Hanlon et al. found that debility was common among stroke survivors. Rodriguez et al. showed an overall prevalence of frailty of 15.2% in older adults and a positive correlation between frailty and stroke in a survey of eight urban and four rural areas in eight countries, including Cuba, the Dominican Republic, Puerto Rico, Venezuela, Peru, Mexico, China, and India.Palmer et al. [27] found that the incidence of frailty was twice as high in stroke patients as in those who did not have a stroke [28]. Rowan et al. found frailty in about a quarter of acute stroke patients through a cross-sectional survey [11]. Stroke increases the risk of debilitation, and the prevalence of debilitation varies widely by region between countries, while debilitation imposes a serious burden on stroke patients and reduces their quality of life.
Exploring the causal relationship between these two comorbidities may be difficult because they share the same risk factors, such as hyperglycemia and Dyslipidemia. In addition,Evans et al showed that neurological deficits after stroke may exacerbate the phenotypic features of frailty, that hemodynamic changes in central and peripheral vasculature occur with age, and that frailty is associated with impaired brain self-regulation. And that a history of previous stroke is an important factor in the transition from robust to debilitated patients and in the worsening of the debilitating trajectory [29]. Hanotier et al. pointed out that prolonged malnutrition can easily lead to electrolyte disorders in the elderly. Once a stroke occurs, due to insufficient nutrition intake, electrolyte disorders worsen, and body mass sharply decreases, ultimately leading to frailty in the elderly [30]. Stroke patients have varying degrees of neurological dysfunction, which can affect the number of skeletal muscles to a certain extent [31], leading to the occurrence of sarcopenia, thereby reducing limb muscle strength and grip strength, and increasing the risk of physical weakness.
Zhu et al. [8] conducted a two-way Mendelian randomisation study of FI and stroke and found that FI was significantly associated with stroke in both directions of the outcome, but stroke-related subgroup analyses were not performed, which somewhat supports our findings. Liu et al. [32] found an implied association between FI and any stroke, and FI was associated with a high risk of LAS, but there was no causal association between FI and IS and small-artery stroke. There was no causal relationship.However, our study found that stroke, IS, and LAS were all causally related to FI, which may be due to the fact that our study was conducted in a different direction than theirs.
The main strengths of this study include the use of MR for causal inference and analyzing a large sample group. MR methods can effectively avoid the drawbacks of uncertain residual confounding and reverse causality that exist in traditional observational study methods [21]. Data on stroke and frailty are derived from existing large GWAS, which allows for more precise assessment of effect sizes than individual-level data or results from studies with limited sample sizes. Inevitably, there are some limitations. First, the two-sample Mendelian randomization method assumes a correlation between the exposure factor and the outcome, and the MR method is not applicable if the relationship is nonlinear. Second, the results of the analysis in this study are based only on populations of European origin, so further research and validation are needed for generalization to other populations. Finally, database statistics are difficult to analyze stratified by sex or age, which may lead to biased findings.
Conclusions
In summary, we found a causal relationship between stroke and its subtypes and debility by two-sample MR analysis. Further studies are needed to elucidate the potential mechanisms underlying the various causal relationships between stroke subtypes and debility.
Data availability
All data are publicly available. Detailed information for these datasets is summarized in supplementary material.
