Abstract
Background
Heart failure (HF) and frailty are accompanied by a bidirectional relationship, sharing common risk factors including elevated levels of natriuretic peptides and inflammation. The aim of this study was to compare biomarkers associated with poor clinical outcomes, that is, plasma brain natriuretic peptide (BNP), N-terminal-pro B-type natriuretic peptide (NT-proBNP), and C-reactive protein (CRP) in patients with HF and frailty vs. patients with HF without frailty.
Methods
From inception until July 2023, PubMed, Scopus, Web of Science, and Cochrane Library a systematic literature search was conducted. To evaluate whether frailty is linked with greater levels of BNP, NT-proBNP, and CRP, a meta-analysis using a random-effects model was used to calculate the pooled effects (CRD42023446607).
Results
Fifty-three studies were included in this systematic review and meta-analysis. Patients with HF and frailty displayed significantly higher levels of BNP (k = 11; SMD: 0.53, 95%CI 0.30–0.76, I2 = 86%, P < 0.01), NT-proBNP (k = 23; SMD: 0.33, 95%CI 0.25–0.40, I2 = 72%, P < 0.01), and CRP (k = 8; SMD: 0.30, 95%CI 0.12–0.48, I2 = 62%, P < 0.01) vs. patients with HF without frailty. Using meta-regression, body mass index (BMI) and age were deemed potential moderators of these findings.
Conclusions
Frailty in HF is linked to increased concentrations of BNP, NT-proBNP, and CRP, which have been epidemiologically associated with adverse outcomes. The increased risk of NYHA III/IV classification further emphasizes the clinical impact of frailty in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frailty is a dynamic, multidimensional syndrome with an increased risk of presentation with advancing age. It is characterized by an increased vulnerability to external stressors and thus an increased risk of adverse health outcomes [1]. There are currently various different approaches of frailty diagnosis used in clinical practice, on one hand those that define frailty as a risk physical phenotype preceding dependency and on the other hand those that value frailty as an accumulation of deficits (multidimensional frailty), including comorbidities, disabilities, symptoms, and biochemical markers [2].
Heart failure (HF) is a clinical disorder marked by structural and/or functional myocardial abnormalities that result in high intracardiac pressure and insufficient cardiac output [3]. Interestingly, patients with HF display higher inflammatory status [4] and major skeletal muscle abnormalities, including a shift in muscle fibre type distribution with fewer type II muscle fibres and a lower capillary-to-fiber ratio, which may contribute to exercise intolerance and accelerated losses of muscle mass and function [5]. The presence of HF may accelerate the development of frailty with an estimated prevalence of approximately 45% [6].
A biomarker that is associated with HF severity by reflecting mechanical overload and cardiac function is plasma brain natriuretic peptide (BNP), for which research has shown that may be exacerbated by frailty [7]. Likewise, N-terminal-pro B-type natriuretic peptide (NT-proBNP) is a biologically inactive derivative of BNP; a marker commonly used to assess HF severity [8]. In addition, clinical risk stratification for HF has been performed via assessment of the New York Heart Association (NYHA) classification, although it has been deemed an unreliable predictor of negative outcomes in HF, poorly distinguishing patients across a range of functional disabilities [9]. The association between inflammation and HF has been consistent over time, in both clinical and basic research [10].
Considering the negative impact of frailty on HF outcomes and vice versa, it is important to know this potential relationship and examine the degree by which natriuretic peptides, C-reactive protein (CRP), and NYHA scores may differ in patients with HF with or without frailty. In this systematic review and meta-analysis, we aim to compare the differences in plasma BNP, NT-proBNP, CRP, and NYHA classification, in patients with HF and frailty vs. patients with HF without frailty.
Methods
The revised 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to conduct this systematic review and meta-analysis [11]. The protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42023446607).
Search strategy
From the beginning until July 2023, PubMed, Scopus, Web of Science, and Cochrane Library were searched independently by KP and KI. The search phrases “(heart failure OR ejection fraction) AND frail*” were employed. All article duplicates were removed prior to screening.
Inclusion and exclusion criteria
Studies were included based on the following criteria: (i) data from observational studies (i.e., cross-sectional, longitudinal, and case–control); (ii) patients with HF irrespective of ejection fraction and clinical setting (i.e., inpatients or outpatients); (iii) patients aged ≥ 18 years; (iv) studies including data related to BNP, NT-proBNP, CRP, and NYHA classification for both patients with and without frailty; and (iv) the following criteria for the definition of frailty: Fried’s criteria, Clinical Frailty Scale, FRAIL scale, Rockwood index. Published articles were excluded if they (i) included participants with terminal conditions (i.e., end-stage cancer); (ii) criteria for frailty not specified in inclusion criteria; (iii) were reviews, letters, in vivo or in vitro experiments, commentaries, or posters; and (iv) were not published as a full text and in English.
