Abstract
Background
Fatigue is a common and often debilitating symptom experienced by many stroke survivors. Significant post stroke fatigue may predispose individuals to other health complications, such as falls, which can lead to fractures and soft tissue injuries. Only limited research has examined the association between fatigue and falls in stroke survivors.
Methods
Data were obtained from the Sax Institute’s 45 and Up Study, from a subset of individuals who had experienced a stroke. The Modified Fatigue Impact Scale—5-item version (MFIS-5) was used to measure the level of fatigue. A logistic regression model, adjusted for stroke characteristics and comorbidities, was used to determine the magnitude of association between change in fatigue score and odds of having had a fall.
Results
A total of 576 participants completed the questionnaire. A total of 214 (37.2%) participants reported having had a fall in the previous 12 months. There was a statistically significant association between fatigue scores and fall status (p < 0.001). Specifically, for every 1-point increase in the fatigue score (MFIS-5) (i.e. higher level of fatigue), the odds of a person having a fall is 1.10 times greater (AOR = 1.10; 95% CI 1.05, 1.15; p < 0.001).
Conclusion
This study revealed an association between an increasing risk of falls with increasing severity of post stroke fatigue. Accurate detection and management of fatigue may help reduce the risk of falls and should be the focus of future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term stroke is typically used to describe a neurological injury to the central nervous system (CNS) by a vascular cause which can be divided into: cerebral infarction; intracerebral haemorrhage (ICH); and subarachnoid haemorrhage (SAH) [1]. Stroke is a major source of morbidity and mortality and is the second leading cause of death and second most common cause of disability adjusted life years (DALY’s) worldwide [2]. Research predicts a rise in the prevalence of stroke, based on evidence of an aging population in countries such as Australia [3]. This could lead to an increased burden across all areas of stroke care, from acute management to rehabilitation and long-term care [3].
Recent advances in stroke management and outcomes have concentrated on hyper-acute and acute care while the post-acute phase has received much less attention [4]. It is estimated that over 30 million stroke survivors are living with post-stroke disability worldwide [5]. Recent research found that one in five people live at least 15 years after a stroke and concluded that poor functional, cognitive and psychological outcomes affect a substantial proportion of these long-term survivors [6]. Common comorbidities among stroke survivors include pain, depression, and fatigue which can significantly affect health related quality of life [7].
Fatigue is common and often debilitating with 40% of stroke patients affected by fatigue [7]. Physical and cognitive fatigue can impact gait control [8], impair balance and limit mobility, which can increase the risk of falls [9]. Post-stroke fatigue can adversely affect daily activities, the ability to return to work, and the participation in rehabilitation programmes [10]. Many factors are thought to contribute to post stroke fatigue, including physical factors such as an increased level of disability, neurophysiological factors such as corticomotor excitability, and psychological factors such as depression and anxiety [10]. Inflammation is also thought to play a significant role, with research demonstrating that inflammatory cytokines are upregulated in the brain after stroke [11]. Specifically, IL-1β appears to be a predictor of post stroke fatigue [12] with researchers suggesting that fatigue after stroke may be due to inflammation-induced sickness behaviour [11]. Significant post stroke fatigue may predispose stroke survivors to other health complications, such as falls.
Falls among stroke survivors are common, with 40–58% of individuals experiencing a fall within 1 year of their stroke [13,14,15]. Falls can lead to further physical health complications such as fractures [16], soft tissue injuries and impairment to functional activities [17]. Additionally, if a hip fracture is sustained as a result of the fall, stroke survivors are less likely to regain independent mobility than the general population [18]. Consequently, stroke survivors often develop psychosocial issues after a fall such as depression [19] and a fear of falling again [20]. This can lead to individuals reducing their level of activity further, causing more physical deconditioning and, loss of independence, resulting in poorer quality of life [17].