References
Collaborators GBDS (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 20:795–820
Paul S, Candelario-Jalil E (2021) Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol 335:113518
Myint PK, Sinha S, Luben RN et al (2008) Risk factors for first-ever stroke in the EPIC-Norfolk prospective population-based study. Eur J Cardiovasc Prev Rehabil 15:663–9
Sanuade OA, Dodoo FN, Koram K et al (2019) Prevalence and correlates of stroke among older adults in Ghana: evidence from the study on global AGEing and adult health (SAGE). PLoS ONE 14:e0212623
Nguyen TV, Le D, Tran KD et al (2019) Frailty in older patients with acute coronary syndrome in Vietnam. Clin Interv Aging 14:2213–22
Blanco S, Ferrieres J, Bongard V et al (2017) Prognosis impact of frailty assessed by the edmonton frail scale in the setting of acute coronary syndrome in the elderly. Can J Cardiol 33:933–9
Proietti M, Cesari M (2020) Frailty: what is it? Adv Exp Med Biol 1216:1–7
Zhu J, Zhou D, Wang J et al (2022) Frailty and cardiometabolic diseases: a bidirectional Mendelian randomisation study. Age Ageing 51:afac256
Burton JK, Stewart J, Blair M et al (2022) Prevalence and implications of frailty in acute stroke: systematic review & meta-analysis. Age Ageing 51:afac064
Sonny A, Kurz A, Skolaris LA et al (2020) Deficit accumulation and phenotype assessments of frailty both poorly predict duration of hospitalization and serious complications after noncardiac surgery. Anesthesiology 132:82–94
Taylor-Rowan M, Cuthbertson G, Keir R et al (2019) The prevalence of frailty among acute stroke patients, and evaluation of method of assessment. Clin Rehabil 33:1688–96
Davies NM, Holmes MV, Davey Smith G (2018) Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362:k601
Burgess S, Timpson NJ, Ebrahim S et al (2015) Mendelian randomization: where are we now and where are we going? Int J Epidemiol 44:379–88
Larsson SC, Traylor M, Markus HS (2019) Homocysteine and small vessel stroke: a mendelian randomization analysis. Ann Neurol 85:495–501
Zheng J, Baird D, Borges MC et al (2017) Recent developments in Mendelian randomization studies. Curr Epidemiol Rep 4:330–45
Atkins JL, Jylhava J, Pedersen NL et al (2021) A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell 20:e13459
Gao L, Di X, Gao L et al (2023) The Frailty Index and colon cancer: a 2-sample Mendelian-randomization study. J Gastrointest Oncol 14:798–805
Hemani G, Tilling K, Davey Smith G (2017) Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet 13:e1007081
Bowden J, Davey Smith G, Haycock PC et al (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40:304–14
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–25
Zhao H, Zhu J, Ju L et al (2022) Osteoarthritis & stroke: a bidirectional mendelian randomization study. Osteoarthr Cartil 30:1390–7
Martin S, Tyrrell J, Thomas EL et al (2022) Disease consequences of higher adiposity uncoupled from its adverse metabolic effects using Mendelian randomisation. Elife 11:e72452
Bowden J, Spiller W, Del Greco MF et al (2018) Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int J Epidemiol 47:2100
Gronau QF, Wagenmakers EJ (2019) Limitations of bayesian leave-one-out cross-validation for model selection. Comput Brain Behav 2:1–11
Calado LB, Ferriolli E, Moriguti JC et al (2016) Frailty syndrome in an independent urban population in Brazil (FIBRA study): a cross-sectional populational study. Sao Paulo Med J 134:385–392
Kanai M, Noguchi M, Kubo H et al (2020) Pre-stroke frailty and stroke severity in elderly patients with acute stroke. J Stroke Cerebrovasc Dis 29:105346
Llibre Rodriguez JJ, Prina AM, Acosta D et al (2018) The prevalence and correlates of frailty in urban and rural populations in latin America, China, and India: a 10/66 population-based survey. J Am Med Dir Assoc 19:287–95.e4
Palmer K, Vetrano DL, Padua L et al (2019) Frailty syndromes in persons with cerebrovascular disease: a systematic review and meta-analysis. Front Neurol 10:1255
Evans NR, Todd OM, Minhas JS et al (2022) Frailty and cerebrovascular disease: concepts and clinical implications for stroke medicine. Int J Stroke 17:251–9
Hanotier P (2015) Hyponatremia in the elderly: its role in frailty. Rev Med Brux 36:475–84
Jung H, Kim M, Lee Y et al (2020) Prevalence of physical frailty and its multidimensional risk factors in Korean community-dwelling older adults: findings from Korean frailty and aging cohort study. Int J Environ Res Public Health 17:7883
Liu W, Zhang L, Fang H et al (2022) Genetically predicted frailty index and risk of stroke and Alzheimer’s disease. Eur J Neurol 29:1913–21
Funding
This study was supported by the National Natural Science Foundation of China (Project No. 82260281) and the Science and Technology Fund Project of Guizhou Provincial Health Commission (gzwkj2023-588);Scientific study on the Construction of shared Medical Model and Optimization Mechanism for patients with Stroke Depression in low Resource area. Guizhou Science and Technology Plan Project, Guizhou Science and Technology Cooperation (Qiankehe) Foundation(NO:. ZK [2024] key Project 069).
Author information
Authors and Affiliations
Contributions
Jiangnan Wei wrote the main manuscript text, Jiaxian Wang drew the images and tables, Jiayin Chen performed the data collection, Kezhou Yang organized the information, and Ning Liu revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
For this study, publicly available summarized data from the GWAS database were used and the data were approved by the ethical review board.
Informed consent
Informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, J., Wang, J., Chen, J. et al. Stroke and frailty index: a two-sample Mendelian randomisation study. Aging Clin Exp Res 36, 114 (2024). https://doi.org/10.1007/s40520-024-02777-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40520-024-02777-9