Abstract
Background
Leukocyte telomere length (LTL) is a robust marker of biological aging, which is associated with obesity. Recently, the visceral adiposity index (VAI) has been proposed as an indicator of adipose distribution and function.
Objective
To evaluated the association between VAI and LTL in adult Americans.
Methods
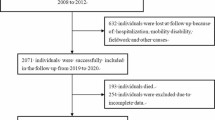
There were 3193 participants in U.S. National Health and Nutrition Examination Surveys (1999–2002) included in this analysis. LTL was measured using quantitative PCR (qPCR) and expressed as telomere to single-gene copy ratio (T/S ratio). We performed multiple logistic regression models to explore the association between VAI and LTL by adjusting for potential confounders.
Results
Among all participants, VAI was associated with the shorter LTL (β: – 14.81, 95% CI – 22.28 to – 7.34, p < 0.001). There were significant differences of LTL in VAI tertiles (p < 0.001). Participants in the higher VAI tertile had the shorter LTL (1.26 ≤ VAI < 2.46: β = – 130.16, 95% CI [ – 183.44, – 76.87]; VAI ≥ 2.46: β = – 216.12, 95% CI [ – 216.12, – 81.42], p for trend: < 0.001) comparing with the lower VAI tertile. We also found a non-linear relationship between VAI and LTL. VAI was negatively correlated with LTL when VAI was less than 2.84.
Conclusions
The present study demonstrates that VAI is independently associated with telomere length. A higher VAI is associated with shorter LTL. The results suggest that VAI may provide prediction for LTL and account for accelerating the biological aging.


Similar content being viewed by others
Data availability
The data that support the results of this research are available from the corresponding author upon reasonable request.
References
Aubert G, Lansdorp PM (2008) Telomeres and aging. Physiol Rev 88:557–579. https://doi.org/10.1152/physrev.00026.2007
Müezzinler A, Zaineddin AK, Brenner H (2013) A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 12:509–519. https://doi.org/10.1016/j.arr.2013.01.003
Haycock PC, Heydon EE, Kaptoge S et al (2014) Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349:g4227. https://doi.org/10.1136/bmj.g4227
Salpea KD, Talmud PJ, Cooper JA et al (2010) Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 209:42–50. https://doi.org/10.1016/j.atherosclerosis.2009.09.070
Cohen S, Janicki-Deverts D, Turner RB et al (2013) Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA 309:699–705. https://doi.org/10.1001/jama.2013.613
Zhang X, Zhao Q, Zhu W et al (2017) The association of telomere length in peripheral blood cells with cancer risk: a systematic review and meta-analysis of prospective studies. Cancer Epidem Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 26:1381–1390. https://doi.org/10.1158/1055-9965.EPI-16-0968
Wentzensen IM, Mirabello L, Pfeiffer RM et al (2011) The association of telomere length and cancer: a meta-analysis. Cancer Epidem Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 20:1238–1250. https://doi.org/10.1158/1055-9965.EPI-11-0005
Zhang J, Rane G, Dai X et al (2016) Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res Rev 25:55–69. https://doi.org/10.1016/j.arr.2015.11.006
Masi S, D’Aiuto F, Cooper J et al (2016) Telomere length, antioxidant status and incidence of ischaemic heart disease in type 2 diabetes. Int J Cardiol 216:159–164. https://doi.org/10.1016/j.ijcard.2016.04.130
Saltiel AR, Olefsky JM (2017) Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127:1–4. https://doi.org/10.1172/JCI92035
Vona R, Gambardella L, Cittadini C et al (2019) Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev 2019:8267234. https://doi.org/10.1155/2019/8267234
Lee M, Martin H, Firpo MA et al (2011) Inverse association between adiposity and telomere length: The fels longitudinal study. Am J Hum Biol Off J Hum Biol Counc 23:100–106. https://doi.org/10.1002/ajhb.21109
Batsis JA, Mackenzie TA, Vasquez E et al (2005) (2018) Association of adiposity, telomere length and mortality: data from the NHANES 1999–2002. Int J Obes 42:198–204. https://doi.org/10.1038/ijo.2017.202
Abbasalizad-Farhangi M (2021) Central obesity accelerates leukocyte telomere length (LTL) shortening in apparently healthy adults: A systematic review and meta-analysis. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1971155
Mundstock E, Sarria EE, Zatti H et al (2015) Effect of obesity on telomere length: Systematic review and meta-analysis. Obes Silver Spring Md 23:2165–2174. https://doi.org/10.1002/oby.21183