To date, only limited research has examined the association between fatigue and falls in stroke survivors. A qualitative review which examined the experiences of recurrent fallers in the first year after stroke reported that participants view themselves at higher risk of falls when they feel tired [21]. However, a small scale observational case–control study which included a fatigue rating scale found no association between fatigue and falls [20]. More research is needed to explore if a relationship between fatigue and risk of falling exists in stroke survivors. The purpose of this research is to address this research gap with the hope of identifying risk factors and therapeutic targets for stroke care and management.
Methods
Sample
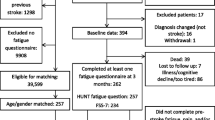
Data were obtained from a sub-study of the Sax Institute’s 45 and Up Study, which is conducted in Australia[22]. The baseline questionnaire collected information from 267,153 men and women aged 45 and above who resided in the state of New South Wales, Australia. Participants were randomly sampled from the Services Australia (formerly the Australian Government Department of Human Services) Medicare enrolment database. The sample represented approximately 11% of the NSW population aged 45 years and over with a response rate of about 18%. The 45 and Up Study was approved by the University of New South Wales Human Research Ethics Committee. People aged 80+ years and residents of rural and remote areas were oversampled. Participants joined the Study by completing a baseline questionnaire between January 2006 and December 2009. The sub-study survey of participants from this cohort occurred between April and October 2017. For this sub-study, 1,300 participants who had previously indicated on the baseline questionnaire that a doctor had diagnosed them as having had a stroke were mailed a sub-study questionnaire, with 576 (44.3% response) returning a completed questionnaire.
Demographic measures
Demographic measures included age, gender, marital status and education. Participants were also asked how they managed on their available income. In addition, area of residence was defined using the Accessibility/Remoteness Index of Australia Plus (ARIA+) remoteness score, which uses postcode to determine road distances to service centres, and thus participants were categorised as residing in a major city, inner regional area, or outer regional/remote area [23].
Stroke-related measures
Participants were asked to specify the time (years/months) since they were first diagnosed with stroke and also the number of strokes they had experienced. The Modified Fatigue Impact Scale—5-item version (MFIS-5) was used to measure the level of fatigue[24]. The total score ranges from 0 to 20, with a higher score indicating more severe fatigue. A MFIS-5 score of > 10 is considered high fatigue[25]. The participants were asked to rate the degree of disability or dependence in the daily activities using the modified Rankin Scale (mRS)[26].
Health status measures
The survey collected self‐reported measures of height and weight, which were used to calculate body mass index (BMI). BMI was used to categorised participants according to WHO recommendations: underweight as BMI < 18.5 kg m2; healthy weight as BMI from 18.5 to 24.99 kg m2; overweight as BMI from 25 to 29.99 kg m2; and obese as BMI ≥ 30 kg m2 [27]. Participants were asked about their consumption of alcohol. Based on National Health and Medical Research Council (NHMRC) guidelines, a variable for alcohol status was derived from the frequency and quantity of alcohol consumption, comprising of 4 categories: ‘non-drinkers’; ‘low risk’ (up to14 drinks per week); ‘risky’ (15–28 drinks per week); and ‘high risk’ (more than 28 drinks per week) [28].
In addition, participants were asked about their smoking status, which was defined as either non-smoking (i.e. never smoked or not smoking now but have smoked in the past) or smoking. Participants were also asked to report the number of times as well as the time (hours and minutes) spent in the last week: walking briskly, in moderate intensity leisure activities (e.g. social tennis, recreational swimming), in vigorous leisure activity (e.g. competitive sport, running), or vigorous household chores (i.e. that make you breathe harder or puff and pant). Responses to these questions were summed and assigned a metabolic equivalent (MET) value. Based on the MET value, participants were categorised to indicate the overall volume of physical activity, as followed: 0 to < 500 (inactive or low); 500 to < 1000 (moderately active); ≥ 1000 (highly active) [29].