Data extraction and risk of bias
Two authors (KP and KI) extracted data independently, which included the name of the first author, year of publication, country of origin, study design, definition of frailty, patient characteristics (sample size, age, gender, body mass index (BMI)), left ventricular ejection fraction (LVEF) rate, and reported comorbidities. Disagreements between authors were resolved by a third investigator (KN).
The quality of the included studies was evaluated using the Methodological index for non-randomized studies (MINORS) tool [12] and performed by two independent reviewers (KP and CJ). MINORS is a comprehensive tool used to assess bias in nonrandomized controlled trials based on the following items: a clearly stated aim; inclusion of consecutive patients; prospective data collection; endpoints appropriate to study aim; unbiased assessment of study endpoint; follow-up period appropriate to study aim; < 5% lost to follow-up; prospective calculation of study size; adequate control group; contemporary groups; baseline equivalence of groups; and adequate statistical analyses. According to the scoring system, MINORS’ domains are scored as 0 if they are not reported, 1 when they have been reported but with inadequate details, and 2 when they have been reported while providing adequate information. The global ideal score is 16 for noncomparative studies, and scores below 8 and 10 were deemed as a high risk of bias and of some concerns, respectively.
Statistical analysis
Quantitative data were treated as continuous measurements, and changes in outcomes from sarcopenic and non-sarcopenic individuals were compared between groups to calculate standardised mean differences (SMDs) for the evaluation of differences between groups in relation to BNP, NT-proBNP, and CRP, and the odds ratio (OR) regarding the prevalence of NYHA III and IV levels. SMDs were used due to potentially different methods of assessment, which were not described in the respective manuscripts. When studies provided interquartile ranges (IQR), the formula ‘standard deviation (SD) = width of IQR/1.35’ was used to approximately calculate the missing SDs [13]. Statistical significance was assessed using the random-effects model and inverse-variance method.
Statistical heterogeneity of outcome measurements between different studies was assessed using the overlap of their confidence interval (95% CI) and expressed as measurements of Cochran’s Q (Chi-square test) and I2. The classification of data as having low heterogeneity was based on I2 from 30 to 49%, moderate heterogeneity from 50 to 74% and high heterogeneity from 75% and above [14]. In case of high heterogeneity, meta-regressions were performed using a random-effects model [15] based on BMI, LVEF rate, and age, using STATA/MP 13.0. Subgroup analysis according to different definitions of frailty was also performed. Sensitivity analyses were conducted based on differences in health status (different reported comorbidities between patients with frailty vs. patients without frailty), and studies with increased bias risk. The meta-analysis was synthesized using Review Manager (RevMan 5.4.1) software. A P value of < 0.05 was considered statistically significant.
Results
Search results
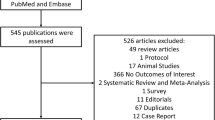
A flow diagram of the selection process is shown in Fig. 1. The initial literature search provided 5191 publications. Following the exclusion of duplicates and abstracts, 53 full texts were identified as eligible for inclusion in the systematic review and meta-analysis. Of these 53 studies, three studies were excluded due to the inclusion of identical, but more recent or more appropriate cohorts that had already been included in our study [16,17,18] and nine studies because of the usage of non-established or non-clear frailty definition [19,20,21,22,23,24,25,26,27]. Overall, 41 studies [7, 18, 24, 28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65] were included in the systematic review and meta-analysis (Fig. 1). Characteristics of the included studies are summarised in Table 1.
