Abstract
Aim
To explore associations of cerebrospinal fluid biomarkers of neurodegeneration and amyloidosis with caregiver burden, cognition and functionality in dementia with Lewy bodies (DLB) paired with late-onset Alzheimer’s disease (AD) and healthy older people.
Methods
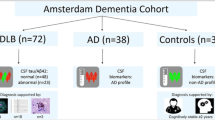
Consecutive outpatients with DLB were matched with outpatients with AD according to sex, cognitive scores and dementia stage, and with cognitively healthy controls according to age and sex to investigate associations of cerebrospinal fluid amyloid-β (Aβ42,Aβ40,Aβ38), tau, phospho-tau Thr181, ubiquitin, α-synuclein and neurofilament light with caregiver burden, functionality, reverse digit span, a clock drawing test, Mini-Mental State Examination (MMSE) and Severe MMSE, adjusted for sex, age, education, dementia duration and APOE-ε4 alleles.
Results
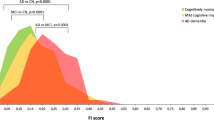
Overall, 27 patients with DLB (78.98 ± 9.0 years-old; eleven APOE-ε4 +) were paired with 27 patients with AD (81.50 ± 5.8 years-old; twelve APOE-ε4 +) and 27 controls (78.98 ± 8.7 years-old; four APOE-ε4 +); two-thirds were women. In AD, Aβ42/Aβ38 and Aβ42 were lower, while tau/Aβ42 and phospho-tau Thr181/Aβ42 were higher; α-synuclein/Aβ42 was lower in DLB and higher in AD. The following corrected associations remained significant: in DLB, instrumental functionality was inversely associated with tau/phospho-tau Thr181 and tau/Aβ42, and reverse digit span associated with α-synuclein; in AD, instrumental functionality was inversely associated with neurofilament light, clock drawing test scores inversely associated with phospho-tau Thr181/Aβ42 and α-synuclein/Aβ42, and Severe MMSE inversely associated with tau/Aβ42 and tau/phospho-tau Thr181.
Conclusions
Cerebrospinal fluid phospho-tau Thr181 in DLB was similar to AD, but not Aβ42. In associations with test scores, biomarker ratios were superior to isolated biomarkers, while worse functionality was associated with axonal degeneration only in AD.

Similar content being viewed by others
Data availability
All data generated during this study have been published and are freely available for download from Mendeley Data at https://doi.org/10.17632/39xyyjdsf3.1.
References
Silverberg N, Elliott C, Ryan L et al (2018) NIA commentary on the NIA-AA research framework: towards a biological definition of Alzheimer’s disease. Alzheimers Dement 14:576–578
Paraskevas GP, Bougea A, Constantinides VC et al (2019) In vivo prevalence of Alzheimer biomarkers in dementia with Lewy bodies. Dement Geriatr Cogn Disord 47:289–296
McKeith IG, Boeve BF, Dickson DW et al (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 89:88–100
De Oliveira FF, Machado FC, Sampaio G et al (2020) Neuropsychiatric feature profiles of patients with Lewy body dementia. Clin Neurol Neurosurg 194:105832
Bertolucci PHF, Brucki SMD, Campacci SR et al (1994) The mini-mental state examination in an outpatient population: influence of literacy. Arq Neuropsiquiatr 52:1–7
Wajman JR, Oliveira FF, Schultz RR et al (2014) Educational bias in the assessment of severe dementia: Brazilian cutoffs for severe mini-mental state examination. Arq Neuropsiquiatr 72:273–277
De Oliveira FF, Wajman JR, Bertolucci PHF et al (2015) Correlations among cognitive and behavioural assessments in patients with dementia due to Alzheimer’s disease. Clin Neurol Neurosurg 135:27–33
Katz S, Akpom CA (1976) A measure of primary socio-biological functions. Int J Health Serv 6:493–508
Lawton MP (1988) Instrumental activities of daily living (IADL) scale: self-reported version. Psychopharmacol Bull 24:789–791
Taub A, Andreoli SB, Bertolucci PHF (2004) Dementia caregiver burden: reliability of the Brazilian version of the Zarit caregiver burden interview. Cad Saude Publica 20:372–376
Boccardi M, Monsch A, Ferrari C et al (2022) Harmonizing neuropsychological assessment for mild neurocognitive disorders in Europe. Alzheimers Dement 18:29–42
Soysal P, Tan SG (2021) The prevalence and co-incidence of geriatric syndromes in older patients with early-stage Alzheimer’s disease and dementia with Lewy bodies. Aging Clin Exp Res 33:2599–2603
Parnetti L, Paciotti S, Farotti L et al (2019) Parkinson’s and Lewy body dementia CSF biomarkers. Clin Chim Acta 495:318–325
Sala A, Nordberg A, Rodriguez-Vieitez E (2021) Longitudinal pathways of cerebrospinal fluid and positron emission tomography biomarkers of amyloid-β positivity. Mol Psychiatry 26:5864–5874