The participants were asked if they had been diagnosed or treated by a doctor for any of the following conditions: anxiety/nervous disorder, asthma, cancer, dementia/Alzheimer’s disease, depression, diabetes, heart disease, hypertension, osteoarthritis, and/or osteoporosis. Participants were also asked if they had taken or used any prescription medications, other than those for their stroke, during the past 12 months. These prescription medications were categorised into psychotropic (e.g. Avanza, Epilim, Gabapentin) or non-psychotropic.
Statistical analyses
Chi-square tests were used to examine the association between categorical variables. These included depression status, demographic factors, health status, health care utilisation, and self-care activity measures.. A multiple logistic regression model was then used to determine the magnitude of association between change in fatigue score and odds of having had a fall. Note that this regression model also included potential confounding variables, as determined by the bivariate analyses (i.e. statistically significant demographic and health status characteristics). All analyses were conducted using the statistical software Stata, version 14.1. Statistical significance was set at the α = 0.05 level.
Results
The average age of participants was 75.8 (SD = 9.1) years, with 54.9% being male and 45.1% female. The majority of participants resided in a major city (52.2%), followed by an inner regional area (33.9%) and outer regional or remote area (13.9%).
The associations between stroke characteristics and having had a fall in the previous 12 months are presented in Table 1. It can be seen that a participant was more likely to have had a fall if they had had three or more strokes, compare to those who only had one stroke (p = 0.035). Participants with a high degree of stroke disability were more likely to have had a fall, compared to those with a low degree of disability (p = 0.012). There were 60 (10.4%) participants considered to have high fatigue (MFIS-5 > 10) and there was a statistically significant difference in fatigue scores between those participants who had a fall (mean = 5.5) and those participants who did not have a fall (mean = 3.4) (p < 0.001).
Overall, 214 (37.2%) participants reported having had a fall in the previous 12 months. Details of these falls were as follows: 207 (35.9%) had a fall to the ground; 104 (18.1%) had been injured as a result of a fall; and 89 (15.5%) needed to seek medical attention for an injury from a fall. Table 2 shows the associations between demographic characteristics and having had a fall in the previous 12 months. It can be seen that none of the demographic characteristics were statistically significant associated with having had a fall.
Table 3 shows the association between health status and having had a fall in the previous 12 months. It can be seen that participants with anxiety or nervous disorder were more likely to have had a fall, compared to those without anxiety or nervous disorder (p = 0.001). Participants with depression were more likely to have had a fall, compared to those without depression (p < 0.001). Participants with diabetes were more likely to have had a fall, compared to those without diabetes (p < 0.001). Participants with osteoarthritis were more likely to have had a fall, compared to those without osteoarthritis (p = 0.022). In addition, those participants who were taking a psychotropic medication were more likely to have had a fall, compared to those who were not taking a psychotropic medication (p = 0.001).
A logistic regression model was used to determine the magnitude of association between change in fatigue score and odds of having had a fall. The subsequent odds ratio was adjusted for all variables identified as being statistically significantly associated to falls in the bivariate analyses (Tables 1, 2, 3) (i.e. number of strokes, stroke disability, use of psychotropic medications, anxiety, depression, diabetes, osteoarthritis). The resulting model revealed that fatigue is statistically significantly associated with falls. Specifically, for every 1-point increase in the fatigue score (MFIS-5) (i.e. higher level of fatigue), the odds of a person having a fall is 1.10 times greater (AOR = 1.10; 95% CI 1.05, 1.15; p < 0.001).
Discussion
Our study of middle-aged and older Australian adult stroke survivors revealed that the prevalence on falls was 37.2% and severe fatigue was 10.4%. We hypothesised that the risk of falls among stroke survivors increases with higher levels of fatigue. Our results confirm this association, with a 10% increase in risk of falls for every 1-point increase in fatigue score. Our study finding is consistent with previous research in other nervous system conditions such as multiple sclerosis (MS) [30] and Parkinson’s disease [31]. Research has shown that individuals affected by MS who indicated high fatigue levels performed significantly worse on tests of balance and had more falls in a 6-month period compared to those with low fatigue levels [30]. Similar observations have been made in patients with Parkinson’s disease where severity of fatigue was related to an increased number of falls [31].