BNP levels in patients with heart failure and frailty versus without frailty
Patients with heart failure HF and frailty (n = 1551) had significantly higher levels of BNP vs. those without frailty (n = 1487), albeit a high degree of heterogeneity was observed (k = 11; SMD: 0.53, 95%CI 0.30–0.76, I2 = 86%, P < 0.01) (Fig. 2). Subgroup analysis based on Fried (k = 9; SMD: 0.59, 95%CI 0.31–0.87, I2 = 88%, P < 0.01) and Clinical Frailty Scale (CFS) criteria (k = 2; SMD: 0.23, 95%CI 0.03–0.42, I2 = 0%, P = 0.03) (Figure S1) showed identical statistical outcomes. Our sensitivity analysis excluding studies in which patients with frailty had increased reported comorbidities revealed similar results (k = 5; SMD: 0.34, 95%CI 0.10–0.58, I2 = 51%, P < 0.01) (Figure S2), while when we evaluated similar health status alongside similar frailty definition criteria (Fried criteria in this case), we also found statistically significant differences (k = 5; SMD: 0.34, 95%CI 0.10–0.58, I2 = 51%, P < 0.01) (Figure S3). Sensitivity analysis based on studies with a high risk of bias did not alter the findings from the main analysis (k = 8; SMD: 0.53, 95%CI 0.23–0.84, I2 = 89%, P < 0.01) (Figure S4).
NT-proBNP levels in patients with heart failure and frailty versus without frailty
Our main analysis showed that patients with HF and frailty (n = 8389) had significantly higher levels of NT-proBNP vs. those without frailty (n = 10,040) with a moderate degree of heterogeneity (k = 23; SMD: 0.33, 95%CI 0.25–0.40, I2 = 72%, P < 0.01) (Fig. 3). Subgroup analysis based on Fried (k = 11; SMD: 0.38, 95%CI 0.26–0.50, I2 = 44%, P < 0.01), FRAIL scale (k = 5; SMD: 0.28, 95%CI 0.01–0.54, I2 = 75%, P = 0.04), CFS criteria (k = 3; SMD: 0.44, 95%CI 0.30–0.57, I2 = 24%, P < 0.01), and the Rockwood index (k = 3; SMD: 0.18, 95%CI 0.02–0.33, I2 = 92%, P = 0.03) (Figure S5) demonstrated similar results. Our sensitivity analysis excluding studies in which patients with frailty had increased reported comorbidities revealed identical findings (k = 7; SMD: 0.39, 95%CI 0.22–0.56, I2 = 41%, P < 0.01) (Figure S6), while when we evaluated similar health status alongside similar frailty definition criteria (Fried criteria in this case), statistically significant differences were also observed (k = 6; SMD: 0.32, 95%CI 0.16–0.48, I2 = 23%, P < 0.01) (Figure S7). Sensitivity analysis based on studies with a high risk of bias did not alter the findings of the main analysis (k = 15; SMD: 0.30, 95%CI 0.20–0.40, I2 = 68%, P < 0.01) (Figure S8).
CRP levels in patients with heart failure and frailty versus without frailty
Patients with HF and frailty (n = 1039) had significantly higher levels of CRP vs. those without frailty (n = 986) with a moderate degree of heterogeneity (k = 8; SMD: 0.30, 95%CI 0.12–0.48, I2 = 62%, P < 0.01) (Fig. 4).
For this analysis, the younger and older patients with HF in the study conducted by Aguilar-Iglesias et al. (2022) [32] were both included in the analysis and there was no overlap of participants between groups. In addition, subgroup analysis based on Fried (k = 5; SMD: 0.24, 95%CI 0.13–0.35, I2 = 0%, P < 0.01) and CFS criteria (k = 2; SMD: 0.76, 95%CI 0.20–1.32, I2 = 67%, P < 0.01) depicted identical results, but insignificant differences between groups were found when the FRAIL scale was used solely based on the younger and older patients of the Aguilar-Iglesias et al. (2022) study [32] (SMD: 0.10, 95%CI – 0.22 to 0.41, I2 = 0%, P = 0.54) (Figure S9). Our sensitivity analysis excluding studies in which patients between groups had an increased number of comorbidities did not alter the findings of our main analysis (k = 6; SMD: 0.32, 95%CI 0.09–0.55, I2 = 75%, P < 0.01) (Figure S10). Sensitivity analysis based on studies with a high risk of bias did not alter the findings from the main analysis (k = 5; SMD: 0.25, 95%CI 0.13–0.36, I2 = 9%, P < 0.01) (Figure S11).
NYHA levels in patients with heart failure and frailty versus without frailty
Patients with HF and frailty (n = 8009) have a significantly increased risk of higher NYHA classification score vs. patients without frailty (n = 10,225) with a high degree of heterogeneity (k = 24; OR: 4.23, 95%CI 3.04–5.90, I2 = 91%, P < 0.01) (Fig. 5). Subgroup analysis based on Fried (k = 17; OR: 3.28, 95%CI 2.40–4.49, I2 = 63%, P < 0.01), FRAIL scale (k = 2; OR: 50.35, 95%CI 2.12–1197.89, I2 = 84%, P = 0.02), CFS criteria (k = 2; OR: 4.34, 95%CI 2.87–6.56, I2 = 0%, P < 0.01), and Rockwood index (k = 3; OR: 5.09, 95%CI 2.15–12.08, I2 = 99%, P < 0.01) similarly showed statistically significant results (Figure S12). Sensitivity analysis based on studies with high risk of bias did not alter the findings from the main analysis (k = 14; OR: 3.40, 95%CI 2.58–4.47, I2 = 72%, P < 0.01) (Figure S13).