De Oliveira FF, Miraldo MC, Castro-Neto EF et al (2021) Associations of neuropsychiatric features with cerebrospinal fluid biomarkers of amyloidogenesis and neurodegeneration in dementia with Lewy bodies compared with Alzheimer’s disease and cognitively healthy people. J Alzheimers Dis 81:1295–1309
van Steenoven I, Majbour NK, Vaikath NN et al (2018) α-Synuclein species as potential cerebrospinal fluid biomarkers for dementia with Lewy bodies. Mov Disord 33:1724–1733
Pereira JB, Janelidze S, Ossenkoppele R et al (2021) Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer’s disease. Brain 144:310–324
Toledo JB, Korff A, Shaw LM et al (2013) CSF α-synuclein improves diagnostic and prognostic performance of CSF tau and Aβ in Alzheimer’s disease. Acta Neuropathol 126:683–697
Jung JH, Jeon S, Baik K et al (2021) Apolipoprotein E4, amyloid, and cognition in Alzheimer’s and Lewy body disease. Neurobiol Aging 106:45–54
Leonenko G, Shoai M, Bellou E et al (2019) Genetic risk for Alzheimer disease is distinct from genetic risk for amyloid deposition. Ann Neurol 86:427–435
Lautner R, Palmqvist S, Mattson N et al (2014) Apolipoprotein E genotype and the diagnostic accuracy of cerebrospinal fluid biomarkers for Alzheimer disease. JAMA Psychiat 71:1183–1191
Okello A, Koivunen J, Edison P et al (2009) Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology 73:754–760
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the national institute on aging and the Alzheimer’s association workgroup. Alzheimers Dement 7:263–269
Chaves MLF, Camozzato AL, Godinho C et al (2007) Validity of the clinical dementia rating scale for the detection and staging of dementia in Brazilian patients. Alzheimer Dis Assoc Disord 21:210–217
Loeb C, Gandolfo C (1983) Diagnostic evaluation of degenerative and vascular dementia. Stroke 14:399–401
Petersen RC (2011) Mild cognitive impairment. N Engl J Med 364:2227–2234
Teunissen CE, Petzold A, Bennett JL et al (2009) A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73:1914–1922
Oliveira F (2023) Data for: differential associations of clinical features with cerebrospinal fluid biomarkers in dementia with Lewy bodies and Alzheimer’s disease. Mendeley Data V1. https://doi.org/10.17632/39xyyjdsf3.1
Budson AE, Price BH (2007) Memory dysfunction in neurological practice. Pract Neurol 7:42–47
Paterson RW, Slattery CF, Poole T et al (2018) Cerebrospinal fluid in the differential diagnosis of Alzheimer’s disease: clinical utility of an extended panel of biomarkers in a specialist cognitive clinic. Alzheimers Res Ther 10:32
Duits FH, Teunissen CE, Bouwman FH et al (2014) The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement 10:713–723
Kaerst L, Kuhlmann A, Wedekind D et al (2014) Using cerebrospinal fluid marker profiles in clinical diagnosis of dementia with Lewy bodies, Parkinson’s disease, and Alzheimer’s disease. J Alzheimers Dis 38:63–73
van de Beek M, Mofrad RB, van Steenoven I et al (2020) Sex-specific associations with cerebrospinal fluid biomarkers in dementia with Lewy bodies. Alzheimers Res Ther 12:44
Lim X, Yeo JM, Green A et al (2013) The diagnostic utility of cerebrospinal fluid alpha-synuclein analysis in dementia with Lewy bodies—a systematic review and meta-analysis. Parkinsonism Relat Disord 19:851–858
Zetterberg H, Skillbäck T, Mattson N et al (2016) Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol 73:60–67
Ibarra R, Radanovic M, Pais MV et al (2021) AD-related CSF biomarkers across distinct levels of cognitive impairment: correlations with global cognitive state. J Geriatr Psychiatry Neurol 34:659–667
Burns JM, Galvin JE, Roe CM et al (2005) The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology 64:1397–1403
Seo EH, Lim HJ, Yoon HJ et al (2021) Visuospatial memory impairment as a potential neurocognitive marker to predict tau pathology in Alzheimer’s continuum. Alzheimers Res Ther 13:167
Murray ME, Graff-Radford NR, Ross OA et al (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796
Dieks JK, Gawinecka J, Asif AR et al (2013) Low-abundant cerebrospinal fluid proteome alterations in dementia with Lewy bodies. J Alzheimers Dis 34:387–397
Oliveira FF, Machado FC, Sampaio G et al (2015) Contrasts between patients with Lewy body dementia syndromes and APOE-ε3/ε3 patients with late-onset Alzheimer disease dementia. Neurologist 20:35–41
Nalls MA, Duran R, Lopez G et al (2013) A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol 70:727–735
Nielsen HM, Hall S, Surova Y et al (2014) Low levels of soluble NG2 in cerebrospinal fluid from patients with dementia with Lewy bodies. J Alzheimers Dis 40:343–350
Acknowledgements