Several explanations have been hypothesised for the association between fatigue and risk of falls in older adults including gait control, daytime sleepiness, pain, anaemia, and metabolic disorders [32]. Previous research has shown that physical fatigue also has an impact on walking and gait control in older people [8] and that impaired balance, decreased lower extremity strength, and limited mobility are common physiological risk factors for falling [9]. These are common complaints experienced by stroke survivors which may partially explain the high incidence of falls among this population.
Excessive daytime sleepiness (EDS) becomes a chronic problem in 34% of stroke survivors and can significantly affect a stroke survivor’s daytime functional performance [33]. EDS can increase the frequency of falls through several different mechanisms [34]. For example, for healthy mobilisation and balance coordination, there must be effective integration of visual, vestibular, and proprioceptive senses [35]. This requires a high level of attention which can be lacking in those suffering from EDS [35].
Other explanations postulated for the association between fatigue and falls is the presence of other underlying health conditions such as anaemia [36]. Anaemia is relatively common among stroke survivors with prevalence ranging from 15 to 30% of individuals [37]. A recent systematic review and meta-analysis demonstrated an association between anaemia and poor outcomes in stroke survivors [38]. Research has also demonstrated a relationship between falls during hospitalisation in older patients and the presence of anaemia [36]. Anaemia can lead to weakness, fatigue and limitations to activity [36] which may explain the increased risk of falls.
It is interesting to note that alcohol consumption did not affect the risk of falling in our cohort of stoke survivors. One possible explanation for this finding is that risky consumption of alcohol was low, only 15% of the sample, and as such there may be an insufficient number of risky consumers of alcohol to result in statically significant findings.
This research has highlighted the significant relationship between fatigue and falls in stroke survivors. Due to the many long-term consequences of falls in this demographic, identifying and measuring fatigue should be considered in all fall risk assessments. Management of fatigue in stoke survivors should be the focus of future research, as this may contribute to fall prevention. Unfortunately, evidence-based pharmacological treatment options for treating fatigue in neurological conditions such as stroke are scarce [39]. There are some promising non-pharmaceutical treatments, but more research is needed to determine if these treatments also reduce the risk of falls. Non-pharmacological strategies such as mindfulness-based stress reduction [40], yoga, exercise training and cognitive rehabilitation [41] have shown to be effective in treating fatigue resulting from neurological diseases. But again, more research is needed to determine if these fatigue interventions also impact falls and risk of falling.
This study has utilised widely used, validated instruments to measure key variables used in our analyses and is nested within the largest ongoing cohort study of healthy ageing in the Southern Hemisphere. However, there are some limitations which need to be taken into consideration when interpreting these findings. The cohort data are based on respondents' self-reporting and may have the potential for recall bias. Additionally, the limited information about the type and severity of stroke may impact these results. Although several studies have validated the MFIS-5 instrument in Multiple Sclerosis populations, there has been no such validation of the instrument in stroke populations. However, D’Souza [24], in her review of the MFIS-5 instrument, stated that she found it useful in her assessment of patients with conditions in which fatigue is a dominant symptom, such as stroke [24]. Note also that Larson [42] states that the MFIS-5 does have some limitations when interpreting the scores, particularly when trying to interpret what a change in score means [42].
Conclusion
This study found a significant, positive association between risk of falls and fatigue among older Australian stroke survivors. Accurate detection and management of fatigue may help reduce the risk of falls in stoke survivors and should be the focus of future research.
Data sharing statement
The data set could potentially be made available to other researchers if they obtain the necessary approvals. Further information on this process can be obtained from the 45 and Up Study (45andUp.research@ saxinstitute.org.au).