Meta-regression analyses
Variance among studies for the differences observed in BNP levels was detected in relation to age and BMI (P < 0.01), while in NT-proBNP levels, differences were observed only due to age (P < 0.01). In addition, age (P = 0.04) and BMI (P = 0.047) mediated the response of the association with CRP between groups, whereas for NYHA classification changes, age (P < 0.01) and LVEF% (P = 0.01) were significant moderators (Table S1).
Risk of bias
Of the included studies, 13 studies were considered of having a high risk of bias [7, 31, 32, 39, 41, 43, 46, 52, 53, 60, 61, 63, 64], 16 as moderate risk of bias [18, 29, 33, 36, 37, 42, 44, 45, 48,49,50, 55, 56, 58, 59, 62], and 12 studies had a low risk [28, 30, 34, 35, 38, 40, 47, 51, 54, 57, 65, 66] (Table S2).
Discussion
In this present study, we found that frailty is associated with higher levels of BNP, NT-proBNP, and CRP concentrations, and a worsened functional class (NYHA III/IV) in patients with HF. Age and BMI were covariates that mediated this relationship, partially explaining the aforementioned findings.
Natriuretic peptides and frailty
The prevalence of frailty in individuals with HF is notably elevated, as a result of a common association between frailty and HF, sharing several risk factors [67]. The concurrent progression of these conditions involves shared mechanisms, including systemic inflammation, a higher burden of comorbidities, and abnormal skeletal muscle function and structure [68]. Sarcopenia is highly prevalent in patients with chronic HF, who are predisposed to skeletal muscle atrophy, accompanied by a relatively high proportion of non-muscular constituents such as intramuscular adipose tissue or fibrosis, exacerbating frailty [68]. In addition to the recognized utility of both BNP and NT-proBNP as diagnostic and prognostic indicators for HF patients, these biomarkers have been elucidated to be associated with frailty severity [7, 69, 70].
Our study demonstrates significantly elevated levels of BNP/NT-proBNP in patients with HF, as substantiated by several meta-analyses. In contrast to other meta-analyses focusing on the prevalence and prognostic impact of frailty in HF patients, our analysis extends to the association of BNP/NT-proBNP levels with frailty severity [71,72,73]. Li et al. (2023) highlighted BNP as an influential factor of frailty in older patients with HF, for which our data aligns with their findings. Interestingly, we identified age and BMI as potential covariates that may act as intermediates between frailty and elevated BNP, but not NT-proBNP. This may be explained, in part, due to a more pronounced link between altered BNP levels and adiposity, considering that NT-proBNP is not primarily degraded by natriuretic peptide receptors in adipose tissues [74, 75]. Furthermore, age was a common determinant in both BNP and NT-proBNP analyses, suggesting that older patients may be prone to increased BNP/NT-proBNP levels due to a higher burden of comorbidities such as renal function impairments [76, 77]. Although our subgroup analysis based on studies including commonly reported comorbidities between patients with and without frailty did not alter our observations, multiple studies did not assess for kidney or liver diseases that are contributors to elevated BNP and NT-proBNP concentrations to varied degrees [78, 79]. Lastly, elevation of BNP/NT-proBNP levels in frail patients was discernible across various frailty scales, despite the limitations of quick assessment scales used in clinical settings.
CRP, NYHA class, and frailty
In the context of inflammation and frailty, chronic inflammation, characterized by higher oxidative stress and pro-inflammatory cytokines, is recognized as an important mechanism underpinning frailty, impacting multiple organs [67]. Neurohormonal factors activated in HF, such as the renin–angiotensin–aldosterone system, may further contribute to a pro-inflammatory state [80]. Elevation of CRP in patients with frailty has been well-documented, and our study further corroborated the significant elevation of CRP levels in a cohort of patients with HF and frailty. Our results also suggest that age and BMI could mediate this association, aligning with findings from previous meta-analyses [81, 82]. Moreover, symptoms of HF categorized by NYHA class, despite inherent subjectivity, remain fundamental. For instance, fatigue, a principal characteristic of frailty, complicates the differentiation of symptoms between frailty and HF. Meta-analyses have demonstrated that a preponderance of patients concomitantly exhibiting frailty and HF manifest elevated symptomatology (NYHA III/IV class), aligning with our findings of a significant association between frailty and NYHA class for each frailty score.