Hegazi RAF, Sutton-Tyrrell K, Evans RW et al (2003) Relationship of adiposity to subclinical atherosclerosis in obese patients with type 2 diabetes. Obes Res 11:1597–1605. https://doi.org/10.1038/oby.2003.212
Janghorbani M, Salamat MR, Aminorroaya A et al (2017) Utility of the visceral adiposity index and hypertriglyceridemic waist phenotype for predicting incident hypertension. Endocrinol Metab Seoul Korea 32:221–229. https://doi.org/10.3803/EnM.2017.32.2.221
Son D-H, Ha H-S, Lee HS et al (2021) Association of the new visceral adiposity index with coronary artery calcification and arterial stiffness in Korean population. Nutr Metab Cardiovasc Dis NMCD 31:1774–1781. https://doi.org/10.1016/j.numecd.2021.02.032
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47. https://doi.org/10.1093/nar/30.10.e47
Rehkopf DH, Needham BL, Lin J et al (2016) Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: a cross-sectional study of US adults. PLoS Med 13:e1002188. https://doi.org/10.1371/journal.pmed.1002188
Bosello O, Vanzo A (2021) Obesity paradox and aging. Eat Weight Disord EWD 26:27–35. https://doi.org/10.1007/s40519-019-00815-4
Kammerlander AA, Lyass A, Mahoney TF et al (2021) Sex differences in the associations of visceral adipose tissue and cardiometabolic and cardiovascular disease risk: the framingham heart study. J Am Heart Assoc 10:e019968. https://doi.org/10.1161/JAHA.120.019968
Sanders JL, Newman AB (2013) Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 35:112–131. https://doi.org/10.1093/epirev/mxs008
German CA, Laughey B, Bertoni AG et al (2020) Associations between BMI, waist circumference, central obesity and outcomes in type II diabetes mellitus: The ACCORD Trial. J Diabetes Compl 34:107499. https://doi.org/10.1016/j.jdiacomp.2019.107499
Njajou OT, Cawthon RM, Blackburn EH et al (2005) (2012) Shorter telomeres are associated with obesity and weight gain in the elderly. Int J Obes 36:1176–1179. https://doi.org/10.1038/ijo.2011.196
Müezzinler A, Mons U, Dieffenbach AK et al (2016) Body mass index and leukocyte telomere length dynamics among older adults: Results from the ESTHER cohort. Exp Gerontol 74:1–8. https://doi.org/10.1016/j.exger.2015.11.019
Diaz VA, Mainous AG, Player MS et al (2005) (2010) Telomere length and adiposity in a racially diverse sample. Int J Obes 34:261–265. https://doi.org/10.1038/ijo.2009.198
MacEneaney OJ, Kushner EJ, Westby CM et al (2010) Endothelial progenitor cell function, apoptosis, and telomere length in overweight/obese humans. Obes Silver Spring Md 18:1677–1682. https://doi.org/10.1038/oby.2009.494
Amato MC, Giordano C, Galia M et al (2010) Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 33:920–922. https://doi.org/10.2337/dc09-1825
Matsuda M, Shimomura I (2013) Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 7:e330-341. https://doi.org/10.1016/j.orcp.2013.05.004
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344. https://doi.org/10.1016/s0968-0004(02)02110-2
Erusalimsky JD (2020) Oxidative stress, telomeres and cellular senescence: What non-drug interventions might break the link? Free Radic Biol Med 150:87–95. https://doi.org/10.1016/j.freeradbiomed.2020.02.008
Vaiserman A, Krasnienkov D (2020) Telomere length as a marker of biological age: state-of-the-art, open issues, and future perspectives. Front Genet 11:630186. https://doi.org/10.3389/fgene.2020.630186
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81870310 and 81200194).
Author information
Authors and Affiliations
Contributions
The authors’ contributions were as follows—conception and design of the research: YF, JG, MC. Analysis and interpretation of data: YF, JG, YG. Assistant in data analysis: XZ and HC. Draft the manuscript: YF. Assistant in the manuscript preparation: JZ, PS. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
None to declare.
Ethical approval
NHANES program was approved by the NCHS Ethics Review Board.
Informed consent
All the participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., Guo, Y., Zhong, J. et al. The association between visceral adiposity index and leukocyte telomere length in adults: results from National Health and Nutrition Examination Survey. Aging Clin Exp Res 34, 2177–2183 (2022). https://doi.org/10.1007/s40520-022-02168-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-022-02168-y