This work was sponsored by FAPESP – The State of São Paulo Research Foundation (grant #2015/10109-5 and grant #2015/18125-0). The sponsor had no role in study design, in data collection, in the analysis and interpretation of the data, in the writing of the report, or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report. All authors have read the paper and agreed to be listed as authors, having approved it and validated the accuracy of the data. Individual author contributions include, as follows: Fabricio Ferreira de Oliveira, MD, MSc, PhD, is a medical researcher of the Department of Neurology and Neurosurgery of the Federal University of São Paulo—UNIFESP, a Member of the Committee of Experts of the European Science Foundation, of the American Academy of Neurology Global Strategies Subcommittee, of the Awards Committee of the International Parkinson and Movement Disorder Society (MDS, 2021–2023), and of the Executive Committee of the ISTAART Biofluid Based Biomarkers Professional Interest Area (Alzheimer’s Association, 2018–2025); he has received research support from CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and FAPESP—The State of São Paulo Research Foundation, and serves as a healthcare council member for Gerson Lehrman Group, for Atheneum Partners, for Guidepoint, and for Lionbridge; he was involved in conceptualization, methodology, formal analysis, investigation, data curation, writing of the original draft, review and editing, visualization, and project administration. Marjorie Câmara Miraldo, MSc, is a researcher at the Department of Neurology and Neurosurgery of the Federal University of São Paulo—UNIFESP; she was involved in methodology, formal analysis, investigation, data curation, review and editing of the draft. Eduardo Ferreira de Castro-Neto, MSc, PhD, is a researcher at the Department of Neurology and Neurosurgery of the Federal University of São Paulo—UNIFESP; he was involved in methodology, formal analysis, resources, review and editing of the draft. Sandro Soares de Almeida, MSc, PhD, is a researcher at the Department of Biophysics of the Federal University of São Paulo—UNIFESP, and has received grants from CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico and FAPESP—The State of São Paulo Research Foundation; he was involved in conceptualization, methodology, validation, formal analysis, resources, data curation, review and editing of the draft. Sandro Luiz de Andrade Matas, MD, MSc, PhD, is a medical researcher at the Department of Neurology and Neurosurgery of the Federal University of São Paulo—UNIFESP; he was involved in methodology, investigation, resources, review and editing of the draft. Paulo Henrique Ferreira Bertolucci, MD, MSc, PhD, is a full professor of the Department of Neurology and Neurosurgery of the Federal University of São Paulo—UNIFESP; he has received grants from CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and FAPESP—The State of São Paulo Research Foundation, and serves as a consultant for Janssen, Lundbeck, Novartis, Pfizer and Support; he was involved in conceptualization, methodology, formal analysis, resources, review and editing of the draft, project administration and funding acquisition. Maria da Graça Naffah-Mazzacoratti, MSc, PhD, is a full professor of the Department of Neurology and Neurosurgery of the Federal University of São Paulo—UNIFESP, and has received grants from CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico, and FAPESP—The State of São Paulo Research Foundation; she was involved in conceptualization, methodology, formal analysis, resources, review and editing of the draft, supervision, project administration and funding acquisition.
Ethical approval
This study is part of the research project 0370/2015 (CAAE 43868615.5.0000.5505) approved by the Ethics Committee of Hospital São Paulo, Federal University of São Paulo (UNIFESP), in June 2015, following the tenets of the Declaration of Helsinki.
Statement of human and animal rights
This study only included human participants.
Informed consent
All invited patients and their legal representatives agreed to participate and signed the Informed Consent Form before the assessment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, F.F., Miraldo, M.C., de Castro-Neto, E.F. et al. Differential associations of clinical features with cerebrospinal fluid biomarkers in dementia with Lewy bodies and Alzheimer’s disease. Aging Clin Exp Res 35, 1741–1752 (2023). https://doi.org/10.1007/s40520-023-02452-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02452-5