Limitations
The inclusion of studies with a diverse age demographic may impact the extrapolation of results to studies predominantly comprised of older-aged cohorts, where elevated BNP/NT-proBNP levels may be influenced by comorbidities, which may had not been reported sufficiently in several trials. In addition, these results cannot be extrapolated in relation to a particular sex, considering that the prevalence of frailty is more pronounced in women compared to men [83]. Likewise, we did not differentiate between HF with reduced (HFrEF) and preserved (HFpEF) ejection fraction, that are characterized by different levels of natriuretic peptides, potentially displaying distinct outcomes linked to frailty. In addition, we were unable to ascertain the potential ramifications of hospitalized versus non-hospitalized patients, given the potential variations in settings, rehabilitation regimens, and severity of HF. Finally, our analyses relied on cross-sectional data, precluding the establishment of causal relationships.
Conclusions
In conclusion, frailty in HF is linked to increased concentrations of BNP, NT-proBNP, and CRP, which have been epidemiologically associated with adverse outcomes. The increased risk of NYHA III/IV classification further emphasizes the clinical impact of frailty in this population.
Data availability
Data are available upon request.
References
Walsh B, Fogg C, Harris S et al (2023) Frailty transitions and prevalence in an ageing population: longitudinal analysis of primary care data from an open cohort of adults aged 50 and over in England, 2006–2017. Age Ageing. 52:afad058
Díez-Villanueva P, Arizá-Solé A, Vidán MT et al (2019) Recommendations of the geriatric cardiology section of the Spanish Society of cardiology for the assessment of frailty in elderly patients with heart disease. Revista Española de Cardiología (English Edition) 72:63–71
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 42:3599–3726
Van Linthout S, Tschöpe C (2017) Inflammation–cause or consequence of heart failure or both? Curr Heart Fail Rep 14:251–265
Kitzman DW, Nicklas B, Kraus WE et al (2014) Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circulat Physiol 306:H1364–H1370
Denfeld QE, Winters-Stone K, Mudd JO et al (2017) The prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol 236:283–289
Nishiguchi S, Nozaki Y, Yamaji M et al (2016) Plasma brain natriuretic peptide level in older outpatients with heart failure is associated with physical frailty, especially with the slowness domain. J Geriatric Cardiol JGC 13:608
Tsutsui H, Albert NM, Coats AJ et al (2023) Natriuretic peptides: role in the diagnosis and management of heart failure: a scientific statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur J Heart Fail 25:616–631
Caraballo C, Desai NR, Mulder H et al (2019) Clinical implications of the New York heart association classification. J Am Heart Assoc 8:e014240
Pascual‐Figal D, Fuster JJ, Bayes‐Genis A (2023) Personalizing Anti‐Inflammatory Therapy in Heart Failure: A New Way. Wiley Online Library
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Slim K, Nini E, Forestier D et al (2003) Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 73:712–716
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:1–10
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database System Rev https://doi.org/10.1002/14651858.ED000142
Zheng P-P, Yao S-M, He W et al (2021) Frailty related all-cause mortality or hospital readmission among adults aged 65 and older with stage-B heart failure inpatients. BMC Geriatr 21:1–9
Tanaka S, Kamiya K, Saito H et al (2021) Prevalence and prognostic value of the coexistence of anaemia and frailty in older patients with heart failure. ESC Heart Failure 8:625–633
Nozaki K, Kamiya K, Hamazaki N et al (2021) Validity and utility of the questionnaire-based FRAIL scale in older patients with heart failure: findings from the FRAGILE-HF. J Am Med Direct Assoc 22:1621-1626.e2
Hamada T, Kubo T, Kawai K et al (2021) Frailty in patients with acute decompensated heart failure in a super-aged regional Japanese cohort. ESC Heart Failure 8:2876–2888
Kałużna-Oleksy M, Kukfisz A, Migaj J et al (2021) A simple risk score based on routine clinical parameters can predict frailty in hospitalized heart failure patients. J Clin Med 10:5963
Son YJ, Kim SW, Lee WS et al (2022) Prevalence and factors associated with pre-frailty and frailty among Korean older adults with heart failure. J Adv Nurs 78:3235–3246