References
Sacco RL, Kasner SE, Broderick JP et al (2013) An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44:2064–2089
Gorelick PB (2019) The global burden of stroke: persistent and disabling. Lancet Neurol 18:417–418
Clissold BB, Sundararajan V, Cameron P et al (2017) Stroke incidence in Victoria, australia—emerging improvements. Front Neurol 8:180
Gattellari M, Goumas C, Jalaludin B et al (2020) Population-based stroke surveillance using big data: state-wide epidemiological trends in admissions and mortality in New South Wales, Australia. Neurol Res 42:587–596
Tse T, Binte Yusoff SZ, Churilov L et al (2017) Increased work and social engagement is associated with increased stroke specific quality of life in stroke survivors at 3 months and 12 months post-stroke: a longitudinal study of an Australian stroke cohort. Top Stroke Rehabil 24:405–414
Crichton SL, Bray BD, McKevitt C et al (2016) Patient outcomes up to 15 years after stroke: survival, disability, quality of life, cognition and mental health. J Neurol Neurosurg Psychiatry 87:1091–1098
Naess H, Lunde L, Brogger J (2012) The effects of fatigue, pain, and depression on quality of life in ischemic stroke patients: the Bergen Stroke Study. Vasc Health Risk Manag 8:407
Helbostad JL, Leirfall S, Moe-Nilssen R et al (2007) Physical fatigue affects gait characteristics in older persons. J Gerontol A Biol Sci Med Sci 62:1010–1015
Sousa LM, Marques-Vieira CM, Caldevilla MN, Henriques CM, Severino SS, Caldeira SM (2017) Risk for falls among community-dwelling older people: systematic literature review. Rev Gaucha Enferm 37:e55030
Drummond A, Hawkins L, Sprigg N et al (2017) The Nottingham Fatigue after Stroke (NotFAST) study: factors associated with severity of fatigue in stroke patients without depression. Clin Rehabil 31:1406–1415
De Doncker W, Dantzer R, Ormstad H et al (2018) Mechanisms of poststroke fatigue. J Neurol Neurosurg Psychiatry 89:287–293
Ormstad H, Aass HCD, Amthor K-F et al (2011) Serum cytokine and glucose levels as predictors of poststroke fatigue in acute ischemic stroke patients. J Neurol 258:670–676
Alemdaroğlu E, Uçan H, Topçuoğlu AM et al (2012) In-hospital predictors of falls in community-dwelling individuals after stroke in the first 6 months after a baseline evaluation: a prospective cohort study. Arch Phys Med Rehabil 93:2244–2250
Mackintosh SF, Hill KD, Dodd KJ et al (2006) Balance score and a history of falls in hospital predict recurrent falls in the 6 months following stroke rehabilitation. Arch Phys Med Rehabil 87:1583–1589
Forster A, Young J (1995) Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ 311:83–86
Mackintosh SF, Hill K, Dodd KJ et al (2005) Falls and injury prevention should be part of every stroke rehabilitation plan. Clin Rehabil 19:441–451
Xu T, Clemson L, O’Loughlin K et al (2018) Risk factors for falls in community stroke survivors: a systematic review and meta-analysis. Arch Phys Med Rehabil 99:563-573.e5
Ramnemark A, Nilsson M, Borssén B et al (2000) Stroke, a major and increasing risk factor for femoral neck fracture. Stroke 31:1572–1577
Hackett ML, Yapa C, Parag V et al (2005) Frequency of depression after stroke: a systematic review of observational studies. Stroke 36:1330–1340
Watanabe Y (2005) Fear of falling among stroke survivors after discharge from inpatient rehabilitation. Int J Rehabil Res 28:149–152
Walsh ME, Galvin R, Williams DJ et al (2019) The experience of recurrent fallers in the first year after stroke. Disabil Rehabil 41:142–149
Collaborators US (2008) Cohort profile: the 45 and up study. Int J Epidemiol 37:941–947
Rural A (2004) Regional and remote health: a guide to remoteness classifications. Australian Institute of Health and Welfare AIHW Cat. No. PHE, Canberra, vol 53
D’Souza E (2016) Modified fatigue impact scale–5-item version (MFIS-5). Occup Med 66:256–257
Garg H, Bush S, Gappmaier E (2016) Associations between fatigue and disability, functional mobility, depression, and quality of life in people with multiple sclerosis. Int J MS Care 18:71–77
Wilson JL, Hareendran A, Grant M et al (2002) Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke 33:2243–2246