Hornsby WE, Sareini M-A, Golbus JR et al (2019) Lower extremity function is independently associated with hospitalization burden in heart failure with preserved ejection fraction. J Cardiac Fail 25:2–9
Chi J, Chen F, Zhang J et al (2022) Frailty is associated with 90-day unplanned readmissions and death in patients with heart failure: a longitudinal study in China. Heart Lung 53:25–31
Kawashima K, Hirashiki A, Nomoto K et al (2019) Peak work rate during exercise could detect frailty status in elderly patients with stable heart failure. Int Heart J 60:1366–1372
Gastelurrutia P, Lupón J, Altimir S et al (2014) Fragility is a key determinant of survival in heart failure patients. Int J Cardiol 175:62–66
Aung T, Qin Y, Tay WT, Binte Salahudin Bamadhaj NS, Chandramouli C, Ouwerkerk W, et al. (2021) Prevalence and prognostic significance of frailty in Asian patients with heart failure: insights from ASIAN-HF. JACC: Asia. 1(3):303–313
Flores-Álvarez F, Sillero-Herrera A, Cuesta-Gaviño J et al (2022) Frailty as a predictor of clinical problems and events that require elderly patients with heart failure to use health resources. Arch Gerontol Geriatr 101:104698
Pandey A, Khan MS, Garcia K et al (2022) Association of baseline and longitudinal changes in frailty burden and risk of heart failure in Type 2 diabetes—findings from the look AHEAD Trial. J GerontolSeries A 77:2489–2497
Matsuda Y, Masuda M, Asai M et al (2021) Clinical frailty score predicts long-term mortality and hospitalization due to heart failure after implantation of cardiac implantable electric device. Circ J 85:1341–1348
McDonagh J, Ferguson C, Prichard R et al (2023) Comparison of six frailty instruments in adults with heart failure: a prospective cohort pilot study. Eur J Cardiovasc Nurs 22:345–354
Ribeiro ÉCT, Sangali TD, Clausell NO et al (2022) C-reactive protein and frailty in heart failure. Am J Cardiol 166:65–71
Aguilar-Iglesias L, Merino-Merino A, Sanchez-Corral E et al (2022) Differences according to age in the diagnostic performance of cardiac biomarkers to predict frailty in patients with acute heart failure. Biomolecules 12:245
Martín-Sánchez FJ, Rodríguez-Adrada E, Mueller C et al (2017) The effect of frailty on 30-day mortality risk in older patients with acute heart failure attended in the emergency department. Acad Emerg Med 24:298–307
Sze S, Pellicori P, Zhang J, Weston J, Squire I, Clark AL (2021) Effect of frailty on treatment, hospitalisation and death in patients with chronic heart failure. Clin Res Cardiol 1–10
Butt JH, Dewan P, Merkely B et al (2022) Efficacy and safety of dapagliflozin according to frailty in heart failure with reduced ejection fraction: a post hoc analysis of the DAPA-HF trial. Ann Intern Med 175:820–830
Aguilar-Iglesias L, Merino-Merino A, Sanchez-Corral E et al (2023) Fast systematic geriatric assessment in acute heart failure patients admitted in Cardiology. Heart Lung 60:133–138
Mollar A, Bonanad C, Diez-Villanueva P et al (2022) Frailty and hospitalization burden in patients with chronic heart failure. Am J Cardiol 183:48–54
Jiménez-Méndez C, Díez-Villanueva P, Bonanad C et al (2022) Frailty and prognosis of older patients with chronic heart failure. Revista Española de Cardiología (English Edition) 75:1011–1019
Rech DA, da Silveira LS, Martins EM et al (2022) Frailty influences the vascular responsiveness of elderly individuals with chronic heart failure. Microvasc Res 141:104316
Lala A, Shah P, Khalatbari S et al (2022) Frailty measures of patient-reported activity and fatigue may predict 1-year outcomes in ambulatory advanced heart failure: a report from the REVIVAL registry. J Cardiac Fail 28:765–774
Sanders NA, Supiano MA, Lewis EF et al (2018) The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail 20:1570–1577
Rodríguez-Pascual C, Paredes-Galán E, Ferrero-Martínez A-I et al (2017) The frailty syndrome is associated with adverse health outcomes in very old patients with stable heart failure: a prospective study in six Spanish hospitals. Int J Cardiol 236:296–303
Denfeld QE, Winters-Stone K, Mudd JO et al (2017) Frequency of and significance of physical frailty in patients with heart failure. Am J Cardiol 119:1243–1249
Kanenawa K, Isotani A, Yamaji K et al (2021) The impact of frailty according to Clinical Frailty Scale on clinical outcome in patients with heart failure. ESC Heart Failure 8:1552–1561
Metze C, Matzik A-S, Scherner M, Körber MI, Michels G, Baldus S, et al. (2017) Impact of frailty on outcomes in patients undergoing percutaneous mitral valve repair. JACC Cardiovasc Intervent 10(19):1920–1929.
Komici K, Gnemmi I, Bencivenga L et al (2020) Impact of galectin-3 circulating levels on frailty in elderly patients with systolic heart failure. J Clin Med 9:2229
Abe T, Jujo K, Maeda D et al (2023) The interaction between physical frailty and prognostic impact of heart failure medication in elderly patients. ESC Heart Failure. https://doi.org/10.1002/ehf2.14114
Kaul P, Rathwell S, Lam CS et al (2023) Patient-reported frailty and functional status in heart failure with preserved ejection fraction: insights from VITALITY-HFpEF. Heart Failure 11:392–403
Kondo T, Adachi T, Kobayashi K et al (2023) Physical frailty and use of guideline-recommended drugs in patients with heart failure and reduced ejection fraction. J Am Heart Assoc. https://doi.org/10.1161/JAHA.122.026844
Ashikawa H, Adachi T, Iwatsu K et al (2023) Physical frailty may predict 2-year prognosis in elderly patients with acute myocardial infarction-a report from the FLAGSHIP study-. Circ J 87:490–497
Testa G, Curcio F, Liguori I et al (2020) Physical vs. multidimensional frailty in older adults with and without heart failure. ESC Heart Failure. 7:1371–80
Dewan P, Jackson A, Jhund PS et al (2020) The prevalence and importance of frailty in heart failure with reduced ejection fraction–an analysis of PARADIGM-HF and ATMOSPHERE. Eur J Heart Fail 22:2123–2133
Meng C, Chai K, Li YY et al (2023) Prevalence and prognosis of frailty in older patients with stage B heart failure with preserved ejection fraction. ESC Heart Failure 10:1133–1143
Vidán MT, Blaya-Novakova V, Sánchez E et al (2016) Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail 18:869–875
Sunaga A, Hikoso S, Yamada T et al (2021) Prognostic impact of clinical frailty scale in patients with heart failure with preserved ejection fraction. ESC Heart Failure 8:3316–3326
Moayedi Y, Duero Posada JG, Foroutan F et al (2018) The prognostic significance of frailty compared to peak oxygen consumption and B-type natriuretic peptide in patients with advanced heart failure. Clin Transplant 32:e13158
Kusunose K, Okushi Y, Yamada H et al (2018) Prognostic value of frailty and diastolic dysfunction in elderly patients. Circ J 82:2103–2110
Uzun HG, Simsek E, Engin C et al (2022) Relation between frailty and 1-year outcomes after implantation of a left ventricular assist device. Am J Cardiol 173:88–93
Kleipool EE, Wiersinga JH, Trappenburg MC et al (2020) The relevance of a multidomain geriatric assessment in older patients with heart failure. ESC Heart Failure 7:1264–1272
Nozaki K, Hamazaki N, Kamiya K et al (2020) Rising time from bed in acute phase after hospitalization predicts frailty at hospital discharge in patients with acute heart failure. J Cardiol 75:587–593
Archer SH, Lee CS, Gupta N et al (2023) Sex differences in the impact of physical frailty on outcomes in heart failure. Heart Lung 61:66–71
Villarreal D, Ramírez H, Sierra V et al (2023) Sodium-glucose cotransporter 2 inhibitors in frail patients with heart failure: clinical experience of a heart failure unit. Drugs Aging 40:293–299
Boxer RS, Wang Z, Walsh SJ et al (2008) The utility of the 6-minute walk test as a measure of frailty in older adults with heart failure. Am J Geriatr Cardiol 17:7–12
Woo J, Yang X, Lui LT et al (2019) Utility of the FRAIL questionnaire in detecting heart failure with preserved ejection fraction. J Nutr Health Aging 23:373–377
Wang Q, Wang H, Tian H et al (2023) A prospective case-control study examining the relationship between frailty and serum myostatin in older persons with chronic heart failure. Risk Manag Healthcare Policy. 16:1343–1349
Khan MS, Segar MW, Usman MS et al (2022) Frailty, guideline-directed medical therapy, and outcomes in HFrEF: from the GUIDE-IT trial. Heart Failure 10:266–275