W. H. Organization (2000) Obesity: preventing and managing the global epidemic
National Health and Medical Research Council (NHMRC) (2001) Australian alcohol guidelines: health risks and benefits. Endorsed October 2001. Commonwealth of Australia, Canberra (ACT)
Australian Government Department of Health (AGDH) (2014) Australia’s physical activity and sedentary behaviour guidelines for adults (18–64 years). Australia Department of Health, Canberra
Vister E, Tijsma ME, Hoang PD et al (2017) Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care 19:91–98
Khalil H, Alissa N, Al-Sharman A et al (2021) Understanding the influence of pain and fatigue on physical performance, fear of falling and falls in people with Parkinson’s disease: a pilot study. Neurodegener Dis Manag 11:113–124
Hedman AMR, Fonad E, Sandmark H (2013) Older people living at home: associations between falls and health complaints in men and women. J Clin Nurs 22:2945–2952
Ding Q, Whittemore R, Redeker N (2016) Excessive daytime sleepiness in stroke survivors: an integrative review. Biol Res Nurs 18:420–431
Soysal P, Smith L, Tan SG et al (2021) Excessive daytime sleepiness is associated with an increased frequency of falls and sarcopenia. Exp Gerontol 150:111364
Robillard R, Prince F, Filipini D et al (2011) Aging worsens the effects of sleep deprivation on postural control. PLoS ONE 6:e28731
Dharmarajan T, Avula S, Norkus EP (2006) Anemia increases risk for falls in hospitalized older adults: an evaluation of falls in 362 hospitalized, ambulatory, long-term care, and community patients. J Am Med Dir Assoc 7:287–293
Hao Z, Wu B, Wang D et al (2013) A cohort study of patients with anemia on admission and fatality after acute ischemic stroke. J Clin Neurosci 20:37–42
Barlas RS, Honney K, Loke YK et al (2016) Impact of hemoglobin levels and anemia on mortality in acute stroke: analysis of UK regional registry data, systematic review, and meta-analysis. J Am Heart Assoc 5:e003019
Penner I-K, Paul F (2017) Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol 13:662–675
Johansson B, Bjuhr H, Rönnbäck L (2012) Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Inj 26:1621–1628
Zedlitz AM, Rietveld TC, Geurts AC et al (2012) Cognitive and graded activity training can alleviate persistent fatigue after stroke: a randomized, controlled trial. Stroke 43:1046–1051
Larson RD (2013) Psychometric properties of the modified fatigue impact scale. Int J MS Care 15:15–20
Acknowledgements
This research was completed using data collected through the 45 and Up Study (http://www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW; and partners: the Heart Foundation; NSW Ministry of Health; NSW Department of Communities and Justice; and the Australian Red Cross Lifeblood. We thank the many thousands of people participating in the 45 and Up Study. Distinguished Professor Jon Adams was supported by the Australian Research Council (ARC) Professorial Future Fellowship (FT140100195) while working on this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
DS, JA, JM conceptualised and designed the study; WP analysed the data and performed the statistical analysis. JB drafted the manuscript with edits from DS and JA. All authors contributed to the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics statements
Ethical approval for the use of the sub-study dataset from the 45 and Up Study was gained from the Human Research Ethics Committees at the University of Technology Sydney on 7th October 2015 (UTS HREC REF NO. 2015000683).
Informed consent
All participants gave their written informed consent prior to their inclusion in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Distinguished Professor Jon Adams was supported by the Australian Research Council (ARC) Professorial Future Fellowship (FT140100195) while working on this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sibbritt, D., Bayes, J., Peng, W. et al. The association between fatigue severity and risk of falls among middle-aged and older Australian stroke survivors. Aging Clin Exp Res 34, 2457–2463 (2022). https://doi.org/10.1007/s40520-022-02179-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-022-02179-9