Richter D, Guasti L, Walker D et al (2022) Frailty in cardiology: definition, assessment and clinical implications for general cardiology. A consensus document of the Council for cardiology practice (CCP), association for acute cardio vascular care (ACVC), association of cardiovascular nursing and allied professions (ACNAP), European association of preventive cardiology (EAPC), European heart rhythm association (EHRA), Council on valvular heart diseases (VHD), Council on hypertension (CHT), Council of Cardio-Oncology (CCO), working Group (WG) aorta and peripheral vascular diseases, Wg e-Cardiology, Wg thrombosis, of the European Society of cardiology, European primary care cardiology Society (EPCCS). Eur J Prevent Cardiol 29:216–227
Pandey A, Kitzman D, Reeves G (2019) Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment, and management. JACC Heart Failure. 7:1001–1011
Ikeda M, Honda H, Takahashi K et al (2016) N-terminal pro-B-type natriuretic peptide as a biomarker for loss of muscle mass in prevalent hemodialysis patients. PLoS One 11:e0166804
Yao S, Guo J, Shi G et al (2019) Association of BNP with frailty in elderly population: Rugao longevity and ageing study. J Nutr Health Aging 23:73–78
Li T, Jiang Y-L, Kang J, Song S, Du Q-F, Yi X-D (2023) Prevalence and risk factors of frailty in older patients with chronic heart failure: a systematic review and meta-analysis. Aging Clin Experim Res 1–11
Marengoni A, Zucchelli A, Vetrano DL et al (2020) Heart failure, frailty, and pre-frailty: a systematic review and meta-analysis of observational studies. Int J Cardiol 316:161–171
Yang X, Lupón J, Vidán MT et al (2018) Impact of frailty on mortality and hospitalization in chronic heart failure: a systematic review and meta-analysis. J Am Heart Assoc 7:e008251
Krauser DG, Lloyd-Jones DM, Chae CU et al (2005) Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J 149:744–750
Kozhuharov N, Martin J, Wussler D et al (2022) Clinical effect of obesity on N-terminal pro-B-type natriuretic peptide cut-off concentrations for the diagnosis of acute heart failure. Eur J Heart Fail 24:1545–1554
Marinescu M, Oprea VD, Nechita A et al (2023) The use of brain natriuretic peptide in the evaluation of heart failure in geriatric patients. Diagnostics 13:1512
Sarzani R, Spannella F, Giulietti F et al (2016) NT-proBNP and its correlation with in-hospital mortality in the very elderly without an admission diagnosis of heart failure. PLoS ONE 11:e0153759
Araujo T, Vohra I, Palacios P et al (2021) B-type natriuretic peptide (BNP) predicts 90-day mortality and need for paracentesis in cirrhotic patients without systolic heart failure. Sci Rep 11:1697
Mihailovici AR, Donoiu I, Gheonea DI et al (2019) NT-proBNP and echocardiographic parameters in liver cirrhosis: correlations with disease severity. Med Princ Pract 28:432–441
Pacurari M, Kafoury R, Tchounwou PB et al (2014) The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inflamm. https://doi.org/10.1155/2014/689360
Byrne T, Cooke J, Bambrick P et al (2023) Circulating inflammatory biomarker responses in intervention trials in frail and sarcopenic older adults: a systematic review and meta-analysis. Exp Gerontol 177:112199
Marcos-Pérez D, Sánchez-Flores M, Proietti S et al (2020) Association of inflammatory mediators with frailty status in older adults: results from a systematic review and meta-analysis. GeroScience 42:1451–1473
Davis MR, Lee CS, Corcoran A et al (2021) Gender differences in the prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol 333:133–140
Acknowledgements
We would like to thank Dunhill Medical Trust for supporting this study.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
Study concept and design: KP, KI, and KN. Acquisition of data: KP and KI. Analysis and interpretation of data: KP and HI. Statistical analysis: KP. Drafting of the manuscript: KP, HI, and CJ. Risk of bias assessment: CJ, KI, and KN. Critical revision of the manuscript for important intellectual content: FF, RS, GYHL, and MI.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Statement of human and animal rights
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prokopidis, K., Ishiguchi, H., Jordan, C. et al. Association between natriuretic peptides and C-reactive protein with frailty in heart failure: a systematic review and meta-analysis. Aging Clin Exp Res 36, 57 (2024). https://doi.org/10.1007/s40520-024-02713-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40520-024-02713-x